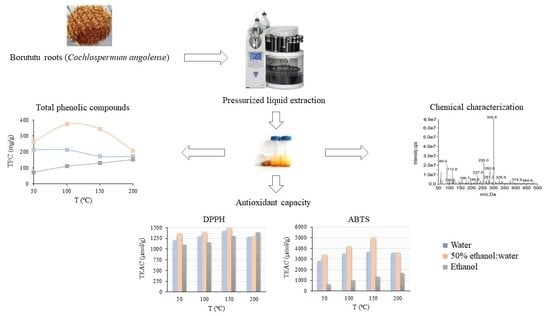
Pressurized Liquid Extraction for the Production of Extracts with Antioxidant Activity from Borututu (Cochlospermum angolense Welw.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Chemicals
2.2. Pressurized Liquid Extraction (PLE)
2.3. Total Phenolic Compound (TPC) Content
2.4. Characterization of the Phenolic Profile
2.5. Antioxidant Activity
2.6. Statistical Analysis
3. Results and Discussion
3.1. PLE of Borututu and TPC Content of the Extracts
3.2. Antioxidant Activity
3.3. Chemical Analysis of the Extracts
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lamien-Meda, A.; Kiendrebeogo, M.; Compaoré, M.; Meda, R.N.T.; Bacher, M.; Koenig, K.; Pacher, T.; Fuehrer, H.P.; Noedl, H.; Willcox, M.; et al. Quality assessment and antiplasmodial activity of West African Cochlospermum species. Phytochemistry 2015, 119, 51–61. [Google Scholar] [CrossRef]
- Pedroso, T.F.M.; Bonamigo, T.R.; da Silva, J.; Vasconcelos, P.; Ramon, M.; Félix, J.M.; Cardoso, C.A.L.; Souza, R.I.C.; dos Santos, A.C.; Volobuff, C.R.F.; et al. Chemical constituents of Cochlospermum regium (Schrank) Pilg. root and its antioxidant, antidiabetic, antiglycation, and anticholinesterase effects in Wistar rats. Biomed. Pharmacother. 2019, 111, 1383–1392. [Google Scholar] [CrossRef]
- Nergard, C.S.; Diallo, D.; Inngjerdingen, K.; Michaelsen, T.E.; Matsumoto, T.; Kiyohara, H.; Yamada, H.; Paulsen, B.S. Medicinal use of Cochlospermum tinctorium in Mali. J. Ethnopharmacol. 2005, 96, 255–269. [Google Scholar] [CrossRef]
- Napane, F.; Kossi, M.; Yendube, T.K.; Yaovi-Gameli, A.; Povi, L.-E.; Aklesso, M.; Kwashie, E.G.; Kodjo, A.A. Phytochemical screening and antimicrobial activities of hydroethanolic extracts from leaves and roots of Cochlospermum planchonii (Bixaceae). J. Pharmacogn. Phytother. 2020, 12, 94–101. [Google Scholar] [CrossRef]
- Presber, H.W.; Hegenscheid, B.; Hernandez-Alvarez, H.; Herrmann, D.; Brendel, C.; Al, E. Inhibition of the growth of Plasmodium falciparum and Plasmodium berghei in vitro by an extract of Cochlospermum angolense (Welw.). Acta Trop. 1992, 50, 331–338. [Google Scholar] [CrossRef]
- Pereira, C.; Calhelha, R.C.; Barros, L.; Ferreira, I.C.F.R. Antioxidant properties, anti-hepatocellular carcinoma activity and hepatotoxicity of artichoke, milk thistle and borututu. Ind. Crops Prod. 2013, 49, 61–65. [Google Scholar] [CrossRef]
- Leonardi, M.; Giovanelli, S.; Cioni, P.L.; Flamini, G.; Pistelli, L. Evaluation of volatile constituents of Cochlospermum angolense. Nat. Prod. Commun. 2012, 7, 629–632. [Google Scholar] [PubMed] [Green Version]
- Abourashed, E.A.; Fu, H.W. Hydroxybenzoic acids are significant contributors to the antioxidant effect of borututu bark, Cochlospermum angolensis Welw. ex Oliv. Antioxidants 2017, 6, 9. [Google Scholar] [CrossRef] [Green Version]
- Pereira, C.; Calhelha, R.C.; Barros, L.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R.; Al, E. Synergisms in antioxidant and anti-hepatocellular carcinoma activities of artichoke, milk thistle and borututu syrups. Ind. Crops Prod. 2014, 52, 709–713. [Google Scholar] [CrossRef] [Green Version]
