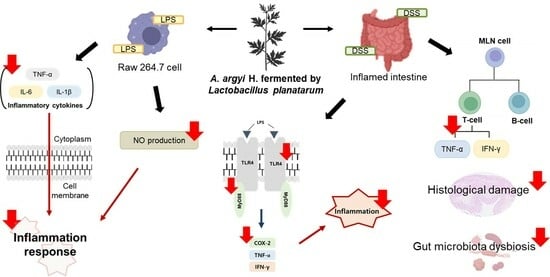
Anti-Inflammatory Effects of Artemisia argyi H. Fermented by Lactobacillus plantarum in the LPS-Induced RAW 264.7 Cells and DSS-Induced Colitis Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Sample Preparation
2.3. Cell Culture
2.4. Measurement of Nitric Oxide (NO)
2.5. Measurement of Cytokines Production
2.6. Animals and Experimental Designs
2.7. Measurement of Colon Length and Disease Activity Index (DAI)
2.8. Isolation of Mesenteric Lymph Node (MLN)
2.9. Measurement of Cytokine Production
2.10. Histological Analysis
2.11. Fecal Collection and DNA Extraction
2.12. Quantitative Real-Time PCR (qPCR)
2.13. Western Blot Analysis
2.14. Statistical Analysis
3. Results
3.1. Effects of FAA on NO Production in the LPS-Induced RAW 264.7 Cells
3.2. Effects of FAA on Cytokine Production in the LPS-Induced RAW 264.7 Cells
3.3. Effects of FAA on Colon Length in the DSS-Induced Colitis Model
3.4. Effects of FAA on DAI in the DSS-Induced Colitis Model
3.5. Effects of FAA on the Production of Cytokines in the DSS-Induced Colitis Model
3.6. Effects of FAA on Histopathology in the DSS-Induced Colitis Model
3.7. Effects of FAA on the Gut Microbiota Composition in the DSS-Induced Colitis Model
3.8. Effects of FAA on Inflammation in the DSS-Induced Colitis Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wehkamp, J.; Götz, M.; Herrlinger, K.; Steurer, W.; Stange, E.F. Inflammatory bowel disease: Crohn’s disease and ulcerative colitis. Dtsch. Arztebl. Int. 2016, 113, 72. [Google Scholar] [CrossRef] [PubMed]
- Yue, B.; Luo, X.; Yu, Z.; Mani, S.; Wang, Z.; Dou, W. Inflammatory bowel disease: A potential result from the collusion between gut microbiota and mucosal immune system. Microorganisms 2019, 7, 440. [Google Scholar] [CrossRef] [PubMed]
- Loddo, I.; Romano, C. Inflammatory bowel disease: Genetics, epigenetics, and pathogenesis. Front. Immunol. 2015, 6, 551. [Google Scholar] [CrossRef] [PubMed]
- Atreya, R.; Siegmund, B. Location is important: Differentiation between ileal and colonic Crohn’s disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 544–558. [Google Scholar] [CrossRef] [PubMed]
- Abu-Freha, N.; Ealiwa, N.; Abu-Tailakh, M.; Abu-Abed, M.; Bader, S.; Tabu, R.; Schwartz, D. Ethnic issues and disparities in inflammatory bowel diseases: What can we learn from the arab population in israel? J. Pers. Med. 2023, 13, 1008. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, P.; Zhang, Y.; Du, X.Y.; Zhang, Y.J. Comparison of effects of aminosalicylic acid, glucocorticoids and immunosuppressive agents on the expression of multidrug-resistant genes in ulcerative colitis. Sci. Rep. 2022, 12, 20656. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, P.; Colombel, J.F.; Aboubakr, A.; Narula, N. Systematic review: Safety of mesalazine in ulcerative colitis. Aliment. Pharmacol. Ther. 2018, 47, 1597–1609. [Google Scholar] [CrossRef]
- Navegantes, K.C.; de Souza Gomes, R.; Pereira, P.A.T.; Czaikoski, P.G.; Azevedo, C.H.M.; Monteiro, M.C. Immune modulation of some autoimmune diseases: The critical role of macrophages and neutrophils in the innate and adaptive immunity. J. Transl. Med. 2017, 15, 36. [Google Scholar] [CrossRef]
