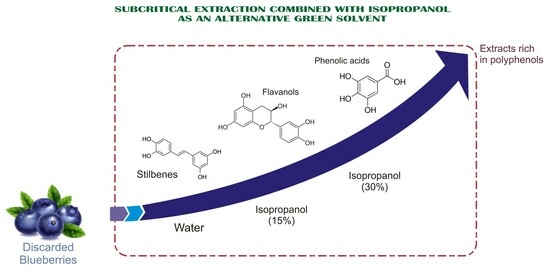
Selective Recovery of Polyphenols from Discarded Blueberries (Vaccinium corymbosum L.) Using Hot Pressurized Liquid Extraction Combined with Isopropanol as an Environmentally Friendly Solvent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Chemical Reagents
2.3. Hot Pressurized Liquid Extraction (HPLE)
2.4. Total Polyphenol Content (TPC)
2.5. Antioxidant Capacity by 2,2 Diphenyl 1 Picrylhydrazyl (DPPH) Analysis
2.6. Antioxidant Capacity by Oxygen Radical Absorbance Capacity (ORAC) Analysis
2.7. Quantification of Fructose and Glucose
2.8. Quantification of Target Polyphenols
2.9. Statistical Analysis
3. Results and Discussion
3.1. Polyphenol Extraction
3.2. Antioxidant Capacity
3.3. Reducing Sugar Content
3.4. Impact of the Use of Isopropanol to Recover Specific Polyphenols
3.4.1. Flavonols
3.4.2. Flavanols
3.4.3. Phenolic Acids
3.4.4. Stilbenes
3.5. Impact of the Use of Isopropanol versus Ethanol in HPLE and Conventional Extraction with Acetone
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- INEI Informe Técnico de Producción Nacional-Diciembre 2019 [Report]. Available online: https://www.inei.gob.pe/media/MenuRecursivo/boletines/02-informe-tecnico-n02_produccion-nacional-dic-2019.pdf/ (accessed on 20 April 2023).
- Li, D.; Li, B.; Ma, Y.; Sun, X.; Lin, Y.; Meng, X. Polyphenols, Anthocyanins, and Flavonoids Contents and the Antioxidant Capacity of Various Cultivars of Highbush and Half-High Blueberries. J. Food Compos. Anal. 2017, 62, 84–93. [Google Scholar] [CrossRef]
- Wang, S.Y.; Chen, C.T.; Sciarappa, W.; Wang, C.Y.; Camp, M.J. Fruit Quality, Antioxidant Capacity, and Flavonoid Content of Organically and Conventionally Grown Blueberries. J. Agric. Food Chem. 2008, 56, 5788–5794. [Google Scholar] [CrossRef]
- He, K.; Li, X.; Chen, X.; Ye, X.; Huang, J.; Jin, Y.; Li, P.; Deng, Y.; Jin, Q.; Shi, Q.; et al. Evaluation of Antidiabetic Potential of Selected Traditional Chinese Medicines in STZ-Induced Diabetic Mice. J. Ethnopharmacol. 2011, 137, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, A.R.; Ema, T.I.; Siddiquee, M.F.R.; Shahriar, A.; Ahmed, H.; Mosfeq-Ul-Hasan, M.; Rahman, N.; Islam, R.; Uddin, M.R.; Mizan, M.F.R. Natural Flavonols: Actions, Mechanisms, and Potential Therapeutic Utility for Various Diseases. Beni-Suef Univ. J. Basic Appl. Sci. 2023, 12, 47. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.Y.; Sang, L.X.; Jiang, M.; McPhee, D.J. Catechins and Their Therapeutic Benefits to Inflammatory Bowel Disease. Molecules 2017, 22, 484. [Google Scholar] [CrossRef] [PubMed]
- Cvjetko Bubalo, M.; Vidović, S.; Radojčić Redovniković, I.; Jokić, S. Green Solvents for Green Technologies. J. Chem. Technol. Biotechnol. 2015, 90, 1631–1639. [Google Scholar] [CrossRef]
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef]
- Sulaiman, S.F.; Sajak, A.A.B.; Ooi, K.L.; Supriatno; Seow, E.M. Effect of Solvents in Extracting Polyphenols and Antioxidants of Selected Raw Vegetables. J. Food Compos. Anal. 2011, 24, 506–515. [Google Scholar] [CrossRef]
- Nile, S.H.; Park, S.W. Edible Berries: Bioactive Components and Their Effect on Human Health. Nutrition 2014, 30, 134–144. [Google Scholar] [CrossRef]
- Joshi, D.R.; Adhikari, N. An Overview on Common Organic Solvents and Their Toxicity. J. Pharm. Res. Int. 2019, 28, 1–18. [Google Scholar] [CrossRef]
