Chemometric Discrimination of Cichorium glandulosum Boiss. et Huet and Cichorium intybus L. via Their Metabolic Profiling, Antioxidative, and Hypoglycemic Activities
Abstract
:1. Introduction
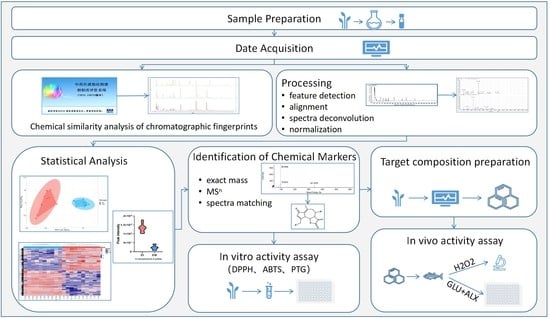
2. Materials and Methods
2.1. Chemicals
2.2. Sample and Sample Preparation
2.3. HPLC Apparatus and Chromatographic Conditions
2.4. LC-QTOF MS Apparatus and Chromatographic Conditions
2.5. Data Pretreatment and Multivariate Data Analysis
2.6. In vitro Activity Assay
2.6.1. Assays for Antioxidant Activities In Vitro
2.6.2. Assays for Hypoglycemic Activities In Vitro
2.7. In Vivo Activity Assay
2.7.1. Antioxidant Activity in Larval Zebrafish
2.7.2. Hypoglycemic Effect Determination on Zebrafish Diabetic Model
2.8. Statistical Analysis
3. Results and Discussion
3.1. Identification of the Common Peaks and HPLC Fingerprint Similarity Analysis
3.2. Non-Targeted LC-QTOF-MS Analysis
3.3. Multivariate Statistical Analysis
3.4. Identification of Chemical Markers
3.5. In Vitro Activity Assay
3.6. Composition Preparation and Pharmacodynamic Evaluation
3.6.1. Composition Preparation Using Preparative-HPLC
3.6.2. Effect of CG and CI on H2O2-Induced Larval Zebrafish Injury Model In Vivo
3.6.3. Effects of CG and CI on Hypoglycemic Effect Induced by High GLU and ALX in Zebrafish Model
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- China Pharmacopoeia Committee. Pharmacopoeia of the People’s Republic of China; Chemical Industry Press: Beijing, China, 2015.
- Perović, J.; Tumbas Šaponjac, V.; Kojić, J.; Krulj, J.; Moreno, D.A.; García-Viguera, C.; Bodroža-Solarov, M.; Ilić, N. Chicory (Cichorium Intybus L.) as a Food Ingredient—Nutritional Composition, Bioactivity, Safety, and Health Claims: A Review. Food Chem. 2021, 336, 127676. [Google Scholar] [CrossRef] [PubMed]
- Muthusamy, V.S.; Anand, S.; Sangeetha, K.N.; Sujatha, S.; Arun, B.; Lakshmi, B.S. Tannins Present in Cichorium Intybus Enhance Glucose Uptake and Inhibit Adipogenesis in 3T3-L1 Adipocytes through PTP1B Inhibition. Chem. Biol. Interact. 2008, 174, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, M.; Ohkawara, T.; Kanayama, T.; Kitagawa, K.; Nishimura, H.; Nishihira, J. Effects of the Extract from Roasted Chicory (Cichorium Intybus L.) Root Containing Inulin-Type Fructans on Blood Glucose, Lipid Metabolism, and Fecal Properties. J. Tradit. Complement. Med. 2015, 5, 161–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, K.M.P.; Rathinasabapathy, T.; Esposito, D.; Komarnytsky, S. Structural Constraints and Importance of Caffeic Acid Moiety for Anti-Hyperglycemic Effects of Caffeoylquinic Acids from Chicory. Mol. Nutr. Food Res. 2017, 61, 1601118. [Google Scholar] [CrossRef] [PubMed]
- Heimler, D.; Isolani, L.; Vignolini, P.; Romani, A. Polyphenol Content and Antiradical Activity of Cichorium Intybus L. from Biodynamic and Conventional Farming. Food Chem. 2009, 114, 765–770. [Google Scholar] [CrossRef]
