Production and Characterization of k-Carrageenan Films Incorporating Cymbopogon winterianus Essential Oil as New Food Packaging Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biopolymer and Essential Oil
2.2. Essential Oil Chemical Analysis: Gas Chromatography-Mass Spectrometry (GC-MS)
2.3. Antioxidant Activity Evaluation
2.4. Antibacterial and Anti-Quorum Sensing Activities Evaluation
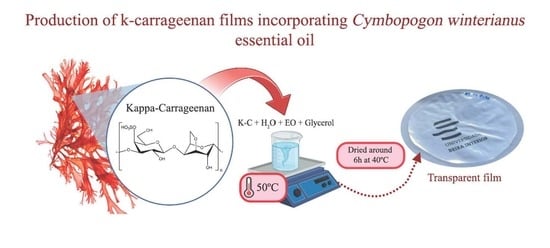
2.5. Production of Bioactive Films
2.6. Film Characterization
2.6.1. Fourier-Transform Infrared (FTIR) Spectroscopy
2.6.2. Physical Properties
2.6.3. Contact Angles and Surface Free Energy
2.6.4. Barrier Properties
2.6.5. Antioxidant Activity
2.6.6. Antibacterial and Anti-Quorum Sensing Activities
2.6.7. Anti-Biofilm Properties
2.7. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition of C. winterianus Essential Oil
3.2. Antioxidant Activity of the Essential Oil
3.3. Antibacterial and Anti-Quorum Sensing Properties of the Essential Oil
3.4. FTIR Spectra of the Films
3.5. Physical Properties of the Films
3.6. Contact Angle and Surface Free Energies
3.7. Barrier Properties
3.8. Antioxidant Activity of the Films
3.9. Antibacterial and Anti-Quorum Sensing Properties of the Films
3.10. Anti-Biofilm Activity of Film against Listeria monocytogenes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abou-Zeid, D.M.; Müller, R.J.; Deckwer, W.D. Degradation of natural and synthetic polyesters under anaerobic conditions. J. Biotechnol. 2001, 86, 113–126. [Google Scholar] [CrossRef]
- Vroman, I.; Tighzert, L. Biodegradable polymers. Materials 2009, 2, 307–344. [Google Scholar] [CrossRef]
- Borrelle, S.B.; Ringma, J.; Law, K.L.; Monnahan, C.C.; Lebreton, L.; McGivern, A.; Murphy, E.; Jambeck, J.; Leonard, G.H.; Rochman, C.M. Mitigate Plastic Pollution. Science 2020, 1518, 1515–1518. [Google Scholar] [CrossRef] [PubMed]
- Brandon, J.A.; Jones, W.; Ohman, M.D. Multidecadal increase in plastic particles in coastal ocean sediments. Sci. Adv. 2019, 5, eaax0587. [Google Scholar] [CrossRef] [PubMed]
- Lim, X.Z. Microplastics are everywhere-but are they harmful? Nature 2021, 593, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Delangiz, N.; Aliyar, S.; Pashapoor, N.; Nobaharan, K.; Lajayer, B.A.; Rodríguez-Couto, S. Can polymer-degrading microorganisms solve the bottleneck of plastics’ environmental challenges? Chemosphere 2022, 294, 133709. [Google Scholar] [CrossRef]
- Xie, Q.; Liu, G.; Zhang, Y.; Yu, J.; Wang, Y.; Ma, X. Active edible films with plant extracts: A updated review of their types, preparations, reinforcing properties, and applications in muscle foods packaging and preservation. Crit. Rev. Food Sci. Nutr. 2022. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yang, Z.; Wang, Z.; Li, J.; Zhang, X. Flexible sensing enabled packaging performance optimization system (FS-PPOS) for lamb loss reduction control in E-commerce supply chain. Food Control 2023, 145, 109394. [Google Scholar] [CrossRef]
- Man, C.M.D. Food Storage Trials; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 9780081004357. [Google Scholar] [CrossRef]
- Ansar, B.S.K.; Kavusi, E.; Dehghanian, Z.; Pandey, J.; Lajayer, B.A.; Price, G.W.; Astatkie, T. Removal of organic and inorganic contaminants from the air, soil, and water by algae. Environ. Sci. Pollut. Res. 2022. [Google Scholar] [CrossRef]
- Zarina, S.; Ahmad, I. Biodegradable composite films based on k-carrageenan reinforced by cellulose nanocrystal from kenaf fibers. Bioresources 2015, 10, 256–271. [Google Scholar] [CrossRef]
- Pereira, L. Carrageenans: Sources and Extraction Methods, Molecular Structure, Bioactive Properties and Health Effects; Nova Science Publishers: Hauppauge, NY, USA, 2016. [Google Scholar]