- Drosou, C.; Kyriakopoulou, K.; Bimpilas, A.; Tsimogiannis, D.; Krokida, M. A Comparative Study on Different Extraction Techniques to Recover Red Grape Pomace Polyphenols from Vinification Byproducts. Ind. Crops Prod. 2015, 75, 141–149. [Google Scholar] [CrossRef]
- Aaby, K.; Grimmer, S.; Holtung, L. Extraction of Phenolic Compounds from Bilberry (Vaccinium myrtillus L.) Press Residue: Effects on Phenolic Composition and Cell Proliferation. LWT—Food Sci. Technol. 2013, 54, 257–264. [Google Scholar] [CrossRef]
- Ameer, K.; Shahbaz, H.M.; Kwon, J.H. Green Extraction Methods for Polyphenols from Plant Matrices and Their Byproducts: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 295–315. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.T.; Veggi, P.C.; Meireles, M.A.A. Extraction of Antioxidant Compounds from Jabuticaba (Myrciaria Cauliflora) Skins: Yield, Composition and Economical Evaluation. J. Food Eng. 2010, 101, 23–31. [Google Scholar] [CrossRef]
- Santos, D.T.; Veggi, P.C.; Meireles, M.A.A. Optimization and Economic Evaluation of Pressurized Liquid Extraction of Phenolic Compounds from Jabuticaba Skins. J. Food Eng. 2012, 108, 444–452. [Google Scholar] [CrossRef]
- Plaza, M.; Turner, C. Trends in Analytical Chemistry Pressurized Hot Water Extraction of Bioactives. Trends Anal. Chem. 2015, 71, 39–54. [Google Scholar] [CrossRef]
- Mariotti-Celis, M.S.; Martínez-Cifuentes, M.; Huamán-Castilla, N.; Pedreschi, F.; Iglesias-Rebolledo, N.; Pérez-Correa, J.R. Impact of an Integrated Process of Hot Pressurised Liquid Extraction–Macroporous Resin Purification over the Polyphenols, Hydroxymethylfurfural and Reducing Sugars Content of Vitis vinifera ‘Carménère’ Pomace Extracts. Int. J. Food Sci. Technol. 2018, 53, 1072–1078. [Google Scholar] [CrossRef]
- Allcca-Alca, E.E.; León-Calvo, N.C.; Luque-Vilca, O.M.; Martínez-Cifuentes, M.; Pérez-Correa, J.R.; Mariotti-Celis, M.S.; Huamán-Castilla, N.L. Hot Pressurized Liquid Extraction of Polyphenols from the Skin and Seeds of Vitis vinifera L. cv. Negra Criolla Pomace a Peruvian Native Pisco Industry Waste. Agronomy 2021, 11, 866. [Google Scholar] [CrossRef]
- Huaman-Castilla, N.L.; Martinez, M.; Camilo, C.; Pedreschi, F.; Mariotti-Celis, M.S.; Perez-Correa, J.R. The Impact of Temperature and Ethanol Concentration on the Global Recovery of Specific Polyphenols in an Integrated HPLE/RP Process on Carm é n è Re Pomace Extracts. Molecules 2019, 24, 3145. [Google Scholar] [CrossRef]
- Mariotti-Celis, M.; Martínez-Cifuentes, M.; Huamán-Castilla, N.; Vargas-González, M.; Pedreschi, F.; Pérez-Correa, J. The Antioxidant and Safety Properties of Spent Coffee Ground Extracts Impacted by the Combined Hot Pressurized Liquid Extraction–Resin Purification Process. Molecules 2017, 23, 21. [Google Scholar] [CrossRef]
- Jayakumar, C.; Devi, V.M.; Sridar, R. A Study on the Extraction of Bioactive Compounds from Capparis zeylanica. AIP Conf. Proc. 2020, 2225, 070002. [Google Scholar]
- Vimercati, W.C.; Araújo, C.d.S.; Macedo, L.L.; Pimenta, C.J. Optimal Extraction Condition for the Recovery of Bioactive Compounds and Antioxidants from Coffee Silverskin. J. Food Process Eng. 2022, 45, e14009. [Google Scholar] [CrossRef]
- Merck: Precio Solventes Organicos (Isopropanol). Available online: https://www.sigmaaldrich.com/PE/es/search/2-propanol?focus=products&page=1&perpage=30&sort=relevance&term=2%20propanol&type=product/ (accessed on 15 June 2023).