- Silva, O.; Barbosa, S.; Diniz, A.; Valdeira, M.L.; Gomes, E. Plant extracts antiviral activity against herpes simplex virus type 1 and African swine fever virus. Int. J. Pharmacogn. 1997, 35, 12–16. [Google Scholar] [CrossRef]
- Ferreres, F.; Grosso, C.; Gil-Izquierdo, A.; Valentão, P.; Andrade, P.B.; Al, E. Ellagic acid and derivatives from Cochlospermum angolensis welw. extracts: HPLC-DAD-ESI/MSn profiling, quantification and in vitro anti-depressant, anti-cholinesterase and anti-oxidant activities. Phytochem. Anal. 2013, 24, 534–540. [Google Scholar] [CrossRef]
- Costa, A.S.G.; Nunes, M.A.; Almeida, I.M.C.; Carvalho, M.R.; Barroso, M.F.; Alves, R.C.; Oliveira, M.B.P.P. Teas, dietary supplements and fruit juices: A comparative study regarding antioxidant activity and bioactive compounds. LWT-Food Sci. Technol. 2012, 49, 324–328. [Google Scholar] [CrossRef] [Green Version]
- Barreira, J.C.M.; Morais, A.L.; Ferreira, I.C.F.R.; Oliveria, M.B.P.P. Insights on the formulation of herbal beverages with medicinal claims according with their antioxidant properties. Molecules 2013, 18, 2851–2863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, C.; Barreira, J.C.M.; Calhelha, R.C.; Lopes, M.; Queiroz, M.J.R.P.; Vilas-Boas, M.; Barros, L.; Ferreira, I.C.F.R. Is honey able to potentiate the antioxidant and cytotoxic properties of medicinal plants consumed as infusions for hepatoprotective effects? Food Funct. 2015, 6, 1435–1442. [Google Scholar] [CrossRef] [Green Version]
- Pereira, C.; Barros, L.; Alves, M.J.; Pereira, L.; Santos-Buelga, C.; Ferreira, I.C.F.R. Phenolic profile and antimicrobial activity of different dietary supplements based on Cochlospermum angolensis Welw. Ind. Crops Prod. 2015, 74, 412–416. [Google Scholar] [CrossRef] [Green Version]
- Gallego, R.; Bueno, M.; Herrero, M. Sub-and supercritical fluid extraction of bioactive compounds from plants, food-by-products, seaweeds and microalgae—An update. TrAC Trends Anal. Chem. 2019, 116, 198–213. [Google Scholar] [CrossRef]
- Richter, B.E.; Jones, B.A.; Ezzell, J.L.; Porter, N.L.; Avdalovic, N.; Pohl, C. Accelerated solvent extraction: A technique for sample preparation. Anal. Chem. 1996, 68, 1033–1039. [Google Scholar] [CrossRef]
- Villanueva Bermejo, D.; Mendiola, J.A.; Ibañez, E.; Reglero, G.; Fornari, T. Pressurized liquid extraction of caffeine and catechins from green tea leaves using ethyl lactate, water and ethyl lactate + water mixtures. Food Bioprod. Process. 2015, 96, 106–112. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Padilla, A.; Martin, D.; Villanueva Bermejo, D.; Jaime, L.; Ruiz-Rodriguez, A.; Restrepo Florez, C.E.; Rivero Barrios, D.M.; Fornari, T. Vaccinium meridionale Swartz extracts and their addition in beef burgers as antioxidant ingredient. J. Sci. Food Agric. 2018, 98, 377–383. [Google Scholar] [CrossRef]