- Necas, J.; Bartosikova, L. Carrageenan: A review. Vet. Med. 2013, 58, 187–205. [Google Scholar] [CrossRef]
- Zia, K.M.; Tabasum, S.T.; Nasif, M.; Sultan, N.; Aslam, N.; Noreen, A.; Zuber, M. A review on synthesis, properties and applications of natural polyme- based carrageenan blends and composites. Int. J. Biol. Macromol. 2017, 96, 282–301. [Google Scholar] [CrossRef] [PubMed]
- Campo, V.L.; Kawano, D.F.; Silva, D.B.; Carvalho, I. Carrageenans: Biological properties, chemical modifications and structural analysis—A review. Carbohydr. Polym. 2009, 77, 167–180. [Google Scholar] [CrossRef]
- Fatima, S.; Abad, A.H.F.; Sharma, S. Physiological and metabolic responses of different genotypes of Cymbopogon martinii and C. winterianus to water stress. Plant Growth Regul. 2002, 37, 143–149. [Google Scholar] [CrossRef]
- Blank, A.F.; Costa, A.G.; Arrigoni-Blank, M.F.; Cavalcanti, S.C.H.; Alvez, P.B.; Innecco, R.; Ehlert, P.A.D.; Sousa, I.F. Influence of season, harvest time and drying on Java citronella (Cymbopogon winterianus Jowitt) volatile oil. Bras. Farmacogn. 2007, 17, 557–564. [Google Scholar] [CrossRef]
- Curtis, C.F.; Lines, J.D.; Ijumba, J.; Callaghan, A.; Hill, N. The relative efficacy of repellents against mosquito vectors of disease. Med. Vet. Etomol. 1987, 1, 109–119. [Google Scholar] [CrossRef]
- Wany, A.; Jha, S.; Nigam, V.K.; Pandey, D.M. Chemical analysis and therapeutic uses of citronella oil from Cymbopogon winterianus: A short review. Int. J. Adv. Res. 2013, 1, 504–521. [Google Scholar]
- Sedikelo, G.K.; Lenetha, G.G.; Malebo, N.J. Chromatography-mass spectrometry and chemical characteristics of Thymus zygis and Cymbopogon winterianus essential oils: Possible insect repellents. Sci. Afr. 2022, 15, e01095. [Google Scholar] [CrossRef]
- Verma, R.S.; Verma, S.K.; Tandon, S.; Padalia, R.C.; Darokar, M.P. Chemical composition and antimicrobial activity of Java citronella (Cymbopogon winterianus Jowitt ex Bor) essential oil extracted by different methods. J. Essent. Oil Res. 2020, 1, 449–455. [Google Scholar] [CrossRef]
- Luís, Â.; Pereira, L.; Domingues, F.; Ramos, A. Development of a carboxymethyl xylan film containing licorice essential oil with antioxidant properties to inhibit the growth of foodborne pathogens. LWT Food Sci. Technol. 2019, 111, 218–225. [Google Scholar] [CrossRef]
- Luís, Â.; Ramos, A.; Domingues, F. Pullulan films containing rockrose essential oil for potential food packaging applications. Antibiotics 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Kamath, A.; Shukla, A.; Patel, D. Quorum Sensing and Quorum Quenching: Two sides of the same coin. Physiol. Mol. Plant Pathol. 2023, 123, 101927. [Google Scholar] [CrossRef]
- Luís, Â.; Domingues, F.; Ramos, A. Production of hydrophobic zein-based films bioinspired by the lotus leaf surface: Characterization and bioactive properties. Microorganisms 2019, 7, 267. [Google Scholar] [CrossRef]
- Luís, Â.; Gallardo, E.; Ramos, A.; Domingues, F. Design and characterization of bioactive bilayer films: Release kinetics of isopropyl palmitate. Antibiotics 2020, 9, 443. [Google Scholar] [CrossRef]
- Luís, Â.; Ramos, A.; Domingues, F. Pullulan–apple fiber biocomposite films: Optical, mechanical, barrier, antioxidant and antibacterial properties. Polymers 2021, 13, 870. [Google Scholar] [CrossRef] [PubMed]
- Luthra, R.; Singh, N.; Sharma, S. Changes in monoterpene content accompanying development of Cymbopogon winterianus Jowitt leaves. J. Essent. Oil Res. 1991, 3, 349–354. [Google Scholar] [CrossRef]
- Rodrigues, K.A.F.; Dias, C.N.; Amaral, F.M.M.A.; Moraes, D.F.C.; Filho, V.E.M.; Andrade, E.H.A.; Maia, J.G.S. Molluscicidal and larvicidal activities and essential oil composition of Cymbopogon winterianus. Pharm. Biol. 2013, 1, 1293–1297. [Google Scholar] [CrossRef]