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Brand-Williams; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. Food Sci. Technol. 1995, 28, 25–30. [Google Scholar]
- Chambia, F.; Chirinosa, R.; Pedreschic, R.; Betalleluz-Pallardela, I.; Debasteb, F.; Campos, D. Antioxidant Potential of Hydrolyzed Polyphenolic Extracts from Tara (Caesalpinia spinosa) Pods. Ind. Crops Prod. 2013, 47, 168–175. [Google Scholar] [CrossRef]
- Maldonado, I.; Vega Quispe, A.P.; Merma Chacca, D.; Zirena Vilca, F. Optimization of the Elimination of Antibiotics by Lemna Gibba and Azolla Filiculoides Using Response Surface Methodology (RSM). Front. Environ. Sci. 2022, 10, 1–13. [Google Scholar] [CrossRef]
- Vergara-Salinas, J.R.; Bulnes, P.; Zúñiga, M.C.; Pérez-Jiménez, J.; Torres, J.L.; Mateos-Martín, M.L.; Agosin, E.; Pérez-Correa, J.R. Effect of Pressurized Hot Water Extraction on Antioxidants from Grape Pomace before and after Enological Fermentation. J. Agric. Food Chem. 2013, 61, 6929–6936. [Google Scholar] [CrossRef]
- Bánvölgyi, S.; Dusza, E.; Namukwambi, F.K.; Kiss, I.; Stefanovits-Bányai, É.; Vatai, G. Optimization of Extraction of Phenolic Compounds from Tokaji Aszú Marc Using Response Surface Methodology. Prog. Agric. Eng. Sci. 2020, 16, 1–9. [Google Scholar] [CrossRef]
- Subra-Paternault, P.; Garcia-Mendoza, M.D.P.; Savoire, R.; Harscoat-Schiavo, C. Impact of Hydro-Alcoholic Solvents on the Oil and Phenolics Extraction from Walnut (Juglans regia L.) Press-Cake and the Self-Emulsification of Extracts. Foods 2022, 11, 186. [Google Scholar] [CrossRef]
- Stranathan, J.D. The Dielectric Constant of Isopropyl Alcohol Vapor. J. Chem. Phys. 1937, 5, 828–830. [Google Scholar] [CrossRef]
- Åkerlöf, G. Dielectric Constants of Some Organic Solvent-Water Mixtures at Various Temperatures. J. Am. Chem. Soc. 1932, 54, 4125–4139. [Google Scholar] [CrossRef]
- Zhang, H.; Birch, J.; Yang, H.; Xie, C.; Kong, L.; Dias, G.; Bekhit, A.E.D. Effect of Solvents on Polyphenol Recovery and Antioxidant Activity of Isolates of Asparagus Officinalis Roots from Chinese and New Zealand Cultivars. Int. J. Food Sci. Technol. 2018, 53, 2369–2377. [Google Scholar] [CrossRef]
- Bánvölgyi1, S.; Namukwambi1, F.K.; István, K.; Stefanovits-Bányai, E.; Vatai1, G. Extraction of antioxidant and polyphenol compounds from Tokaji aszú marc with iso-propanol–water solvent. In Proceedings of the 23rd International Symposium on Analytical and Environmental Problems, University of Szeged, Department of Inorganic and Analytical Chemistry, Szeged, Hungary, 10 October 2017. [Google Scholar]
