Stability Kinetics of Anthocyanins of Grumixama Berries (Eugenia brasiliensis Lam.) during Thermal and Light Treatments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Proximate Composition, Physicochemical Properties, and Oxidase Enzymes Activities
2.3. Total Monomeric Anthocyanins, Total Phenolic Compounds and Antioxidant Capacity
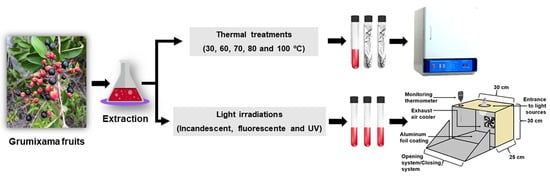
2.4. Thermal and Light Stability of Anthocyanin Extracts
2.5. Degradation Kinetics of Anthocyanins
2.6. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Grumixama Fruits
3.2. Thermal Stability of Total Anthocyanins and Total Phenolic Compounds
3.3. Light Stability of Total Anthocyanins and Total Phenolic Compounds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fischer, D.C.H.; Limberger, R.P.; Henriques, A.T.; Moreno, P.R.H. Essential oils from leaves of two Eugenia brasiliensis specimens from southeastern Brazil. J. Essent. Oil Res. 2005, 17, 499–500. [Google Scholar] [CrossRef]
- Reynertson, K.A.; Yang, H.; Jiang, B.; Basile, M.J.; Kennelly, E.J. Quantitative analysis of antiradical phenolic constituents from fourteen edible Myrtaceae fruits. Food Chem. 2008, 109, 883–890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santiago, M.C.P.A.; Gouvêa, A.C.M.S.; Peixoto, F.M.; Borguini, R.G.; Godoy, R.L.O.; Pacheco, S. Characterization of jamelão (Syzygium cumini L.) skeels fruit peel powder for use as natural colorant. Fruits 2016, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, N.U.; Park, J.I.; Jung, H.J.; Yang, T.J.; Hur, Y.; Nou, I.S. Characterization of dihydroflavonol 4-reductase (DFR) genes and their association with cold and freezing stress in Brassica rapa. Gene 2014, 550, 46–55. [Google Scholar] [CrossRef]
- Echegaray, N.; Munekata, P.E.S.; Gullón, P.; Dzuvor, C.K.O.; Gullón, B.; Kubi, F.; Lorenzo, J.M. Recent advances in food products fortification with anthocyanins. Crit. Rev. Food Sci. Nutr. 2022, 62, 1553–1567. [Google Scholar] [CrossRef]
- Cortez, R.; Luna-Vital, D.A.; Margulis, D.; Gonzalez de Mejia, E. Natural pigments: Stabilization methods of anthocyanins for food applications. Compr. Rev. Food Sci. Food Saf. 2017, 16, 180–198. [Google Scholar] [CrossRef]
- Amogne, N.Y.; Ayele, D.W.; Tsigie, Y.A. Recent advances in anthocyanin dyes extracted from plants for dye sensitized solar cell. Mater. Renew. Sustain. Energy 2020, 9, 23. [Google Scholar] [CrossRef]
- Arruda, H.S.; Silva, E.K.; Peixoto Araujo, N.M.; Pereira, G.A.; Pastore, G.M.; Marostica Júnior, M.R. Anthocyanins recovered from agri-food by-products using innovative processes: Trends, challenges, and perspectives for their application in food systems. Molecules 2021, 9, 2632. [Google Scholar] [CrossRef]
- Kšonžeková, P.; Mariychuk, R.; Eliašová, A.; Mudroňová, D.; Csank, T.; Király, J.; Marcinčáková, D.; Pistl, J.; Tkáčiková, L. In vitro study of biological activities of anthocyanin-rich berry extracts on porcine intestinal epithelial cells. J. Sci. Food Agric. 2016, 96, 1093–1100. [Google Scholar] [CrossRef]