- Leite, B.L.S.; Bonfim, R.R.; Antoniolli, A.R.; Thomazzi, S.M.; Araújo, A.A.S.; Blank, A.F.; Estevam, C.S.; Cambui, E.V.F.; Bonjardim, L.R.; Júnior, R.L.C.A.; et al. Assessment of antinociceptive, anti-inflammatory and antioxidant properties of Cymbopogon winterianus leaf essential oil. Pharm. Biol. 2010, 48, 1164–1169. [Google Scholar] [CrossRef]
- Kaur, H.; Bhardwaj, U.; Kaur, R. Cymbopogon nardus essential oil: A comprehensive review on its chemistry and bioactivity. J. Essent. Oil Res. 2021, 33, 205–220. [Google Scholar] [CrossRef]
- Victoria, F.N.; Radatz, C.S.; Sachini, M.; Jacob, R.G.; Alves, D.; Savegnago, L.; Perin, G.; Motta, A.S.; Silva, W.P.; Lenardão, E.J. Further analysis of the antimicrobial activity of α-phenylseleno citronellal and α-phenylseleno citronellol. Food Control 2012, 23, 95–99. [Google Scholar] [CrossRef]
- Fatima, K.; Luqman, S. Citronellal suppress the activity of ornithine decarboxylase in hypopharyngeal carcinoma cells. S. Afr. J. Bot. 2021, 143, 443–448. [Google Scholar] [CrossRef]
- Nakahara, K.; Alzoreky, N.S.; Yoshihashi, T.; Nguyen, H.T.T.; Trakoontivakorn, G. Chemical Composition and Antifungal Activity of Essential Oil from Cymbopogon nardus (Citronella Grass). Jpn. Agric. Res. Q. 2013, 37, 249–252. [Google Scholar] [CrossRef]
- Xie, S.; Wu, G.; Ren, R.; Xie, R.; Yin, H.; Chen, H.; Yang, B.; Zhang, Z.; Maosheng, G. Transcriptomic and metabolic analyses reveal differences in monoterpene profiles and the underlying molecular mechanisms in six grape varieties with different flavors. LWT Food Sci. Technol. 2023, 174, 114442. [Google Scholar] [CrossRef]
- Zielińska-Błajet, M.; Feder-Kubis, J. Monoterpenes and their derivatives—Recent development in biological and medical applications. Int. J. Mol. Sci. 2020, 21, 7078. [Google Scholar] [CrossRef] [PubMed]
- Waterman, P.G. Volatile Oil Crops: Their Biology, Biochemistry, and Production; John Wiley and Sons Inc.: New York, NY, USA, 1993; ISBN 0-470-22087-2. [Google Scholar]
- Ishnava, K.B.; Chauhan, J.B.; Barad, M.B. Anticariogenic and phytochemical evaluation of Eucalyptus globules Labill. Saudi J. Biol. Sci. 2013, 20, 69–74. [Google Scholar] [CrossRef]
- Domínguez, R.; Barba, F.J.; Gómez, B.; Putnik, P.; Kovaceviv, D.B.; Pateiro, M.; Santos, E.M.; Lorenzo, J.M. Active packaging films with natural antioxidants to be used in meat industry: A review. Food Res. Int. 2018, 113, 93–101. [Google Scholar] [CrossRef]
- Sacchetti, G.; Maietti, S.; Muzzoli, M.; Scaglianti, M.; Manfredini, S.; Radice, M.; Bruni, R. Comparative evaluation of 11 essential oils of different origin as functional antioxidants, antiradicals and antimicrobials in foods. Food Chem. 2005, 91, 621–632. [Google Scholar] [CrossRef]
- Ribeiro-Santos, R.; Andrade, M.; Melo, N.R.; Sanches-Silva, A. Use of essential oils in active food packaging: Recent advances and future trends. Trends Food Sci. Technol. 2017, 61, 132–140. [Google Scholar] [CrossRef]
- Simic, A.; Rancic, A.; Sokovic, M.D.; Ristic, M.; Grujic-Jovanovic, S.; Vukojevic, J.; Marin, P.D. Essential oil composition of Cymbopogon winterianus and Carum carvi and their antimicrobial activities. Pharm. Biol. 2008, 46, 437–441. [Google Scholar] [CrossRef]
- Camargo, A.C.; Woodward, J.J.; Call, D.R.; Nero, L.A. Listeria monocytogenes in Food-Processing Facilities, Food Contamination, and Human Listeriosis: The Brazilian Scenario. Foodborne Pathog. Dis. 2017, 14, 623–636. [Google Scholar] [CrossRef]
- Ragon, M.; Wirth, T.; Hollandt, F.; Lavenir, R.; Lecuit, M.; Monnier, A.L.; Brisse, S. A new perspective on Listeria monocytogenes evolution. PLoS Pathog. 2008, 4, e1000146. [Google Scholar] [CrossRef] [PubMed]