- Tong, J.; Yao, X.; Zeng, H.; Zhou, G.; Chen, Y.; Ma, B.; Wang, Y. Hepatoprotective Activity of Flavonoids from Cichorium Glandulosum Seeds in Vitro and in Vivo Carbon Tetrachloride-Induced Hepatotoxicity. J. Ethnopharmacol. 2015, 174, 355–363. [Google Scholar] [CrossRef]
- Nwafor, I.C.; Shale, K.; Achilonu, M.C. Chemical Composition and Nutritive Benefits of Chicory (Cichorium Intybus) as an Ideal Complementary and/or Alternative Livestock Feed Supplement. Sci. World J. 2017, 2017, 7343928. [Google Scholar] [CrossRef] [Green Version]
- Ding, L.; Liu, J.-L.; Hassan, W.; Wang, L.-L.; Yan, F.-R.; Shang, J. Lipid Modulatory Activities of Cichorium Glandulosum Boiss et Huet Are Mediated by Multiple Components within Hepatocytes. Sci. Rep. 2014, 4, 4715. [Google Scholar] [CrossRef] [Green Version]
- Tong, J.; Ma, B.; Ge, L.; Mo, Q.; Zhou, G.; He, J.; Wang, Y. Dicaffeoylquinic Acid-Enriched Fraction of Cichorium Glandulosum Seeds Attenuates Experimental Type 1 Diabetes via Multipathway Protection. J. Agric. Food Chem. 2015, 63, 10791–10802. [Google Scholar] [CrossRef]
- Saggu, S.; Sakeran, M.I.; Zidan, N.; Tousson, E.; Mohan, A.; Rehman, H. Ameliorating Effect of Chicory (Chichorium Intybus L.) Fruit Extract against 4-Tert-Octylphenol Induced Liver Injury and Oxidative Stress in Male Rats. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2014, 72, 138–146. [Google Scholar] [CrossRef]
- Abbas, Z.K.; Saggu, S.; Sakeran, M.I.; Zidan, N.; Rehman, H.; Ansari, A.A. Phytochemical, Antioxidant and Mineral Composition of Hydroalcoholic Extract of Chicory (Cichorium Intybus L.) Leaves. Saudi J. Biol. Sci. 2015, 22, 322–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, X.; Feng, S.; Zhang, J.; Sun, S.; Geng, Y.; Yang, M.; Liu, Y.; Qin, L.; Zhao, T.; Wang, C.; et al. Application of HPLC Fingerprint Combined with Chemical Pattern Recognition and Multi-Component Determination in Quality Evaluation of Echinacea Purpurea (L.) Moench. Molecules 2022, 27, 6463. [Google Scholar] [CrossRef] [PubMed]
- Fragallah, S.A.D.A.; Wang, P.; Li, N.; Chen, Y.; Lin, S. Metabolomic Analysis of Pollen Grains with Different Germination Abilities from Two Clones of Chinese Fir (Cunninghamia Lanceolata (Lamb) Hook). Molecules 2018, 23, 3162. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Li, Y.; Huang, Y.; Tian, Y.; Xu, F.; Zhang, Z. Chemomic and Chemometric Approach Based on Ultra-Fast Liquid Chromatography with Ion Trap Time-of-Flight Mass Spectrometry to Reveal the Difference in the Chemical Composition between Da-Cheng-Qi Decoction and Its Three Constitutional Herbal Medicines. J. Sep. Sci. 2014, 37, 1148–1154. [Google Scholar] [CrossRef] [PubMed]
- Kind, T.; Tsugawa, H.; Cajka, T.; Ma, Y.; Lai, Z.; Mehta, S.S.; Wohlgemuth, G.; Barupal, D.K.; Showalter, M.R.; Arita, M.; et al. Identification of Small Molecules Using Accurate Mass MS/MS Search. Mass Spectrom. Rev. 2018, 37, 513–532. [Google Scholar] [CrossRef]