- Jackman, R.L.; Yada, R.Y.; Tung, M.A.; Speers, R.A. Anthocyanins as food colorants? A review. J. Food Biochem. 1987, 11, 201–247. [Google Scholar] [CrossRef]
- Carocho, M.; Barreiro, M.F.; Morales, P.; Ferreira, I.C.F.R. Adding molecules to food, pros and cons: A review on synthetic and natural food additives. Compr. Rev. Food Sci. Food Saf. 2014, 13, 377–399. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Rhim, J.W. Anthocyanin food colorant and its application in pH-responsive color change indicator films. Crit. Rev. Food Sci. Nutr. 2021, 61, 2297–2325. [Google Scholar] [CrossRef] [PubMed]
- Peighambardoust, S.H.; Yaghoubi, M.; Hosseinpour, A.; Alirezalu, K.; Soltanzadeh, M.; Dadpour, M. Development and application of dual-sensors label in combination with active chitosan-based coating incorporating yarrow essential oil for freshness monitoring and shelf-life extension of chicken fillet. Foods 2022, 11, 3533. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official methods of analysis AOAC International, 16th ed.; AOAC International: Gaitherburg, MD, USA, 1997. [Google Scholar]
- FAO/WHO. Food and Agriculture Organization of the United Nations; Food energy: Methods of analysis and conversion factors. Food and Nutrition, 2002. Available online: http://www.fao.org/uploads/media/FAO_2003_Food_Energy_02.pdf (accessed on 25 May 2022).
- Oktay, M.; Küfreviolu, I.; Kocaçalişkan, I.; Sakirolu, H. Polyphenoloxidase from Amasya apple. J. Food Sci. 1995, 3, 60. [Google Scholar] [CrossRef]
- Khan, A.A.; Robinson, D.S. Hydrogen donor specificity of mango isoperoxidases. Food Chem. 1994, 49, 407–410. [Google Scholar] [CrossRef]
- Chisté, R.C.; Lopes, A.S.; De Faria, L.J.G. Thermal and light degradation kinetics of anthocyanin extracts from mangosteen peel (Garcinia mangostana L.). Int. J. Food Sci. Technol. 2010, 45, 1902–1908. [Google Scholar] [CrossRef]
- Wrolstad, R.E.; Durst, R.W.; Lee, J. Tracking color and pigment changes in anthocyanin products. Trends Food Sci. Technol. 2005, 16, 423–428. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Teixeira, L.L.; Hassimotto, N.M.A.; Lajolo, F.M. Grumixama—Eugenia brasiliensis Lam. In Exotic Fruits; Sueli, R., Ebenezer, O.S., Edy, S.B., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 219–224. [Google Scholar]
- Ramos, A.L.C.C.; Mendes, D.D.; Silva, M.R.; Augusti, R.; Melo, J.O.F.; Araújo, R.L.B.; Lacerda, I.C.A. Chemical profile of Eugenia brasiliensis (Grumixama) pulp by PS/MS paper spray and SPME-GC/MS solid-phase microextraction. Res., Soc. Dev. 2020, 9, e318974008. [Google Scholar] [CrossRef]
- Silva, N.A.; Rodrigues, E.; Mercadante, A.Z.; Rosso, V.V. Phenolic compounds and carotenoids from four fruits native from the Brazilian Atlantic forest. J. Agric. Food Chem. 2014, 62, 5072–5084. [Google Scholar] [CrossRef] [PubMed]