- Babalola, O.O. Beneficial bacteria of agricultural importance. Biotechnol. Lett. 2010, 32, 1559–1570. [Google Scholar] [CrossRef] [PubMed]
- Gulzar, S.; Balange, A.K.; Nagarajarao, R.C.; Zhao, Q.; Benjakul, S. Microcapsules of Shrimp Oil Using Kidney Bean Protein Isolate and k-Carrageenan as Wall Materials with the Aid of Ultrasonication or High-Pressure Microfluidization: Characteristics and Oxidative Stability. Foods 2022, 11, 1431. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Hernández, A.; Aguilar-Flores, C.; Aparicio-Saguilán, A. Fingerprint analysis of FTIR spectra of polymers containing vinyl acetate. DYNA 2019, 86, 198–205. [Google Scholar] [CrossRef]
- Ojagh, S.M.; Rezaei, M.; Razavi, S.H.; Hosseini, S.M.H. Development and evaluation of a novel biodegradable film made from chitosan and cinnamon essential oil with low affinity toward water. Food Chem. 2010, 122, 161–166. [Google Scholar] [CrossRef]
- Zhang, W.; Shu, C.; Chen, Q.; Cao, J.; Jiang, W. The multi-layer film system improved the release and retention properties of cinnamon essential oil and its application as coating in inhibition to penicillium expansion of apple fruit. Food Chem. 2019, 299, 125109. [Google Scholar] [CrossRef]
- Liang, J.; Yan, H.; Zhang, J.; Dai, W.; Gao, X.; Zhou, Y.; Wan, X.; Puligundla, P. Preparation and characterization of antioxidant edible chitosan films incorporated with epigallocatechin gallate nanocapsules. Carbohydr. Polym. 2017, 171, 300–306. [Google Scholar] [CrossRef]
- Sharma, S.; Barkauskaite, S.; Jaiswal, A.K.; Jaiswal, S. Essential oils as additives in active food packaging. Food Chem. 2021, 343, 128403. [Google Scholar] [CrossRef]
- Sánchez-González, L.; Vargas, M.; González-Martínez, C.; Chiralt, A.; Cháfer, M. Characterization of edible films based on hydroxypropylmethylcellulose and tea tree essential oil. Food Hydrocoll. 2009, 23, 2102–2109. [Google Scholar] [CrossRef]
- Barizão, C.L.; Crepaldi, M.I.; Junior, O.O.S.; Oliveira, A.C.; Martins, A.F.; Garcia, P.S.; Bonafé, E.G. Biodegradable films based on commercial k-carrageenan and cassava starch to achieve low production costs. Int. J. Biol. Macromol. 2020, 165, 582–590. [Google Scholar] [CrossRef]
- Atarés, L.; Chiralt, A. Essential oils as additives in biodegradable films and coatings for active food packaging. Trends Food Sci. Technol. 2016, 48, 51–62. [Google Scholar] [CrossRef]
- Szlachetka, O.; Witkowska-Dobrev, J.; Baryła, A.; Dohojda, M. Low-density polyethylene (LDPE) building films–Tensile properties and surface morphology. J. Build. Eng. 2021, 44, 103386. [Google Scholar] [CrossRef]
- Sánchez-González, L.; Cháfer, M.; Chiralt, A.; González-Martínez, C. Physical properties of edible chitosan films containing bergamot essential oil and their inhibitory action on Penicillium italicum. Carbohydr. Polym. 2010, 82, 277–283. [Google Scholar] [CrossRef]
- Niu, B.; Shao, P.; Chen, H.; Sun, P. Structural and physiochemical characterization of novel hydrophobic packaging films based on pullulan derivatives for fruits preservation. Carbohydr. Polym. 2019, 208, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Carpena, M.; Nuñez-Estevez, B.; Soria-Lopez, A.; Garcia-Oliveira, P.; Prieto, M.A. Essential Oils and Their Application on Active Packaging Systems: A Review. Resources 2021, 10, 7. [Google Scholar] [CrossRef]
- Wang, G.; Guo, Z.; Liu, W. Interfacial effects of superhydrophobic plant surfaces: A Review. J. Bionic Eng. 2014, 11, 325–345. [Google Scholar] [CrossRef]
- What is Surface Free Energy? Biolin Scientific. Available online: https://www.biolinscientific.com/blog/what-is-surface-free-energy (accessed on 4 May 2023).