- Huang, C. Pathogenesis of coronaviruses through human monocytes and tissue macrophages. Viral Immunol. 2021, 34, 597–606. [Google Scholar] [CrossRef]
- Yang, Z.; Lin, S.; Feng, W.; Liu, Y.; Song, Z.; Pan, G.; Zhang, Y.; Dai, X.; Ding, X.; Chen, L. A potential therapeutic target in traditional Chinese medicine for ulcerative colitis: Macrophage polarization. Front. Pharmacol. 2022, 13, 999179. [Google Scholar] [CrossRef] [PubMed]
- Marrocco, A.; Ortiz, L.A. Role of metabolic reprogramming in pro-inflammatory cytokine secretion from LPS or silica-activated macrophages. Front. Immunol. 2022, 13, 936167. [Google Scholar] [CrossRef]
- Son, Y.; Shin, J.M.; Ha, I.J.; Erdenebileg, S.; Jung, D.S.; Kim, Y.S.; Kim, S.M.; Nho, C.W. Identification of chemical compounds from Artemisia gmelinii using UPLC-QTOF-MS/MS and their regulatory effects on immune responses in DSS-induced colitis mice. Am. J. Chin. Med. 2021, 49, 941–963. [Google Scholar] [CrossRef]
- Shin, J.M.; Son, Y.; Ha, I.J.; Erdenebileg, S.; Jung, D.S.; Song, D.; Kim, Y.S.; Kim, S.M.; Nho, C.W. Artemisia argyi extract alleviates inflammation in a DSS-induced colitis mouse model and enhances immunomodulatory effects in lymphoid tissues. BMC Complement. Med. Ther. 2022, 22, 64. [Google Scholar] [CrossRef]
- Kawahara, M.; Nemoto, M.; Nakata, T.; Kondo, S.; Takahashi, H.; Kimura, B.; Kuda, T. Anti-inflammatory properties of fermented soy milk with Lactococcus lactis subsp. lactis S-SU2 in murine macrophage RAW 264.7 cells and DSS-induced IBD model mice. Int. Immunopharmacol. 2015, 26, 295–303. [Google Scholar] [CrossRef]
- Kiesler, P.; Fuss, I.J.; Strober, W. Experimental models of inflammatory bowel diseases. Cell. Mol. Gastroenterol. Hepatol. 2015, 1, 154–170. [Google Scholar] [CrossRef]
- Wu, H.; Wu, E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes 2012, 3, 4–14. [Google Scholar] [CrossRef]
- Zhao, Q.; Elson, C.O. Adaptive immune education by gut microbiota antigens. Immunology 2018, 154, 28–37. [Google Scholar] [CrossRef]
- Bisht, D.; Kumar, D.; Kumar, D.; Dua, K.; Chellappan, D.K. Phytochemistry and pharmacological activity of the genus artemisia. Arch. Pharm. Res. 2021, 44, 439–474. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.; Seo, W.; Bae, W.; Kang, M.; Shin, J. Physicochemical characteristics and biological activities of Artemisia Argyi H. J. Life Sci. 2014, 24, 377–385. [Google Scholar] [CrossRef]
- Nigam, M.; Atanassova, M.; Mishra, A.P.; Pezzani, R.; Devkota, H.P.; Plygun, S.; Salehi, B.; Setzer, W.N.; SharifiRad, J. Bioactive compounds and health benefits of Artemisia species. Nat. Prod. Commun. 2019, 14, 1934578X19850354. [Google Scholar]
- Svanberg, U.; Lorri, W. Fermentation and nutrient availability. Food Control 1997, 8, 319–327. [Google Scholar] [CrossRef]
- Shahbazi, R.; Sharifzad, F.; Bagheri, R.; Alsadi, N.; Yasavoli-Sharahi, H.; Matar, C. Anti-inflammatory and immunomodulatory properties of fermented plant foods. Nutrients 2021, 13, 1516. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, N.K.; Lee, A.Y.; Seo, W.T.; Kim, H.Y. Antioxidant activity study of Artemisia argyi H. extract fermented with lactic acid bacteria. J. Korean Med. Obes. Res. 2022, 22, 115–124. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, J.H.; Choi, J.M.; Kim, H.; Seo, W.T.; Cho, E.J.; Kim, H.Y. Anti-oxidant and immune enhancement effects of Artemisia argyi H. fermented with lactic acid bacteria. J. Appl. Biol. Chem. 2023, 66, 492–502. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, L.; Huang, H.; Li, Q.; Wang, X.; Sun, Q.; Wang, Q.; Li, N. In vitro antioxidant analysis of flavonoids extracted from Artemisia argyi stem and their anti-inflammatory activity in lipopolysaccharide-stimulated RAW 264.7 macrophages. Food Chem. 2023, 407, 135198. [Google Scholar] [CrossRef]
- Sainakham, M.; Jantrawut, P.; Kiattisin, K.; Chittasupho, C.; Singh, S. Potential of green extraction using edible deep eutectic solvents on the bioactivities from Curcuma aromatica rhizome extracts for food application. J. Agric. Food Res. 2023, 14, 100868. [Google Scholar] [CrossRef]
- Shin, J.Y.; Kang, E.S.; Park, J.H.; Cho, B.O.; Jang, S.I. Anti-inflammatory effect of red ginseng marc, Artemisia scoparia, Paeonia japonica and Angelica gigas extract mixture in LPS-stimulated RAW 264.7 cells. Biomed. Rep. 2022, 17, 63. [Google Scholar] [CrossRef]
- He, Q.; Niu, M.; Bi, J.; Du, N.; Liu, S.; Yang, K.; Li, H.; Yao, J.; Du, Y.; Duan, Y. Protective effects of a new generation of probiotic Bacteroides fragilis against colitis in vivo and in vitro. Sci. Rep. 2023, 13, 15842. [Google Scholar] [CrossRef]
- Sánchez-Fidaigo, S.; Cárdeno, A.; Villegas, I.; Talero, E.; de la Lastra, C.A. Dietary supplementation of resveratrol attenuates chronic colonic inflammation in mice. Eur. J. Pharmacol. 2010, 633, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Kim, J.W.; Roh, Y.S.; Kim, J.H.; Han, K.M.; Kwon, H.J.; Lim, C.W.; Kim, B. Evaluation of immunomodulatory effects of zearalenone in mice. J. Immunotoxicol. 2017, 14, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Lim, B.O.; Lee, S.H.; Park, D.K.; Choue, R.W. Effect of dietary pectin on the production of immunoglobulins and cytokines by mesenteric lymph node lymphocytes in mouse colitis induced with dextran sulfate sodium. Biosci. Biotechnol. Biochem. 2003, 67, 1706–1712. [Google Scholar] [CrossRef]
- Davaatseren, M.; Hwang, J.T.; Park, J.H.; Kim, M.S.; Wang, S.; Sung, M.J. Poly-γ-glutamic acid attenuates angiogenesis and inflammation in experimental colitis. Mediators Inflamm. 2013, 2013, 982383. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Huang, X.; Yu, H.; Shi, H.; Chen, M.; Song, J.; Tang, W.; Teng, F.; Li, C.; Yi, L.; et al. TMT-based quantitative proteomics revealed protective efficacy of Icariside II against airway inflammation and remodeling via inhibiting LAMP2, CTSD and CTSS expression in OVA-induced chronic asthma mice. Phytomedicine 2023, 118, 154941. [Google Scholar] [CrossRef] [PubMed]
- Noratto, G.D.; Garcia-Mazcorro, J.F.; Markel, M.; Martino, H.S.; Minamoto, Y.; Steiner, J.M.; Byrne, D.; Suchodolski, J.S.; Mertens-Talcott, S.U. Carbohydrate-free peach (Prunus persica) and plum (Prunus domestica) juice affects fecal microbial ecology in an obese animal model. PLoS ONE 2014, 9, e101723. [Google Scholar] [CrossRef] [PubMed]
- TatiyaAphiradee, N.; Chatuphonprasert, W.; Jarukamjorn, K. Immune response and inflammatory pathway of ulcerative colitis. J. Basic Clin. Physiol. Pharmacol. 2018, 30, 1–10. [Google Scholar] [CrossRef]