- Colaric, M.; Veberic, R.; Stampar, F.; Hudina, M. Evaluation of peach and nectarine fruit quality and correlations between sensory and chemical attributes. J. Sci. Food Agric. 2005, 85, 2611–2616. [Google Scholar] [CrossRef]
- Whitaker, J.R. Mechanisms of oxidoreductases important in food component modification. In Chemical Changes in Food during Processing; Richardson, T., Finley, J.W., Eds.; AV Publishing: Westport, CT, USA, 1985; pp. 121–176. [Google Scholar]
- Kim, A.; Lee, K.; Kim, B.G.; Cha, S.W.; Jeong, E.J.; Kerr, W.L.; Choi, S. Thermal processing under oxygen–free condition of blueberry puree: Effect on anthocyanin, ascorbic acid, antioxidant activity, and enzyme activities. Food Chem. 2021, 342, 128345. [Google Scholar] [CrossRef] [PubMed]
- Kader, F.; Irmouli, M.; Nicolas, J.P.; Metche, M. Involvement of blueberry peroxidase in the mechanisms of anthocyanin degradation in blueberry juice. J. Food Sci. 2002, 67, 910–915. [Google Scholar] [CrossRef]
- Kader, F.; Rovel, B.; Girardin, M.; Metche, M. Mechanism of browning in fresh highbush blueberry fruit (Vaccinium corymbosum L). Role of blueberry polyphenol oxidase, chlorogenic acid and anthocyanins. J. Sci. Food Agric. 1997, 74, 31–34. [Google Scholar] [CrossRef]
- Araújo, F.F.; Neri-Numa, I.A.; de Paulo Farias, D.; da Cunha, G.R.M.C.; Pastore., G.M. Wild Brazilian species of Eugenia genera (Myrtaceae) as an innovation hotspot for food and pharmacological purposes. Food Res. Int. 2019, 121, 57–72. [Google Scholar] [CrossRef]
- Flores, G.; Dastmalchi, K.; Paulino, S.; Whalen, K.; Dabo, A.J.; Reynertson, K.A.; Foronjy, R.F.; D’armiento, J.M.; Kennelly, E.J. Anthocyanins from Eugenia brasilienis edible fruits as potential therapeutics for COPD treatment. Food Chem. 2012, 134, 1256–1262. [Google Scholar] [CrossRef] [Green Version]
- Patras, A.; Bruton, N.P.; O’Donnell, C.; Tiwari, B.K. Effect of thermal processing on anthocyanin stability in foods; mechanisms and kinetics of degradation. Trends Food Sci. Technol. 2010, 21, 3–11. [Google Scholar] [CrossRef]
- Tonon, R.V.; Brabet, C.; Hubinger, M.D. Anthocyanin stability and antioxidant activity of spray-dried açai (Euterpe oleracea Mart.) juice produced with different carrier agents. Food Res. Int. 2010, 43, 907–914. [Google Scholar] [CrossRef]
- Das, A.B.; Goud, V.V.; Das, C. Degradation kinetics of anthocyanins from purple rice bran and effect of hydrocolloids on its stability. J. Food Process Eng. 2020, 43, e13360. [Google Scholar] [CrossRef]
- Ursu, M.S.; Aprodu, I.; Milea, S.A.; Enachi, E.; Râpeanu, G.; Bahrim, G.E.; Stănciuc, N. Thermal degradation kinetics of anthocyanins extracted from purple maize flour extract and the effect of heating on selected biological functionality. Foods 2020, 9, 1593. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Qin, P.; Zhang, Y.; Cui, S.; Ren, G. Identification of anthocyanins isolated from black rice (Oryza sativa L.) and their degradation kinetics. Food Res. Inter. 2013, 50, 691–697. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Tao, C.; Liu, M.; Pan, Y.; Lv, Z. Effect of temperature and pH on stability of anthocyanin obtained from blueberry. J. Food Meas. Charact. 2018, 12, 1744–1753. [Google Scholar] [CrossRef]