- Rbihi, S.; Aboulouard, A.; Laallam, L.; Jouaiti, A. Contact Angle Measurements of Cellulose based Thin Film composites: Wettability, surface free energy and surface hardness. Surf. Interfaces 2020, 21, 100708. [Google Scholar] [CrossRef]
- Chibowski, E.; Terpilowski, K. Surface free energy of polypropylene and polycarbonate solidifying at different solid surfaces. Appl. Surf. Sci. 2009, 256, 1573–1581. [Google Scholar] [CrossRef]
- Sousa, A.M.M.; Gonçalves, M.P. Strategies to improve the mechanical strength and water resistance of agar films for food packaging applications. Carbohydr. Polym. 2015, 132, 196–204. [Google Scholar] [CrossRef]
- Farhan, A.; Hani, N.M. Characterization of edible packaging films based on semi-refined k-carrageenan plasticized with glycerol and sorbitol. Food Hydrocoll. 2017, 64, 48–58. [Google Scholar] [CrossRef]
- Tonyali, B.; Cikrikci, S.; Oztop, M.H. Physicochemical and microstructural characterization of gum tragacanth added whey protei- based films. Food Res. Int. 2018, 105, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hadjilouka, A.; Mavrogiannis, G.; Mallouchos, A.; Paramithiotis, S.; Mataragas, M.; Drosinos, E.H. Effect of lemongrass essential oil on Listeria monocytogenes gene expression. LWT Food Sci. Technol. 2017, 77, 510–516. [Google Scholar] [CrossRef]






| Retention Time (min) | Compounds | % Relative | Chemical Family |
|---|---|---|---|
| 6.09 | Acetone | 0.02 | Aliphatic ketone |
| 13.06 | Tricyclene | 0.01 | Monoterpene |
| 13.85 | α-Pinene | 0.22 | Monoterpene |
| 16.49 | Camphene | 0.04 | Monoterpene |
| 19.18 | β-Pinene | 0.01 | Monoterpene |
| 20.04 | Sabinene | 0.01 | Monoterpene |
| 21.99 | Δ-3-Carene | 0.02 | Monoterpene |
| 22.89 | β-Myrcene | 0.06 | Monoterpene |
| 23.13 | α-Phellandrene | 0.01 | Monoterpene |
| 25.58 | Limonene | 3.50 | Monoterpene |
| 26.29 | β-Phellandrene | 0.02 | Monoterpene |
| 26.60 | 1,8-Cineole | 0.07 | Monoterpenic ether |
| 28.02 | cis-β-Ocimene | 0.11 | Monoterpene |
| 28.97 | γ-Terpinene | 0.01 | Monoterpene |
| 29.26 | trans-β-Ocimene | 0.06 | Monoterpene |
| 30.69 | p-Cymene | 0.02 | Monoterpene |
| 31.72 | α-Terpinolene | 0.05 | Monoterpene |
| 35.51 | 6-Methyl-5-Hepten-2-one | 0.07 | Aliphatic ketone |
| 36.77 | Melonal | 0.08 | Aliphatic aldehyde |
| 36.99 | cis-Rose oxide | 0.02 | Monoterpenic ether |
| 38.13 | trans-Rose oxide | 0.01 | Monoterpenic ether |
| 44.62 | α-Cubebene | 0.01 | Sesquiterpene |
| 45.08 | Menthone | 0.04 | Monoterpenic ketone |
| 45.79 | Citronellal | 41.12 | Monoterpenic aldehyde |
| 48.98 | β-Bourbonene | 0.08 | Sesquiterpene |
| 50.00 | Linalool | 0.75 | Monoterpenic alcohol |
| 51.71 | neo-Isopulegol | 0.42 | Monoterpenic alcohol |
| 52.21 | Isopulegol | 1.25 | Monoterpenic alcohol |
| 53.11 | trans-α-Bergamotene | 0.07 | Sesquiterpene |
| 53.45 | β-Elemene | 1.17 | Sesquiterpene |
| 53.87 | β-Copaene | 0.04 | Sesquiterpene |