- Sanchez-Martinez, J.D.; Alvarez-Rivera, G.; Gallego, R.; Bittencourt Fagundes, M.; Valdes, A.; Mendiola, J.A.; Ibañez, E.; Cifuentes, A. Neuroprotective potential of terpenoid-rich extracts from orange juice by-products obtained by pressurized liquid extraction. Food Chem. 2022, 13, 100242. [Google Scholar] [CrossRef]
- Nieto, J.A.; Santoyo, S.; Prodanov, M.; Reglero, G.; Jaime, L. Valorisation of grape stems as a source of phenolic antioxidants by using a sustainable extraction methodology. Foods 2020, 9, 604. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidations substrates and antioxidants by means of Folin–Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Ferreres, F.; Grosso, C.; Gil-Izquierdo, A.; Fernandes, A.; Valentão, P.; Andrade, P.B. Comparing the phenolic profile of Pilocarpus pennatifolius Lem. by HPLC-DAD-ESI/MSn with respect to authentication and enzyme inhibition potential. Ind. Crops Prod. 2015, 77, 391–401. [Google Scholar] [CrossRef]
- García, C.J.; García-Villalba, R.; Gil, M.I.; Tomas-Barberan, F.A. Untargeted metabolomics approach using UPLC-ESI-QTOF-MS to explore the metabolome of fresh-cut iceberg lettuce. Metabolomics 2016, 12, 138. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Bio. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of phenolic compounds: A review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef]
- Paini, M.; Casazza, A.A.; Aliakbarian, B.; Perego, P.; Binello, A.; Cravotto, G. Influence of ethanol/water ratio in ultrasound and high-pressure/high-temperature phenolic compound extraction from agri-food waste. Int. J. Food Sci. Technol. 2016, 51, 349–358. [Google Scholar] [CrossRef]
- Bodoira, R.; Rossi, Y.; Montenegro, M.; Maestri, D.; Velez, A. Extraction of antioxidant polyphenolic compounds from peanut skin using water-ethanol at high pressure and temperature conditions. J. Sup. Fluids 2017, 128, 57–65. [Google Scholar] [CrossRef]
- Dall’Acqua, S.; Kumar, G.; Sinan, K.I.; Sut, S.; Ferrarese, I.; Mahomoodally, M.F.; Seebaluck-Sandoram, R.; Etienne, O.K.; Zengin, G. An insight into Cochlospermum planchonii extracts obtained by traditional and green extraction methods: Relation between chemical compositions and biological properties by multivariate analysis. Ind. Crops Prod. 2020, 147, 112226. [Google Scholar] [CrossRef]
- Sounta Oumar, Y.; Kouadio Nathalie, G.; Souleymane, M.; Karamoko, O.; Gnogbo Alexis, B.; Jean David, G.; Adama, C. In vitro antioxidant activity of extracts of the root Cochlospermum planchonii Hook. f. ex. Planch (Cochlospermaceae). J. Pharmacogn. Phytochem. 2014, 3, 164–170. [Google Scholar]

| Extraction Technique | Solvent | T (°C) | Y (%) | TPC (mg/g) |
|---|---|---|---|---|
| PLE | Water | 50 | 17.94 ± 2.60 d | 213.95 ± 0.87 d |
| 100 | 19.25 ± 2.89 d | 213.71 ± 0.83 d | ||
| 150 | 43.63 ± 0.10 b | 173.02 ± 0.28 e | ||
| 200 | 57.32 ± 0.83 a | 171.65 ± 0.59 e | ||
| 50% ethanol: water | 50 | 18.99 ± 0.24 d | 263.98 ± 0.41 c | |
| 100 | 18.72 ± 3.29 d | 374.11 ± 0.49 a | ||
| 150 | 26.85 ± 0.64 c | 343.80 ± 0.49 b | ||
| 200 | 46.29 ± 2.58 b | 208.11 ± 0.76 d | ||
| Ethanol | 50 | 4.58 ± 0.27 f | 72.49 ± 0.28 i | |