- Peng, J.; Jiab, Y.; Dua, X.; Wanga, Y.; Yangc, Z.; Lia, K. Study of physicochemical stability of anthocyanin extracts from black peanut skin and their digestion enzyme and adipogenesis inhibitory activities. LWT Food Sci. Technol. 2019, 107, 107–116. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. Update on natural food pigments: A mini-review on carotenoids, anthocyanins, and betalains. Food Res. Int. 2019, 124, 200–205. [Google Scholar] [CrossRef]
- Bolea, C.A.; Grigore-Gurgu, L.; Aprodu, I.; Vizireanu, C.; Stănciuc, N. Process-structure-function in association with the main bioactive of black rice flour sieving fractions. Foods 2019, 8, 131. [Google Scholar] [CrossRef] [Green Version]
- Martynenko, A.; Chen, Y. Degradation kinetics of total anthocyanins and formation of polymeric color in blueberry hydrothermodynamic (HTD) processing. J. Food Eng. 2016, 171, 44–51. [Google Scholar] [CrossRef]
- Peron, D.V.; Fraga, S.; Antelo, F. Thermal degradation kinetics of anthocyanins extracted from juçara (Euterpe edulis Martius) and “Italia” grapes (Vitis vinifera L.) and the effect of thermal on the antioxidant capacity. Food Chem. 2017, 232, 836–840. [Google Scholar] [CrossRef]
- Verzelloni, E.; Tagliazucchi, D.; Conte, A. Relationship between the antioxidant properties and the phenolic and flavonoid content in traditional balsamic vinegar. Food Chem. 2007, 105, 564–571. [Google Scholar] [CrossRef]
- Tsali, A.; Goula, A.M. Valorization of grape pomace: Encapsulation and storage stability of its phenolic extract. Powder Technol. 2018, 340, 194–207. [Google Scholar] [CrossRef]
- Koutchma, T.; Forney, L.J.; Moraru, C.I. Ultraviolet light in food technology: Principles and applications. In UV Processing Effects on Quality of Foods; Da-Wen, S., Tatiana, K., Larry, J.F., Carmen, I.M., Eds.; CRC Press: Boca Raton, FL, USA, 2009; pp. 103–123. [Google Scholar]
- Du, C.T.; Francis, F.J. Anthocyanins of mangosteen, Garcinia mangostana. J. Food Sci. 1997, 42, 1667–1668. [Google Scholar] [CrossRef]
- Delgado-Vargas, F.; Jiménez, A.R.; Paredes-López, O. Natural pigments: Carotenoids, anthocyanins, and betalains-characteristics, biosynthesis, processing, and storage. Crit. Rev. Food Sci. Nutr. 2000, 40, 289. [Google Scholar] [CrossRef] [PubMed]
- Del-Toro-Sánchez, C.L.; Gutiérrez-Lomelí, M.; Lugo-Cervantes, E.; Zurita, F.; Robles-García, M.A.; Ruiz-Cruz, S.; Guerrero-Medina, P.J. Storage effect on phenols and on the antioxidant activity of extracts from Anemopsis californica and inhibition of elastase enzyme. J. Chem. 2015, 2015, 602136. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Singh, R.; Quek, S.Y. Extraction of anthocyanins from natural sources—Methods and commercial considerations. In Anthocyanins from Natural Sources: Exploiting Targeted Delivery for Improved Health; Marianne, S.-L.B., Giovana, B.C., Eds.; Royal Society of Chemistry: London, UK, 2019; pp. 77–105. [Google Scholar]