| 54.11 | Terpinen-4-ol | 0.02 | Monoterpenic alcohol |
| 54.24 | trans-β-Caryophyllene | 0.31 | Sesquiterpene |
| 54.91 | Citronellyl formate | 0.04 | Monoterpenic ester |
| 55.75 | cis-β-Terpineol | 0.05 | Monoterpenic alcohol |
| 56.43 | trans-Muurola-3,5-diene | 0.02 | Sesquiterpene |
| 57.63 | Citronellyl acetate | 2.00 | Monoterpenic ester |
| 58.09 | trans-Cadina-1(6),4-diene | 0.03 | Sesquiterpene |
| 58.89 | α-Humulene | 0.08 | Sesquiterpene |
| 58.98 | Neral | 0.63 | Monoterpenic aldehyde |
| 59.88 | α-Amorphene | 0.21 | Sesquiterpene |
| 60.17 | Germacrene-D | 1.09 | Sesquiterpene |
| 61.49 | (Z,E)-α-Farnesene | 0.12 | Sesquiterpene |
| 62.02 | Geranial | 1.28 | Monoterpenic aldehyde |
| 62.76 | Bicyclogermacrene | 0.01 | Sesquiterpene |
| 63.37 | Geranyl acetate | 2.63 | Monoterpenic ester |
| 63.83 | Citronellol | 11.94 | Monoterpenic alcohol |
| 63.93 | Δ-Cadinene | 1.21 | Sesquiterpene |
| 64.25 | γ-Cadinene | 0.51 | Sesquiterpene |
| 65.01 | Lavandul | 0.01 | Monoterpenic alcohol |
| 65.59 | trans-Cadina-1,4-diene | 0.04 | Sesquiterpene |
| 65.86 | Nerol | 0.19 | Monoterpenic alcohol |
| 66.14 | α-Cadinene | 0.12 | Sesquiterpene |
| 68.54 | Geraniol | 19.97 | Monoterpenic alcohol |
| 71.48 | Geranyl butyrate | 0.15 | Monoterpenic alcohol |
| 71.71 | cis-Muurol-5-en-4-β-ol | 0.11 | Sesquiterpenic alcohol |
| 74.59 | trans-Muurol-5-en-4-β-ol | 0.13 | Sesquiterpenic alcohol |
| 77.48 | Methyl eugenol | 0.06 | Phenylpropanoid |
| 80.15 | Germacrene-D-4-ol | 1.11 | Sesquiterpenic alcohol |
| 80.63 | 1,10-Di-Epi-Cubenol | 0.07 | Sesquiterpenic alcohol |
| 81.03 | 1-Epi-Cubenol | 0.02 | Sesquiterpenic alcohol |
| 81.24 | Elemol | 1.98 | Sesquiterpenic alcohol |
| 81.93 | 8-Hidroxy-neo-menthol | 0.18 | Alcohol |
| 84.28 | Eugenol | 0.90 | Phenylpropanoid |
| 84.83 | trans-Methyl-isoeugenol | 0.16 | Alcohol |
| 85.79 | T-Muurolol | 0.25 | Sesquiterpenic alcohol |
| 86.23 | α-Muurolol | 0.07 | Sesquiterpenic alcohol |
| 86.70 | Elemicine | 0.05 | Ether |
| 87.30 | Citronellic acid | 0.26 | Carboxylic acid |
| 87.42 | α-Eudesmol | 0.16 | Sesquiterpenic alcohol |
| 87.75 | α-Cadinol | 0.55 | Sesquiterpenic alcohol |
| 87.87 | β-Eudesmol | 0.15 | Sesquiterpenic alcohol |
| 92.87 | (E,E)-Farnesol | 0.06 | Sesquiterpenic alcohol |
| Cumulative amount | 43.03 | Monoterpenic aldehydes | |
| 34.75 | Monoterpenic alcohols | ||
| 5.12 | Sesquiterpenes | ||
| 4.67 | Monoterpenic esters | ||
| 4.66 | Sesquiterpenic alcohols | ||
| 4.15 | Monoterpenes | ||
| 0.96 | Phenylpropanoids | ||
| 0.34 | Alcohols | ||
| 0.26 | Carboxylic acids | ||
| 0.10 | Monoterpenic ethers | ||
| 0.09 | Aliphatic ketones | ||
| 0.08 | Aliphatic aldehydes | ||
| 0.05 | Ethers | ||
| 0.04 | Monoterpenic ketones | ||
| Method | Parameters | C. winterianus Essential Oil |
|---|---|---|
| DPPH | IC50 (%, v/v) | 0.06 ± 0.01 |
| AAI | 85.60 ± 13.42 | |
| Antioxidant activity | Very strong | |
| β-Carotene bleaching | IC50 (%, v/v) | 3.16 ± 0.48 |