- Marcuccio, G.; Ambrosino, P.; Merola, C.; Manzo, F.; Motta, A.; Rea, G.; Cantone, E.; Maniscalco, M. Clinical applications of nasal nitric oxide in allergic rhinitis: A review of the literature. J. Clin. Med. 2023, 12, 5081. [Google Scholar] [CrossRef] [PubMed]
- Liy, P.M.; Puzi, N.N.A.; Jose, S.; Vidyadaran, S. Nitric oxide modulation in neuroinflammation and the role of mesenchymal stem cells. Exp. Biol. Med. 2021, 246, 2399–2406. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Kell, D.B.; Pretorius, E. The role of lipopolysaccharide-induced cell signalling in chronic inflammation. Chronic Stress 2022, 6, 24705470221076390. [Google Scholar] [CrossRef]
- Ciesielska, A.; Matyjek, M.; Kwiatkowska, K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. 2021, 78, 1233–1261. [Google Scholar] [CrossRef]
- Muzamil, A.; Tahir, H.M.; Ali, S.; Liaqat, I.; Ali, A.; Summer, M. Inflammatory process and role of cytokines in inflammation: An overview. Punjab Univ. J. Zool. 2021, 36, 237–252. [Google Scholar] [CrossRef]
- Bang, B.; Lichtenberger, L.M. Methods of inducing inflammatory bowel disease in mice. Curr. Protoc. Pharmacol. 2016, 72, 5–58. [Google Scholar] [CrossRef]
- Abdelmegid, A.M.; Abdo, F.K.; Ahmed, F.E.; Kattaia, A.A. Therapeutic effect of gold nanoparticles on DSS-induced ulcerative colitis in mice with reference to interleukin-17 expression. Sci. Rep. 2019, 9, 10176. [Google Scholar] [CrossRef] [PubMed]
- Delday, M.; Mulder, I.; Logan, E.T.; Grant, G. Bacteroides thetaiotaomicron ameliorates colon inflammation in preclinical models of Crohn’s disease. Inflamm. Bowel Dis. 2019, 25, 85–96. [Google Scholar] [CrossRef]
- Salaritabar, A.; Darvishi, B.; Hadjiakhoondi, F.; Manayi, A.; Sureda, A.; Nabavi, S.F.; Bishayee, A. Therapeutic potential of flavonoids in inflammatory bowel disease: A comprehensive review. World J. Gastroenterol. 2017, 23, 5097. [Google Scholar] [CrossRef]
- Gajendran, M.; Loganathan, P.; Jimenez, G.; Catinella, A.P.; Ng, N.; Umapathy, C.; Ziade, N.; Hashash, J.G. A comprehensive review and update on ulcerative colitis. Dis. Mon. 2019, 62, 100851. [Google Scholar] [CrossRef] [PubMed]
- Jialing, L.; Yangyang, G.; Jing, Z.; Xiaoyi, T.; Ping, W.; Liwei, S.; Simin, C. Changes in serum inflammatory cytokine levels and intestinal flora in a self-healing dextran sodium sulfate-induced ulcerative colitis murine model. Life Sci. 2020, 263, 118587. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Lin, J.; Gu, T.; Sun, Q.; Xu, W.; Peng, Y. Chicoric acid effectively mitigated dextran sulfate sodium (DSS)-induced colitis in BALB/c mice by modulating the gut microbiota and fecal metabolites. Int. J. Mol. Sci. 2024, 25, 841. [Google Scholar] [CrossRef]
- Seyedian, S.S.; Nokhostin, F.; Malamir, M.D. A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease. J. Med. Life 2019, 12, 113–122. [Google Scholar] [CrossRef]
- Shaikh, H.; Vargas, J.G.; Mokhtari, Z.; Jarick, K.J.; Ulbrich, M.; Mosca, J.P.; Viera, E.A.; Graf, C.; Le, D.; Heinze, K.G. Mesenteric lymph node transplantation in mice to study immune responses of the gastrointestinal tract. Front. Immunol. 2021, 12, 689896. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, L.; Qin, S. Gut microbiota modulation on intestinal mucosal adaptive immunity. J. Immunol. Res. 2019, 2019, 4735040. [Google Scholar] [CrossRef]