- Smith, M.A.L.; Marley, K.A.; Seigler, D.; Singletary, K.W.; Meline, B. Bioactive Properties of Wild Blueberry Fruits. J. Food Sci. 2000, 65, 352–356. [Google Scholar] [CrossRef]
- Wu, X.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L. Concentrations of Anthocyanins in Common Foods in the United States and Estimation of Normal Consumption. J. Agric. Food Chem. 2006, 54, 4069–4075. [Google Scholar] [CrossRef] [PubMed]
- Ju, Z.Y.; Howard, L.R. Effects of Solvent and Temperature on Pressurized Liquid Extraction of Anthocyanins and Total Phenolics from Dried Red Grape Skin. J. Agric. Food Chem. 2003, 51, 5207–5213. [Google Scholar] [CrossRef]
- Wang, Y.; Fong, S.K.; Singh, A.P.; Vorsa, N.; Johnson-Cicalese, J. Variation of Anthocyanins, Proanthocyanidins, Flavonols, and Organic Acids in Cultivated and Wild Diploid Blueberry Species. HortScience 2019, 54, 576–585. [Google Scholar] [CrossRef]
- Huamán-Castilla, N.L.; Mariotti-Celis, M.S.; Martínez-Cifuentes, M.; Pérez-Correa, J.R. Glycerol as Alternative Co-Solvent for Water Extraction of Polyphenols from Carménère Pomace: Hot Pressurized Liquid Extraction and Computational Chemistry Calculations. Biomolecules 2020, 10, 474. [Google Scholar] [CrossRef]
- Cervantes Ceja, M.L. “Potencial Nutracéutico de Cultivos de Arándano (Vaccinum sp.) Seleccionados En México”, Universidad Autónoma de Querétaro. 2009. Available online: https://cdn.blueberriesconsulting.com/2015/09/pdf_310.pdf/ (accessed on 10 June 2023).
- Brambilla, A.; Lo Scalzo, R.; Bertolo, G.; Torreggiani, D. Steam-Blanched Highbush Blueberry (Vaccinium corymbosum L.) Juice: Phenolic Profile and Antioxidant Capacity in Relation to Cultivar Selection. J. Agric. Food Chem. 2008, 56, 2643–2648. [Google Scholar] [CrossRef]
- Al Hasani, S.; Al-attabi, Z.; Waly, M. Polyphenol and Flavonoid Stability of Wild Blueberry (Sideroxylon mascatense) during Air- and Freeze-Drying and Storage Stability as a Function of Temperature. Foods 2023, 12, 871. [Google Scholar] [CrossRef]
- Méndez-Bermúdez, J.G.; Dominguez, H.; Pusztai, L.; Guba, S.; Horváth, B.; Szalai, I. Composition and Temperature Dependence of the Dielectric Constant of 1-Propanol/Water Mixtures: Experiment and Molecular Dynamics Simulations. J. Mol. Liq. 2016, 219, 354–358. [Google Scholar] [CrossRef]
- Jessop, P.G.; Jessop, D.A.; Fu, D.; Phan, L. Solvatochromic Parameters for Solvents of Interest in Green Chemistry. Green Chem. 2012, 14, 1245–1259. [Google Scholar] [CrossRef]
- Jessop, P.G. Searching for Green Solvents. Green Chem. 2011, 13, 1391–1398. [Google Scholar] [CrossRef]