| Strains | Diameter of Inhibition Zones (mm) 1 | MIC Values (µL/mL) 2 |
|---|---|---|
| S. aureus ATCC 25923 | 15.53 ± 2.12 | 32 |
| L. monocytogenes LMG 16779 | 31.67 ± 5.16 | 8 |
| E. faecalis ATCC 29212 | 12.03 ± 0.18 | 16 |
| E. coli ATCC 25922 | 9.98 ± 0.66 | >32 |
| S. Typhimurium ATCC 13311 | 6.00 ± 0.00 | 32 |
| P. aeruginosa ATCC 27853 | 7.77 ± 0.00 | >32 |
| C. violaceum ATCC 12472 | 10.93 ± 0.81 | - |
| Properties | Control a | 62.5 µL Essential Oil b | 125 µL Essential Oil c | 250 µL Essential Oil d | p-Values | |
|---|---|---|---|---|---|---|
| Structural | Grammage (g/m2) | 65.18 ± 2.91 | 65.36 ± 5.26 | 66.43 ± 5.87 | 69.12 ± 4.09 | 0.926 ab 0.557 ac 0.030 ad* |
| Thickness (µm) | 38.51 ± 4.01 | 41.45 ± 4.98 | 44.17 ± 5.51 | 64.93 ±7.89 | 0.002 ab* <0.001 ac* <0.001 ad* | |
| Mechanical | Tensile strength (N/m) | 3006.99 ± 80.56 | 2783.54 ± 236.82 | 2821.52 ± 201.98 | 1432.29 ± 14,38 | 0.242 ab 0.256 ac 0.019 ad* |
| Tensile index (N.m/g) | 46.15 ± 1.20 | 42.60 ± 3.63 | 42.46 ± 3.02 | 20.75 ± 0.21 | 0.230 ab 0.163 ac 0.018 ad* | |
| Peak elongation (%) | 1.91 ± 0.01 | 2.38 ± 0.09 | 3.73 ± 0.47 | 1.46 ± 0.12 | 0.009 ab* 0.021 ac* 0.114 ad | |
| Elastic modulus (MPa) | 7978.67 ± 312.77 | 6946.07 ± 545.55 | 5558.45 ± 337.19 | 3017.49 ± 2.64 | 0.075 ab 0.008 ac* 0.028 ad* | |
| Optical | L* (Lightness) | 93.01 ± 0.23 | 93.15 ± 0.17 | 93.26 ± 0.15 | 94.07 ± 0.51 | 0.448 ab 0.201 ac 0.052 ad |
| a* (Redness) | 1.58 ± 0.04 | 1.60 ± 0.07 | 1.51 ± 0.02 | 1.33 ± 0.08 | 0.676 ab 0.069 ac 0.018 ad* | |
| b* (Yellowness) | −5.94 ± 0.11 | −5.93 ± 0.40 | −5.56 ± 0.11 | −4.38 ± 0.42 | 0.970 ab 0.014 ac* 0.018 ad* | |
| Transparency (%) | 95.72 ± 0.454 | 94.54 ± 0.384 | 93.83 ± 0.348 | 91.65 ± 1.463 | 0.028 ab* 0.006 ac* 0.031 ad* | |
| Films | Water Contact Angle (◦) | Diiodomethane Contact Angle (◦) | Ethyleneglycol Contact Angle (◦) | Dispersive Component, ɤD (mN/m) | Polar Component, ɤP (mN/m) | Total Surface Free Energy, ɤT (mN/m) | |
|---|---|---|---|---|---|---|---|
| Control | Top face a | 93.22 ± 5.02 | 93.16 ± 5.58 | 93.22 ± 5.02 | 9.84 ± 2.08 | 4.35 ± 1.88 | 14.19 ± 2.80 |
| Bottom face b | 93.09 ± 5.60 | 93.90 ± 4.92 | 93.22 ± 5.02 | 9.97 ± 1.87 | 3.98 ± 1.79 | 13.95 ± 2.59 | |
| 62.5 µL essential oil | Top face c | 97.95 ± 6.98 | 47.27 ± 0.96 | 62.81 ± 2.48 | 35.79 ± 0.52 | 0.38 ± 0.25 | 36.17 ± 0.58 |
| Bottom face d | 93.39 ± 4.79 | 47.04 ± 1.70 | 60.00 ± 1.95 | 35.90 ± 0.92 | 0.74 ± 0.30 | 36.64 ± 0.97 | |
| 125 µL essential oil | Top face e | 86.81 ± 8.44 | 41.67 ± 2.86 | 66.82 ± 0.84 | 38.53 ± 1.46 | 0.00 ± 0.01 | 38.53 ± 1.46 |
| Bottom face f | 69.00 ± 8.44 | 41.45 ± 2.83 | 73.77 ± 5.08 | 38.40 ± 1.44 | 0.20 ± 0.37 | 38.60 ± 1.49 | |
| 250 µL essential oil | Top face g | 95.10 ± 4.76 | 44.26 ± 4.74 | 65.50 ± 5.43 | 37.11 ± 2.46 | 0.23 ± 0.36 | 37.35 ± 2.49 |
| Bottom face h | 84.43 ± 5.47 | 50.47 ± 1.38 | 79.09 ± 0.75 | 33.72 ± 0.77 | 0.33 ± 0.12 | 34.05 ± 0.78 | |