- Cichon, M.J.; Riedl, K.M.; Schwartz, S.J. A Metabolomic Evaluation of the Phytochemical Composition of Tomato Juices Being Used in Human Clinical Trials. Food Chem. 2017, 228, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Burits, M.; Bucar, F. Antioxidant Activity of Nigella Sativa Essential Oil. Phytother. Res. PTR 2000, 14, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Mor, M.; Silva, C.; Vacondio, F.; Plazzi, P.V.; Bertoni, S.; Spadoni, G.; Diamantini, G.; Bedini, A.; Tarzia, G.; Zusso, M.; et al. Indole-Based Analogs of Melatonin: In Vitro Antioxidant and Cytoprotective Activities. J. Pineal Res. 2004, 36, 95–102. [Google Scholar] [CrossRef]
- Wang, L.; Chen, C.; Zhang, B.; Huang, Q.; Fu, X.; Li, C. Structural Characterization of a Novel Acidic Polysaccharide from Rosa Roxburghii Tratt Fruit and Its α-Glucosidase Inhibitory Activity. Food Funct. 2018, 9, 3974–3985. [Google Scholar] [CrossRef]
- Ma, J.; Li, M.; Kalavagunta, P.K.; Li, J.; He, Q.; Zhang, Y.; Ahmad, O.; Yin, H.; Wang, T.; Shang, J. Protective Effects of Cichoric Acid on H2O2-Induced Oxidative Injury in Hepatocytes and Larval Zebrafish Models. Biomed. Pharmacother. Biomedecine Pharmacother. 2018, 104, 679–685. [Google Scholar] [CrossRef]
- Sannasimuthu, A.; Arockiaraj, J. Intracellular Free Radical Scavenging Activity and Protective Role of Mammalian Cells by Antioxidant Peptide from Thioredoxin Disulfide Reductase of Arthrospira Platensis. J. Funct. Foods 2019, 61, 103513. [Google Scholar] [CrossRef]
- Liu, J.; Li, Q.; Tan, R. Comparison of the Hypoglycemic Effect of the Aqueous Extract of Moras Folium and Gynostemma Pentaphyllum Leaves of Zebrafish as A Model. China Tea Process. 2014, 4, 74–82. [Google Scholar] [CrossRef]
- Li, M.; Ma, J.; Ahmad, O.; Cao, Y.; Wang, B.; He, Q.; Li, J.; Yin, H.; Zhang, Y.; He, J.; et al. Lipid-Modulate Activity of Cichorium Glandulosum Boiss. et Huet Polysaccharide in Nonalcoholic Fatty Liver Disease Larval Zebrafish Model. J. Pharmacol. Sci. 2018, 138, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Dou, Z.; Dai, Y.; Zhou, Y.; Wang, S. Quality Evaluation of Rhubarb Based on Qualitative Analysis of the HPLC Fingerprint and UFLC-Q-TOF-MS/MS Combined with Quantitative Analysis of Eight Anthraquinone Glycosides by QAMS. Biomed. Chromatogr. BMC 2021, 35, e5074. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Ren, J.L.; Zhang, A.H.; Sun, H.; Yan, G.L.; Han, Y.; Liu, L. Novel Applications of Mass Spectrometry-Based Metabolomics in Herbal Medicines and Its Active Ingredients: Current Evidence. Mass Spectrom. Rev. 2019, 38, 380–402. [Google Scholar] [CrossRef] [PubMed]
- Worley, B.; Powers, R. PCA as a Practical Indicator of OPLS-DA Model Reliability. Curr. Metabolomics 2016, 4, 97–103. [Google Scholar] [CrossRef] [Green Version]
- Rashid, A.; Ali, V.; Khajuria, M.; Faiz, S.; Gairola, S.; Vyas, D. GC-MS Based Metabolomic Approach to Understand Nutraceutical Potential of Cannabis Seeds from Two Different Environments. Food Chem. 2021, 339, 128076. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Su, A.; Yuan, S.; Zhao, H.; Tan, S.; Hu, C.; Deng, H.; Guo, Y. Evaluation of Total Flavonoids, Myricetin, and Quercetin from Hovenia Dulcis Thunb. As Inhibitors of α-Amylase and α-Glucosidase. Plant Foods Hum. Nutr. Dordr. Neth. 2016, 71, 444–449. [Google Scholar] [CrossRef]