- Guan, Q. A comprehensive review and update on the pathogenesis of inflammatory bowel disease. J. Immunol. Res. 2019, 2019, 7247238. [Google Scholar] [CrossRef] [PubMed]
- Marquez-Flores, Y.K.; Villegas, I.; Cárdeno, A.; Rosillo, M.Á.; Alarcon-de-la-Lastra, C. Apigenin supplementation protects the development of dextran sulfate sodium-induced murine experimental colitis by inhibiting canonical and non-canonical inflammasome signaling pathways. J. Nutr. Biochem. 2016, 30, 143–152. [Google Scholar] [CrossRef]
- He, R.; Zhao, S. Cutaneous manifestations of inflammatory bowel disease: Basic characteristics, therapy, and potential pathophysiological associations. Front. Immunol. 2023, 14, 1234535. [Google Scholar] [CrossRef] [PubMed]
- Villanacci, V.; Reggiani-Bonetti, L.; Leoncini, G.; Parente, P.; Cadei, M.; Albarello, L.; Mandelli, G.; Caputo, A. Histopathology of Non-IBD colitis. A practical approach from the Italian group for the study of the gastrointestinal tract (GIPAD). Pathologica 2021, 113, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, G.R.; Mohana, T.; Athesh, K.; Hillary, V.E.; Vasconcelos, A.B.S.; de Franca, M.N.F.; Montalvão, M.M.; Ceasar, S.A.; Jothi, G.; Sridharan, G.; et al. Anti-inflammatory natural products modulate interleukins and their related signaling markers in inflammatory bowel disease: A systematic review. J. Pharm. Anal. 2023, 13, 1408–1428. [Google Scholar] [CrossRef]
- Kellermann, L.; Riis, L.B. A close view on histopathological changes in inflammatory bowel disease, a narrative review. Dig. Med. Res. 2021, 4, 1–15. [Google Scholar] [CrossRef]
- Menconi, A.; Hernandez-Velasco, X.; Vicuña, E.; Kuttappan, V.; Faulkner, O.; Tellez, G.; Hargis, B.; Bielke, L. Histopathological and morphometric changes induced by a dextran sodium sulfate (DSS) model in broilers. Poult. Sci. 2015, 94, 906–911. [Google Scholar] [CrossRef] [PubMed]
- Grondin, J.A.; Kwon, Y.H.; Far, P.M.; Haq, S.; Khan, W.I. Mucins in intestinal mucosal defense and inflammation: Learning from clinical and experimental studies. Front. Immunol. 2020, 11, 2054. [Google Scholar] [CrossRef]
- van der Post, S.; Jabbar, K.S.; Birchenough, G.; Arike, L.; Akhtar, N.; Sjovall, H.; Johansson, M.E.; Hansson, G.C. Structural weakening of the colonic mucus barrier is an early event in ulcerative colitis pathogenesis. Gut 2019, 68, 2142–2151. [Google Scholar] [CrossRef]
- Yeshi, K.; Ruscher, R.; Hunter, L.; Daly, N.L.; Loukas, A.; Wangchuk, P. Revisiting inflammatory bowel disease: Pathology, treatments, challenges and emerging therapeutics including drug leads from natural products. J. Clin. Med. 2020, 9, 1273. [Google Scholar] [CrossRef]
- Zeng, W.; He, D.; Xing, Y.; Liu, J.; Su, N.; Zhang, C.; Wang, Y.; Xing, X. Internal connections between dietary intake and gut microbiota homeostasis in disease progression of ulcerative colitis: A review. Food Sci. Hum. Wellness 2021, 10, 119–130. [Google Scholar] [CrossRef]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The influence of probiotics on the firmicutes/bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Hu, J.; Geng, F.; Nie, S. Bacteroides utilization for dietary polysaccharides and their beneficial effects on gut health. Food Sci. Hum. Wellness 2022, 11, 1101–1110. [Google Scholar] [CrossRef]