| p-values | 0.210 ac 0.933 bd 0.147 ae 0.004 bf* 0.495 ag 0.031 bh* | <0.001 ac* <0.001 bd* <0.001 ae* <0.001 bf* <0.001 ag* <0.001 bh* | <0.001 ac* <0.001 bd* <0.001 ae* <0.001 bf* <0.001 ag* <0.001 bh* | <0.001 ac* <0.001 bd* <0.001 ae* <0.001 bf* <0.001 ag* <0.001 bh* | 0.065 ac 0.085 bd 0.057 ae 0.062 bf 0.058 ag 0.071 bh | <0.001 ac* <0.001 bd* <0.001 ae* <0.001 bf* <0.001 ag* 0.003 bh* | |
| Films | Water Vapor | Oil | |
|---|---|---|---|
| WVTR (g/m2.day) | WVP (g/Pa.day.m) (×10−5) | (g.mm/m2.day) | |
| Control a | 532.58 ± 1.76 | 1.55 ± 0.01 | 4.79 ± 0.39 |
| 62.5 μL essential oil b | 534.14 ± 33.95 | 1.68 ± 0.11 | 13.32 ± 2.65 |
| 125 μL essential oil c | 503.27 ± 12.35 | 1.68 ± 0.04 | 6.93 ± 1.06 |
| 250 μL essential oil d | 539.75 ± 8.38 | 2.65 ± 0.04 | 9.64 ± 2.08 |
| p-values | 0.959 ab 0.178 ac 0.434 ad | 0.348 ab 0.136 ac 0.015 ad* | 0.401 ab 0.127 ac 0.509 ad |
| Films | % Inhibition | p-Values |
|---|---|---|
| Control a | 79.35 ± 5.64 | - |
| 62.5 μL essential oil b | 90.14 ± 2.68 | 0.375 ab |
| 125 μL essential oil c | 100.00 ± 3.29 | 0.068 ac |
| 250 μL essential oil d | 100.00 ± 4.64 | 0.355 ad |
| Strains | Films | |||
|---|---|---|---|---|
| Control | 62.5 μL Essential Oil | 125 μL Essential Oil | 250 μL Essential Oil | |
| S. aureus ATCC 25923 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| L. monocytogenes LMG 16779 | 0.00 ± 0.00 | 0.00 ± 0.00 | 6.00 ± 0.00 | 6.00 ± 0.00 |
| E. faecalis ATCC 29212 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| E. coli ATCC 25922 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| S. Typhimurium ATCC 13311 | 6.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| P. aeruginosa ATCC 27853 | 6.85 ± 1.20 | 7.93 ± 0.67 | 8.17 ± 0.37 | 7.62 ± 0.16 |
| C. violaceum ATCC 12472 | 6.00 ± 0.00 | 6.00 ± 0.00 | 6.00 ± 0.00 | 6.00 ± 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, C.; Ramos, A.; Luís, Â.; Amaral, M.E. Production and Characterization of k-Carrageenan Films Incorporating Cymbopogon winterianus Essential Oil as New Food Packaging Materials. Foods 2023, 12, 2169. https://doi.org/10.3390/foods12112169
Santos C, Ramos A, Luís Â, Amaral ME. Production and Characterization of k-Carrageenan Films Incorporating Cymbopogon winterianus Essential Oil as New Food Packaging Materials. Foods. 2023; 12(11):2169. https://doi.org/10.3390/foods12112169
Chicago/Turabian StyleSantos, Catarina, Ana Ramos, Ângelo Luís, and Maria E. Amaral. 2023. "Production and Characterization of k-Carrageenan Films Incorporating Cymbopogon winterianus Essential Oil as New Food Packaging Materials" Foods 12, no. 11: 2169. https://doi.org/10.3390/foods12112169
APA StyleSantos, C., Ramos, A., Luís, Â., & Amaral, M. E. (2023). Production and Characterization of k-Carrageenan Films Incorporating Cymbopogon winterianus Essential Oil as New Food Packaging Materials. Foods, 12(11), 2169. https://doi.org/10.3390/foods12112169