- Issac, P.K.; Guru, A.; Velayutham, M.; Pachaiappan, R.; Arasu, M.V.; Al-Dhabi, N.A.; Choi, K.C.; Harikrishnan, R.; Arockiaraj, J. Oxidative Stress Induced Antioxidant and Neurotoxicity Demonstrated in Vivo Zebrafish Embryo or Larval Model and Their Normalization Due to Morin Showing Therapeutic Implications. Life Sci. 2021, 283, 119864. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Q.; Liu, Y.; Bi, L.; Jin, L.; Xu, K.; Peng, R. High Glucose-Induced ROS-Accumulation in Embryo-Larval Stages of Zebrafish Leads to Mitochondria-Mediated Apoptosis. Apoptosis Int. J. Program. Cell Death 2022, 27, 509–520. [Google Scholar] [CrossRef]
- He, Y.; Li, W.; Zheng, Z.; Zhao, L.; Li, W.; Wang, Y.; Li, H. Inhibition of Protein Arginine Methyltransferase 6 Reduces Reactive Oxygen Species Production and Attenuates Aminoglycoside- and Cisplatin-Induced Hair Cell Death. Theranostics 2020, 10, 133–150. [Google Scholar] [CrossRef]
- Deng, Y.; Ma, J.; Weng, X.; Wang, Y.; Li, M.; Yang, T.; Dou, Z.; Yin, Z.; Shang, J. Kaempferol-3-O-Glucuronide Ameliorates Non-Alcoholic Steatohepatitis in High-Cholesterol-Diet-Induced Larval Zebrafish and HepG2 Cell Models via Regulating Oxidation Stress. Life 2021, 11, 445. [Google Scholar] [CrossRef] [PubMed]
- Uzun, Y.; Dalar, A.; Konczak, I. Sempervivum Davisii: Phytochemical Composition, Antioxidant and Lipase-Inhibitory Activities. Pharm. Biol. 2017, 55, 532–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, P.; Wang, Y.; Yang, L.; Sui, J.; Liu, Y. Hypoglycemic Effects in Alloxan-Induced Diabetic Rats of the Phenolic Extract from Mongolian Oak Cups Enriched in Ellagic Acid, Kaempferol and Their Derivatives. Molecules 2018, 23, 1046. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Sample Number | Similarity | Sample Number | Similarity | ||
|---|---|---|---|---|---|
| CGR | CIR | CIR | CGR | ||
| CG1 | 0.991 | 0.642 | CI1 | 0.789 | 0.521 |
| CG2 | 0.983 | 0.600 | CI2 | 0.946 | 0.706 |
| CG3 | 0.916 | 0.741 | CI3 | 0.929 | 0.646 |
| CG4 | 0.981 | 0.600 | CI4 | 0.914 | 0.588 |
| CG5 | 0.982 | 0.614 | CI5 | 0.801 | 0.445 |
| CG6 | 0.973 | 0.674 | CI6 | 0.897 | 0.557 |
| CG7 | 0.954 | 0.723 | CI7 | 0.929 | 0.839 |
| CG8 | 0.983 | 0.679 | CI8 | 0.946 | 0.706 |
| CG9 | 0.900 | 0.626 | CI9 | 0.905 | 0.689 |
| CG10 | 0.968 | 0.708 | CI10 | 0.899 | 0.626 |
| NO. | Compound Name | Elemental Composition | tR (min) | Observed [M-H]- | Product Ions (m/z) | Chemical Class |
|---|---|---|---|---|---|---|
| 1 | L-Tryptophan | C11H12N2O2 | 2.36 | 203.0188 | 159.03 | Amino acid derivatives |
| 2 | Malic acid | C4H6O5 | 2.76 | 133.0175 | 71.01, 115.00 | Organic acids and derivatives |
| 3 | Ferulic acid | C10H10O4 | 2.79 | 193.0277 | 160.84, 117.02 | Phenolic acids |
| 4 | Cis-Aconitic acid | C6H6O6 | 2.82 | 173.0154 | 85.02, 129.04 | Organic acids and derivatives |
| 5 | Quinic acid | C7H12O6 | 2.91 | 191.0225 | 111.00, 127.03, 173.04 | Phenolic acids |