- Fang, Z.; Bhandari, B. Effect of spray drying and storage on the stability of bayberry polyphenols. Food Chem. 2011, 129, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- Flores, F.P.; Singh, R.K.; Kong, F. Physical and storage properties of spray-dried blueberry pomace extract with whey protein isolate as wall material. J. Food Eng. 2014, 137, 1–6. [Google Scholar] [CrossRef]
- Zorić, Z.; Pelaić, Z.; Pedisić, S.; Garofulić, I.E.; Bursać Kovačević, D.; Dragović–Uzelac, V. Effect of storage conditions on phenolic content and antioxidant capacity of spray dried sour cherry powder. LWT Food Sci. Technol. 2017, 79, 251–259. [Google Scholar] [CrossRef]



| Property/Component | Grumixama Fruit |
|---|---|
| Proximate composition | |
| Moisture (g/100 g fruit) | 75.15 ± 0.88 |
| Total solids (TS) (g/100 g fruit) | 24.85 ± 0.88 |
| Ash (g/100 g dw) | 4.83 ± 0.08 |
| Total proteins (g/100 g dw) | 3.90 ± 0.20 |
| Total lipids (g/100 g dw) | 0.28 ± 0.04 |
| Total carbohydrates (g/100 g dw) | 38.03 ± 0.16 |
| Total energetic value (kcal/100 g fruit) | 42.31 ± 1.04 |
| Physicochemical properties | |
| pH | 3.83 ± 0.05 |
| TTA (g citric acid/100 g fruit) | 0.31 ± 0.01 |
| TSS (°Brix) | 15.16 ± 0.08 |
| TSS/TTA ratio | 48.90 ± 0.10 |
| Enzyme activity | |
| PPO activity (U/g fruit) | 1.34 ± 0.05 |
| POD activity (U/g fruit) | 9.54 ± 0.14 |
| Bioactive compounds | |
| TMA (mg CGE/100 g dw) | 2590.99 ± 40.32 |
| TPC (mg GAE/100 g dw) | 2340.36 ± 47.48 |
| Antioxidant capacity (µmol TE/100 g fruit) | 728.00 ± 35.69 |
| Property | Temperature | Time (h) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 24 | 48 | 72 | 120 | 168 | 216 | 264 | |||||
| TMA | 30 °C | 1.00 ± <0.01 a | 0.76 ± 0.04 b | 0.72 ± 0.07 b | 0.70 ± <0.01 b | 0.63 ± 0.04 b | 0.50 ± 0.03 c | 0.47 ± <0.01 c | 0.22 ± <0.01 d | |||
| TPC | 1.00 ± 0.09 a | 0.87 ± 0.08 ab | 0.80 ± <0.01 abc | 0.72 ± 0.04 bcd | 0.56 ± 0.01 de | 0.55 ± 0.03 cde | 0.54 ± <0.01 de | 0.37 ± 0.03 e | ||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||
| TMA | 60 °C | 1.00 ± <0.01 a | 0.93 ± 0.03 ab | 0.87 ± 0.04 abc | 0.77 ± 0.01 bcd | 0.74 ± <0.01 d | 0.69 ± <0.01 d | 0.65 ± ≤0.01 def | 0.60 ± ≤0.01 def | 0.56 ± 0.03 ef | 0.52 ± 0.12 ef | 0.46 ± 0.01 d |
| TPC | 1.00 ± 0.08 a | 0.92 ± 0.04 a | 0.90 ± 0.05 a | 0.85 ± 0.07 ab | 0.76 ± 0.03 abc | 0.77 ± 0.03 abc | 0.74 ± 0.01 abc | 0.63 ± 0.01 bc | 0.53 ± ≤0.01 c | 0.49 ± <0.01 d | ||
| 0 | 0.32 | 0.64 | 0.96 | 1.92 | 2.88 | 3.84 | 4.80 | |||||
| TMA | 80 °C | 1.00 ± 0.02 a | 0.78 ± 0.08 b | 0.57 ± 0.03 c | 0.57 ± 0.03 cd | 0.49 ± 0.01 cde | 0.36 ± 0.02 cde | 0.33 ± 0.02 de | 0.26 ± <0.01 e | |||
| TPC | 1.00 ± 0.06 a | 0.92 ± 0.01 a | 0.71 ± 0.04 b | 0.71 ± 0.04 ab | 0.85 ± 0.03 ab | 0.77 ± 0.02 ab | 0.81 ± <0.01 ab | 0.72 ± <0.01 b | ||||