| Specific Polyphenol | Wavelength (nm) | Regression Equation | R2 |
|---|---|---|---|
| Rutin | 270 | Y = 6.6151462X + 1.7802541 | 0.99922 |
| Quercitin | 270 | Y = 24.4618691X + 2.2829876 | 0.99971 |
| Caffeic acid | 270 | Y = 149.119813X + 0.9753017 | 0.99994 |
| Catechin | 280 | Y = 25.251136X − 0.5309875 | 0.99994 |
| Procyanidin B2 | 280 | Y = 41.3596684X − 0.4846145 | 0.99974 |
| Epicatechin | 280 | Y = 43.3950296X − 2.1554659 | 0.99985 |
| Vanillic acid | 280 | Y = 141.991849X − 5.4568242 | 0.99980 |
| Procyanidin A2 | 280 | Y = 59.2803924X − 0.7963507 | 0.99990 |
| Resveratrol | 324 | Y = 78.8100873X − 31.357898 | 0.99978 |
| Kaempferol | 373 | Y = 38.0226353X − 1.5363721 | 0.99971 |
| Conditions | 70 °C | 100 °C | 130 °C | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Isopropanol (%) | TPC | IC50 | ORAC | TPC | IC50 | ORAC | TPC | IC50 | ORAC |
| Mean CV | Mean CV | Mean CV | Mean CV | Mean CV | Mean CV | Mean CV | Mean CV | Mean CV | |
| 0 | 2.32 A,a | 20.44 C,a | 90.85 A,a | 3.61 B,a | 16.49 B,c | 121.85 B,a | 8.13 C,a | 13.61 A,b | 149.90 C,a |
| 0.09 | 0.02 | 0.03 | 0.04 | 0.03 | 0.04 | 0.08 | 0.03 | 0.05 | |
| 15 | 2.66 A,b | 19.22 C,a | 97.59 A,b | 6.30 B,b | 14.24 B,b | 148.59 B,b | 11.26 C,b | 12.23 A,b | 169.33 C,b |
| 0.07 | 0.03 | 0.04 | 0.08 | 0.03 | 0.05 | 0.04 | 0.03 | 0.03 | |
| 30 | 2.71 A,b | 17.99 C,b | 108.52 A,c | 12.18 B,c | 11.43 B,a | 211.47 B,c | 15.57 C,c | 9.97 A,a | 246.29 C,c |
| 0.09 | 0.03 | 0.03 | 0.05 | 0.04 | 0.05 | 0.02 | 0.04 | 0.06 | |
| Temperature | 70 °C | 100 °C | 130 °C | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Isopropanol | 0% | 15% | 30% | 0% | 15% | 30% | 0% | 15% | 30% |
| Flavanols (µg/gdw) | Mean CV | Mean CV | Mean CV | Mean CV | Mean CV | Mean CV | Mean CV | Mean CV | Mean CV |
| Quercetin | 2.03 | 77.09 | 76.96 | 10.63 | 88.93 | 171.12 | 24.98 | 121.05 | 78.1 |
| 0.02 | 0.03 | 0.02 | 0.08 | 0.06 | 0.11 | 0.09 | 0.05 | 0.02 | |
| Rutin | ND | 14.43 | 71.06 | 4.76 | 22.08 | 161.19 | ND | 64.45 | 93.37 |
| 0.10 | 0.01 | 0.10 | 0.10 | 0.10 | 0.02 | 0.03 | |||
| Kaempferol | 0.91 | 0.98 | 0.93 | 1.25 | 1.09 | 1.51 | 1.46 | 1.73 | 1.64 |
| 0.01 | 0.05 | 0.05 | 0.10 | 0.01 | 0.02 | 0.05 | 0.05 | 0.11 | |
| ∑: | 2.92 | 92.5 | 148.92 | 16.64 | 112.11 | 333.82 | 26.44 | 187.23 | 173.11 |
| Flavanols (µg/gdw) | |||||||||
| Catechin | 0.53 | 0.66 | 10.46 | 0.55 | 1.08 | 17.36 | 1.00 | 1.29 | 18.67 |
| 0.10 | 0.09 | 0.08 | 0.01 | 0.06 | 0.03 | 0.01 | 0.01 | 0.02 | |
| Epicatechin | ND | 1.41 | 11.69 | ND | 1.47 | 18.48 | ND | 3.40 | 22.82 |
| 0.01 | 0.05 | 0.09 | 0.04 | 0.03 | 0.05 | ||||