| 6 | Succinate | C4H6O4 | 3.59 | 117.0159 | 59.01, 99.02 | Organic acids and derivatives |
| 7 | Gentiopicroside | C16H20O9 | 3.77 | 355.1281 | 191.01, 177.07 | Terpenoids |
| 8 | Umbelliferone | C9H6O3 | 3.82 | 161.0450 | 117.05 | Phenolic acids |
| 9 | Salvianolic acid A | C26H22O10 | 5.36 | 493.1242 | 295.06 | Phenolic acids |
| 10 | Caftaric acid | C13H12O9 | 6.91 | 311.0416 | 135.04, 179.03, 87.00 | Phenolic acids |
| 11 | 4-Hydroxyphenylpyruvic acid | C9H8O4 | 6.92 | 179.0340 | 135.04 | Phenolic acids |
| 12 | Phenylacetic acid | C8H8O2 | 6.99 | 135.0441 | 91.05 | Phenolic acids |
| 13 | D-tartaric acid | C4H6O6 | 7.07 | 149.0086 | 87.00, 103.01 | Organic acids and derivatives |
| 14 | Pyrocatechuic acid | C7H6O4 | 7.16 | 153.0226 | 109.02 | Phenolic acids |
| 15 | Caffeic acid-hexoside | C15H18O9 | 8.65 | 341.0885 | 179.03, 135.04 | Phenolic acids |
| 16 | Cichoriin | C15H16O9 | 8.98 | 339.0759 | 177.01, 133.02 | Phenolic acids |
| 17 | Intybulide | C15H16O5 | 9.19 | 275.0936 | 194.90, 150.90 | Terpenoids |
| 18 | Lactupicrin ethylester | C25H28O8 | 9.48 | 455.1557 | 275.08 | Terpenoids |
| 19 | 8-Omrthylsenecioylaustricin | C21H26O5 | 10.57 | 357.0709 | 194.9, 173.0, 217.0 | Terpenoids |
| 20 | Chlorogenic acid | C16H18O9 | 11.03 | 353.0935 | 309.09 | Phenolic acids |
| 21 | 4-Hydroxyphenylacetic acid | C8H8O3 | 12.01 | 151.0397 | 151.04, 107.05 | Phenolic acids |
| 22 | Esculetin | C9H6O4 | 12.99 | 177.0221 | 133.02,131.01 | Phenolic acids |
| 23 | Caffeic acid | C9H8O4 | 13.31 | 179.0337 | 135.04 | Phenolic acids |
| 24 | Irigenin | C18H16O8 | 13.71 | 359.0815 | 96.96 | Flavonoids |
| 25 | p-Hydroxybenzaldehyde | C7H6O2 | 13.85 | 121.0294 | 93.03 | Phenolic acids |
| 26 | Nicotinic acid | C6H5NO2 | 13.87 | 122.0316 | 94.05 | Phenolic acids |
| 27 | Cichorioside J | C22H28O10 | 14.48 | 451.0832 | 59.01, 423.07, 361.01 | Terpenoids |
| 28 | Coumaroyl quinic acid | C16H18O8 | 14.50 | 337.1127 | 191.05 | Phenolic acids |
| 29 | 15-Oxalyl-lactucin | C17H16O8 | 14.92 | 347.0851 | 213.09, 257.08, 275.09 | Terpenoids |
| 30 | Caffeoylquinic acid | C16H18O9 | 16.33 | 353.0341 | 191.0, 179.0 | Phenolic acids |
| 31 | Cichoric acid | C22H18O12 | 17.37 | 473.0713 | 179.03 | Phenolic acids |
| 32 | 11,13-Dihydro-8-deoxylactucin | C15H18O4 | 19.94 | 261.1068 | 229.08 | Terpenoids |
| 33 | Artesin | C15H22O5 | 20.71 | 281.1305 | 201.00 | Terpenoids |
| 34 | 11β,13-dihydrolactucin | C15H18O5 | 21.31 | 277.0333 | 260.18 | Terpenoids |
| 35 | 15-Oxalyl-11β,13-dihydrolactucopicrin | C25H24O11 | 21.94 | 483.1583 | 325.05, 179.03 | Terpenoids |
| 36 | Myricetin-7-O-(6″-O-malonyl)-glucoside | C24H22O16 | 22.00 | 565.1735 | 521.1 | Flavonoids |
| 37 | 15-Deoxylactucin-8-sulfate | C15H16O7S | 22.81 | 339.0589 | 96.96, 79.95 | Terpenoids |
| 38 | 8-Deacetylmatricarin-8-O-sulfate | C15H18O7S | 23.39 | 341.0715 | 96.95, 79.95 | Terpenoids |
| 39 | Chlorogenic acid B | C25H24O12 | 24.99 | 515.1215 | 191.05 | Phenolic acids |
| 40 | 28β-Hydroxytaraxaste | C30H52O | 26.14 | 441.0878 | 277.03, 295.04, 259.03 | Steroid |