- Ohkusa, T.; Koido, S. Intestinal microbiota and ulcerative colitis. J. Infect. Chemother. 2015, 21, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Q.; Tang, J.; Wen, J.; Zhu, J. Regulatory mechanism of mesalazine on TLR4/MyD88-dependent pathway in mouse ulcerative colitis model. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 6637–6644. [Google Scholar]
- Kawai, T.; Akira, S. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int. Immunol. 2009, 21, 317–337. [Google Scholar] [CrossRef]
- Lebeis, S.L.; Bommarius, B.; Parkos, C.A.; Sherman, M.A.; Kalman, D. TLR signaling mediated by MyD88 is required for a protective innate immune response by neutrophils to Citrobacter rodentium. J. Immunol. 2007, 179, 566–577. [Google Scholar] [CrossRef]
- Guo, Y.; Wu, X.; Wu, Q.; Lu, Y.; Shi, J.; Chen, X. Dihydrotanshinone I, a natural product, ameliorates DSS-induced experimental ulcerative colitis in mice. Toxicol. Appl. Pharmacol. 2018, 344, 35–45. [Google Scholar] [CrossRef]










| Target Group | Sequences | Annealing Temperature (°C) | |
|---|---|---|---|
| Forward | Reverse | ||
| Uni (F341/R518) | CCTACGGGAGGCAGCAGT | ATTACCGCGGCTGCTGG | 59 |
| Firmicutes (Phylum) | TGAAACTYAAAGGAATTGACG | ACCATGCACCACCTGTC | 60 |
| Bacteroidetes (Phylum) | GGARCATGTGGTTTAATTCGATGAT | AGCTGACGACAACCATGCAG | 60 |
| Score | Rectal Bleeding | Stool Consistency | Weight Loss (% of Initial wt) |
|---|---|---|---|
| 0 | Normal | Normal pellets | <1% |
| 1 | Slightly bloody | Slightly loose feces | 1–4.99% |
| 2 | Bloody | Loose feces | 5–10% |
| 3 | Bloody in whole colon | Watery diarrhea | >10% |
| Score | Inflammation Severity | Inflammation Extent | Crypt Damage |
|---|---|---|---|
| 0 | None | None | Damage to the basal third of the crypt |
| 1 | Slight | Mucosal | Damage to the basal two-thirds of the crypt |
| 2 | Moderate | Submucosal | Only surface epithelium intact |
| 3 | Severe | Transmural | Loss of entire crypt and epithelium |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.Y.; Kim, J.-H.; Choi, J.M.; Noh, B.W.; Kim, H.Y.; Cho, E.J. Anti-Inflammatory Effects of Artemisia argyi H. Fermented by Lactobacillus plantarum in the LPS-Induced RAW 264.7 Cells and DSS-Induced Colitis Model. Foods 2024, 13, 998. https://doi.org/10.3390/foods13070998
Lee JY, Kim J-H, Choi JM, Noh BW, Kim HY, Cho EJ. Anti-Inflammatory Effects of Artemisia argyi H. Fermented by Lactobacillus plantarum in the LPS-Induced RAW 264.7 Cells and DSS-Induced Colitis Model. Foods. 2024; 13(7):998. https://doi.org/10.3390/foods13070998
Chicago/Turabian StyleLee, Ji Yun, Ji-Hyun Kim, Ji Myung Choi, Byeong Wook Noh, Hyun Young Kim, and Eun Ju Cho. 2024. "Anti-Inflammatory Effects of Artemisia argyi H. Fermented by Lactobacillus plantarum in the LPS-Induced RAW 264.7 Cells and DSS-Induced Colitis Model" Foods 13, no. 7: 998. https://doi.org/10.3390/foods13070998
APA StyleLee, J. Y., Kim, J. -H., Choi, J. M., Noh, B. W., Kim, H. Y., & Cho, E. J. (2024). Anti-Inflammatory Effects of Artemisia argyi H. Fermented by Lactobacillus plantarum in the LPS-Induced RAW 264.7 Cells and DSS-Induced Colitis Model. Foods, 13(7), 998. https://doi.org/10.3390/foods13070998