| Procyanidin A2 | 0.45 | 0.71 | 0.79 | 0.71 | 1.50 | 2.55 | 0.74 | 2.15 | 1.10 |
| 0.07 | 0.04 | 0.01 | 0.10 | 0.01 | 0.08 | 0.04 | 0.02 | 0.11 | |
| Procyanidin B2 | ND | 1.87 | 3.20 | 1.28 | 2.09 | 4.11 | 1.46 | 3.09 | 10.91 |
| 0.04 | 0.11 | 0.07 | 0.00 | 0.11 | 0.03 | 0.04 | 0.02 | ||
| ∑: | 0.99 | 4.65 | 26.13 | 2.55 | 6.14 | 42.49 | 3.20 | 9.92 | 53.51 |
| Phenolic acids (ug/gdw) | |||||||||
| Caffeic | ND | 0.02 | 1.26 | ND | 0.05 | 1.43 | 0.01 | 0.30 | 1.71 |
| 0.07 | 0.05 | 0.02 | 0.07 | 0.09 | 0.09 | 0.06 | |||
| Vanillic | 0.96 | 1.01 | 1.30 | 2.02 | 1.83 | 3.50 | 7.39 | 8.59 | 9.49 |
| 0.01 | 0.06 | 0.00 | 0.09 | 0.03 | 0.10 | 0.08 | 0.01 | 0.03 | |
| ∑: | 0.96 | 1.03 | 2.56 | 2.02 | 1.88 | 4.93 | 7.40 | 8.89 | 11.20 |
| Stilbens (µg/gdw) | |||||||||
| Resveratrol | 7.49 | 7.98 | ND | 8.15 | 8.01 | ND | 9.08 | 8.04 | ND |
| 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huamán-Castilla, N.L.; Copa-Chipana, C.; Mamani-Apaza, L.O.; Luque-Vilca, O.M.; Campos-Quiróz, C.N.; Zirena-Vilca, F.; Mariotti-Celis, M.S. Selective Recovery of Polyphenols from Discarded Blueberries (Vaccinium corymbosum L.) Using Hot Pressurized Liquid Extraction Combined with Isopropanol as an Environmentally Friendly Solvent. Foods 2023, 12, 3694. https://doi.org/10.3390/foods12193694
Huamán-Castilla NL, Copa-Chipana C, Mamani-Apaza LO, Luque-Vilca OM, Campos-Quiróz CN, Zirena-Vilca F, Mariotti-Celis MS. Selective Recovery of Polyphenols from Discarded Blueberries (Vaccinium corymbosum L.) Using Hot Pressurized Liquid Extraction Combined with Isopropanol as an Environmentally Friendly Solvent. Foods. 2023; 12(19):3694. https://doi.org/10.3390/foods12193694
Chicago/Turabian StyleHuamán-Castilla, Nils Leander, Cecilia Copa-Chipana, Luis Omar Mamani-Apaza, Olivia Magaly Luque-Vilca, Clara Nely Campos-Quiróz, Franz Zirena-Vilca, and María Salomé Mariotti-Celis. 2023. "Selective Recovery of Polyphenols from Discarded Blueberries (Vaccinium corymbosum L.) Using Hot Pressurized Liquid Extraction Combined with Isopropanol as an Environmentally Friendly Solvent" Foods 12, no. 19: 3694. https://doi.org/10.3390/foods12193694
APA StyleHuamán-Castilla, N. L., Copa-Chipana, C., Mamani-Apaza, L. O., Luque-Vilca, O. M., Campos-Quiróz, C. N., Zirena-Vilca, F., & Mariotti-Celis, M. S. (2023). Selective Recovery of Polyphenols from Discarded Blueberries (Vaccinium corymbosum L.) Using Hot Pressurized Liquid Extraction Combined with Isopropanol as an Environmentally Friendly Solvent. Foods, 12(19), 3694. https://doi.org/10.3390/foods12193694