| 41 | Quercetin-3-O-β-D-Glucuronide | C21H18O13 | 26.53 | 477.0717 | 301.03 | Flavonoids |
| 42 | Quercetin-3-O-β-D-glucopyranoside | C21H20O12 | 27.42 | 463.0915 | 300.02 | Flavonoids |
| 43 | Rutin | C27H30O16 | 27.52 | 609.1516 | 300.02 | Flavonoids |
| 44 | Quercetin 3-O-(6″-malonyl-glucoside) | C24H22O15 | 29.61 | 549.0928 | 505.09, 300.02 | Flavonoids |
| 45 | Quercetin 3-O-(6″-acetyl-glucoside) | C23H22O13 | 29.79 | 505.1033 | 301.03, 59.01 | Flavonoids |
| 46 | Kaempferol-3-O-glucuronoside | C21H18O12 | 33.08 | 461.0787 | 285.04 | Flavonoids |
| 47 | Kaempferol-3-O-glucoside | C21H20O11 | 33.74 | 447.0956 | 284.03, 285.00 | Flavonoids |
| 48 | Azelaic acid | C9H16O4 | 34.46 | 187.0969 | 125.09 | Fatty acids |
| 49 | Syringetin-3-O-glucoside | C23H24O13 | 34.72 | 507.1187 | 151.00, 303.05 | Flavonoids |
| 50 | Isorhamnetin-7-O-glucoside | C22H23O12 | 34.94 | 477.1070 | 314.04 | Flavonoids |
| 51 | Isorahmnetin-7-O-glucuronide | C22H20O13 | 35.25 | 491.0934 | 315.04 | Flavonoids |
| 52 | Narcissoside | C28H32O16 | 35.60 | 623.1667 | 315.04, 314.04, 300.02 | Flavonoids |
| 53 | Naringin | C27H32O14 | 35.81 | 625.1574 | 271.10, 151.00 | Flavonoids |
| 54 | Kaempferol-3-O-(6″-O-malonyl)-glucoside | C24H22O14 | 37.00 | 533.0981 | 285.03, 489.09 | Flavonoids |
| 55 | Lactucopicrin-15-oxalate | C25H22O10 | 42.96 | 481.1142 | 213.09, 257.08, 151.03 | Terpenoids |
| 56 | β-Sitosterol | C29H50O | 45.03 | 413.1624 | 395.07 | Steroid |
| 57 | 11,13-Dihydrolactucopicrin | C23H24O7 | 45.12 | 411.1515 | 215.10, 151.04 | Terpenoids |
| 58 | Lactucopicrin | C23H22O7 | 45.82 | 409.1385 | 213.09, 257.08, 275.09 | Terpenoids |
| 59 | Tricin-3-O-glucoside | C23H24O12 | 45.85 | 491.1452 | 329.23, 311.21 | Flavonoids |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Xie, G.; Ding, Y.; Ma, J.; Liu, Q.; Wang, Y.; Peng, Z.; Sun, J.; Shang, J. Chemometric Discrimination of Cichorium glandulosum Boiss. et Huet and Cichorium intybus L. via Their Metabolic Profiling, Antioxidative, and Hypoglycemic Activities. Foods 2023, 12, 901. https://doi.org/10.3390/foods12040901
Li M, Xie G, Ding Y, Ma J, Liu Q, Wang Y, Peng Z, Sun J, Shang J. Chemometric Discrimination of Cichorium glandulosum Boiss. et Huet and Cichorium intybus L. via Their Metabolic Profiling, Antioxidative, and Hypoglycemic Activities. Foods. 2023; 12(4):901. https://doi.org/10.3390/foods12040901
Chicago/Turabian StyleLi, Maoru, Guoyong Xie, Yadong Ding, Ji Ma, Qiuyan Liu, Yuqin Wang, Zan Peng, Jianbo Sun, and Jing Shang. 2023. "Chemometric Discrimination of Cichorium glandulosum Boiss. et Huet and Cichorium intybus L. via Their Metabolic Profiling, Antioxidative, and Hypoglycemic Activities" Foods 12, no. 4: 901. https://doi.org/10.3390/foods12040901
APA StyleLi, M., Xie, G., Ding, Y., Ma, J., Liu, Q., Wang, Y., Peng, Z., Sun, J., & Shang, J. (2023). Chemometric Discrimination of Cichorium glandulosum Boiss. et Huet and Cichorium intybus L. via Their Metabolic Profiling, Antioxidative, and Hypoglycemic Activities. Foods, 12(4), 901. https://doi.org/10.3390/foods12040901