| 0 | 0.16 | 0.32 | 0.48 | 0.96 | 1.92 | 2.88 | 3.84 | |||||
| TMA | 100 °C | 1.00 ± 0.03 a | 0.90 ± 0.01 a | 0.84 ± 0.09 ab | 0.69 ± 0.06 bc | 0.54 ± 0.03 cd | 0.44 ± <0.01 de | 0.36 ± 0.01 e | 0.23 ± 0.01 f | |||
| TPC | 1.00 ± 0.06 ab | 0.97 ± 0.10 ab | 0.98 ± 0.04 ab | 1.03 ± 0.03 ab | 1.08 ± 0.07 ab | 1.21 ± 0.05 a | 0.87 ± 0.02 b | 0.97 ± 0.02 b | ||||
| T (°C) | k (h−1) | n | R2 | Q10 | t1/2 (h) | Ea (kJ/mol) |
|---|---|---|---|---|---|---|
| 30 | 0.015 | 0.76 | 0.90 | 1.70 (30–60 °C) | 46.82 | 52.67 (R2 = 0.92) |
| 60 | 0.073 | 1.00 | 0.99 | 2.82 (60–80 °C) | 9.50 | |
| 80 | 0.580 | 0.50 | 0.97 | 0.94 (80–100 °C) | 1.19 | |
| 100 | 0.517 | 0.70 | 0.97 | 1.34 |
| Response | Light | Time (h) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 24 | 48 | 72 | 120 | 168 | 216 | ||
| TMA | Fluorescent | 1.00 ± 0.09 a | 0.74 ± 0.09 ab | 0.72 ± 0.11 ab | 0.59 ± 0.01 b | 0.50 ± 0.01 bc | 0.32 ± 0.04 c | 0.23 < 0.01 c |
| TPC | 1.00 ± 0.09 a | 0.73 ± 0.06 ab | 0.67 ± 0.01 abc | 0.62 ± 0.02 bc | 0.38 ± 0.05 d | 0.49 ± 0.06 cd | 0.48 ± 0.06 cd | |
| 0 | 24 | 48 | 72 | 120 | 168 | 216 | ||
| TMA | Incandes-cent | 1.00 ± 0.05 a | 0.78 ± 0.02 ab | 0.74 ± 0.01 bc | 0.69 ± 0.01 bc | 0.50 ± 0.01 cd | 0.37 ± 0.02 de | |
| TPC | 1.00 ± 0.01 a | 0.71 ± 0.04 bc | 0.57 ± <0.01 c | 0.47 ± 0.02 c | 0.63 ± <0.01 bc | 1.11 ± 0.22 ab | 0.92 ± 0.01 ab | |
| 0 | 10 | 20 | 40 | 60 | ||||
| TMA | Ultraviolet | 1.00 ± 0.27 a | 0.46 ± 0.06 b | 0.24 ± 0.02 bc | 0.05 ± 0.01 bc | 0.04 ± <0.01 bc | ||
| TPC | 1.00 ± 0.15 a | 0.60 ± <0.01 ab | 0.88 ± 0.04 ab | 0.60 ± 0.04 ab | 0.53 ± 0.07 b | |||
| Light | k (h−1) | n | t1/2 (h) |
|---|---|---|---|
| Fluorescent | 0.015 | 0.82 | 45.61 |
| Incandescent | 0.012 | 0.85 | 59.63 |
| Ultraviolet | 3.748 | 0.88 | 0.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Modesto Junior, E.N.; Martins, M.G.; Pereira, G.A.; Chisté, R.C.; Pena, R.d.S. Stability Kinetics of Anthocyanins of Grumixama Berries (Eugenia brasiliensis Lam.) during Thermal and Light Treatments. Foods 2023, 12, 565. https://doi.org/10.3390/foods12030565
Modesto Junior EN, Martins MG, Pereira GA, Chisté RC, Pena RdS. Stability Kinetics of Anthocyanins of Grumixama Berries (Eugenia brasiliensis Lam.) during Thermal and Light Treatments. Foods. 2023; 12(3):565. https://doi.org/10.3390/foods12030565
Chicago/Turabian StyleModesto Junior, Elivaldo Nunes, Mayara Galvão Martins, Gustavo Araujo Pereira, Renan Campos Chisté, and Rosinelson da Silva Pena. 2023. "Stability Kinetics of Anthocyanins of Grumixama Berries (Eugenia brasiliensis Lam.) during Thermal and Light Treatments" Foods 12, no. 3: 565. https://doi.org/10.3390/foods12030565
APA StyleModesto Junior, E. N., Martins, M. G., Pereira, G. A., Chisté, R. C., & Pena, R. d. S. (2023). Stability Kinetics of Anthocyanins of Grumixama Berries (Eugenia brasiliensis Lam.) during Thermal and Light Treatments. Foods, 12(3), 565. https://doi.org/10.3390/foods12030565