| 100 | 7.17 ± 0.76 e,f | 110.71 ± 0.22 h | ||
| 150 | 10.24 ± 0.54 e | 130.97 ± 0.58 g | ||
| 200 | 18.89 ± 1.10 d | 152.81 ± 0.10 f | ||
| Decoction | Water | 24.39 ± 2.1 | 175.23 ± 0.13 |
| Extraction Technique | Solvent | T (°C) | DPPH | ABTS |
|---|---|---|---|---|
| PLE | Water | 50 | 1208 ± 41 d,e,f | 2812 ± 49 f |
| 100 | 1286 ± 39 c,d | 3475 ± 22 d,e | ||
| 150 | 1413 ± 34 a,b | 3645 ± 52 c | ||
| 200 | 1276 ± 39 c,d,e | 3590 ± 9 c,d | ||
| 50% ethanol: water | 50 | 1358 ± 55 b,c | 3387 ± 24 e | |
| 100 | 1392 ± 14 a,b,c | 4174 ± 65 b | ||
| 150 | 1488 ± 27 a | 4979 ± 29 a | ||
| 200 | 1284 ± 45 c,d | 3599 ± 128 c,d | ||
| Ethanol | 50 | 1108 ± 31 f | 639 ± 27 j | |
| 100 | 1156 ± 35 e,f | 1005 ± 25 i | ||
| 150 | 1300 ± 58 b,c,d | 1366 ± 42 h | ||
| 200 | 1386 ± 67 a,b,c | 1693 ± 59 g | ||
| Decoction | Water | 1179 ± 2 | 1698 ± 12 |
| Compound | Tentative Identification * | Rt | Molecular Formula | [M-H]− (m/z) | MS2 (m/z) |
|---|---|---|---|---|---|
| 1 | Gallic acid | 2.3 | C5H5O3 | 169.0148 | 125.0242 |
| 2 | Protocatechuic acid | 3.1 | C5H5O2 | 153.0193 | 109.0293 |
| 3 | EA pentoside | 11.0 | C19H14O12 | 433.0402 | 300.9966 |
| 4 | MEA hexoside | 11.6 | C21H18O13 | 477.0682 | 315.0140 |
| 5 | EA | 11.8 | C14H6O8 | 300.9979 | |
| 6 | EA rhamnoside | 12.1 | C20H16O12 | 447.0569 | 300.9970 |
| 7 | MEA pentoside | 13.4 | C20H16O12 | 447.0570 | 315.0145 |
| 8 | MEA pentoside | 15.3 | C20H16O12 | 447.0574 | 315.0142 |
| 9 | MEA pentoside | 16.0 | C20H16O12 | 447.0569 | 315.0147 |
| 10 | MEA rhamnoside | 16.8 | C21H18O12 | 461.0732 | 315.0147 |
| 11 | MEA galloyl pentoside | 20.4 | C27H20O16 | 599.0689 | 447.0567, 315.0146 |
| 12 | MEA galloyl pentoside | 22.8 | C27H20O16 | 599.0691 | 447.0569, 315.0144 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chipaca-Domingos, H.S.; Ferreres, F.; Fornari, T.; Gil-Izquierdo, A.; Pessela, B.C.; Villanueva-Bermejo, D. Pressurized Liquid Extraction for the Production of Extracts with Antioxidant Activity from Borututu (Cochlospermum angolense Welw.). Foods 2023, 12, 1186. https://doi.org/10.3390/foods12061186
Chipaca-Domingos HS, Ferreres F, Fornari T, Gil-Izquierdo A, Pessela BC, Villanueva-Bermejo D. Pressurized Liquid Extraction for the Production of Extracts with Antioxidant Activity from Borututu (Cochlospermum angolense Welw.). Foods. 2023; 12(6):1186. https://doi.org/10.3390/foods12061186
Chicago/Turabian StyleChipaca-Domingos, Honória S., Federico Ferreres, Tiziana Fornari, Angel Gil-Izquierdo, Benevides C. Pessela, and David Villanueva-Bermejo. 2023. "Pressurized Liquid Extraction for the Production of Extracts with Antioxidant Activity from Borututu (Cochlospermum angolense Welw.)" Foods 12, no. 6: 1186. https://doi.org/10.3390/foods12061186
APA StyleChipaca-Domingos, H. S., Ferreres, F., Fornari, T., Gil-Izquierdo, A., Pessela, B. C., & Villanueva-Bermejo, D. (2023). Pressurized Liquid Extraction for the Production of Extracts with Antioxidant Activity from Borututu (Cochlospermum angolense Welw.). Foods, 12(6), 1186. https://doi.org/10.3390/foods12061186