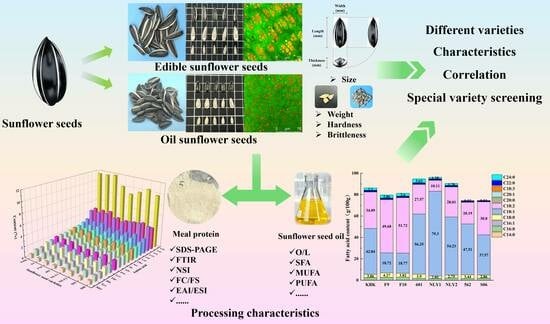
Properties and Characterization of Sunflower Seeds from Different Varieties of Edible and Oil Sunflower Seeds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Quality Determination of Raw Materials
2.2.1. Appearance and Microstructure of Sunflower Seeds
2.2.2. Sunflower Seeds Engineering Characteristics
2.2.3. Basic Composition of Sunflower Seeds
2.3. Analysis of the Fatty Acids of Sunflower Seed Oil
2.4. Amino Acid Composition of Sunflower Seed Meal Protein
2.5. SDS-PAGE of Sunflower Seed Meal Protein
2.6. FTIR of Sunflower Seed Meal Protein
2.7. Free Sulfhydryl Groups and Disulfide Bonds of Sunflower Seed Meal Protein
2.8. Functional Properties of Sunflower Seed Meal Protein
2.8.1. Solubility
2.8.2. Foaming Property
2.8.3. Emulsifying Properties
2.8.4. Water and Oil Holding Capacity
2.9. Statistical Analysis
3. Results and Discussion
3.1. Raw Material Quality Analysis
3.1.1. Appearance and Microstructure of Sunflower Seeds
3.1.2. Sunflower Seeds Engineering Characteristics
3.1.3. Basic Composition of Sunflower Seed
3.2. Fatty Acid Composition of Sunflower Seed Oil
3.3. Amino Acid Composition Analysis of Sunflower Seed Meal Protein
3.4. Correlation Analysis between Raw Materials and Processing Characteristics
3.5. SDS-PAGE Analysis of Sunflower Seed Protein Powder
3.6. FTIR Analysis of Sunflower Seed Protein Powder
3.7. Analysis of Free Sulfhydryl and Disulfide Bonds
3.8. Analysis of Functional Properties of Sunflower Seed Meal Protein
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xiang, F.; Ding, C.; Wang, M.; Hu, H.; Ma, X.; Xu, X.; Zaki Abubakar, B.; Pignitter, M.; Wei, K.-n.; Shi, A.-m.; et al. Vegetable oils: Classification, quality analysis, nutritional value and lipidomics applications. Food Chem. 2024, 439, 138059. [Google Scholar] [CrossRef] [PubMed]
- Harter, A.V.; Gardner, K.A.; Falush, D.; Lentz, D.L.; Bye, R.A.; Rieseberg, L.H. Origin of extant domesticated sunflowers in eastern North America. Nature 2004, 430, 201–205. [Google Scholar] [CrossRef] [PubMed]
- USDA FAS. 2023. Available online: https://apps.fas.usda.gov/psdonline/app/index.html#/app/compositeViz (accessed on 12 December 2023).
- Del Bel, Z.; Andrade, A.; Lindstrom, L.; Alvarez, D.; Vigliocco, A.; Alemano, S. The role of the sunflower seed coat and endosperm in the control of seed dormancy and germination: Phytohormone profile and their interaction with seed tissues. Plant Growth Regul. 2023, 102, 51–64. [Google Scholar] [CrossRef]
- Ji, J.; Zhang, Y.; Wang, Y.; Wang, D.; Jie, H. Influence of seed-roasting degree on quality attributes of sunflower oil. J. Food Sci. 2023, 88, 4028–4045. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Goncalves Filho, J.; Egea, M.B. Sunflower seed byproduct and its fractions for food application: An attempt to improve the sustainability of the oil process. J. Food Sci. 2021, 86, 1497–1510. [Google Scholar] [CrossRef] [PubMed]
- Morya, S.; Menaa, F.; Jimenez-Lopez, C.; Lourenco-Lopes, C.; BinMowyna, M.N.; Alqahtani, A. Nutraceutical and Pharmaceutical Behavior of Bioactive Compounds of Miracle Oilseeds: An Overview. Foods 2022, 11, 1824. [Google Scholar] [CrossRef] [PubMed]
- Petraru, A.; Ursachi, F.; Amariei, S. Nutritional Characteristics Assessment of Sunflower Seeds, Oil and Cake. Perspective of Using Sunflower Oilcakes as a Functional Ingredient. Plants 2021, 10, 2487. [Google Scholar] [CrossRef]
- Niveditha, V.R.; Sridhar, K.R.; Balasubramanian, D. Physical and mechanical properties of seeds and kernels of Canavalia of coastal sand dunes. Int. Food Res. J. 2013, 20, 1547–1554. [Google Scholar]
- Anjum, F.M.; Nadeem, M.; Khan, M.I.; Hussain, S. Nutritional and therapeutic potential of sunflower seeds: A review. Br. Food J. 2012, 114, 544–552. [Google Scholar] [CrossRef]
- Zatonski, W.; Campos, H.; Willett, W. Rapid declines in coronary heart disease mortality in Eastern Europe are associated with increased consumption of oils rich in alpha-linolenic acid. Eur. J. Epidemiol. 2008, 23, 3–10. [Google Scholar] [CrossRef]
- Guallar-Castillon, P.; Rodriguez-Artalejo, F.; Lopez-Garcia, E.; Leon-Munoz, L.M.; Amiano, P.; Ardanaz, E.; Arriola, L.; Barricarte, A.; Buckland, G.; Chirlaque, M.D.; et al. Consumption of fried foods and risk of coronary heart disease: Spanish cohort of the European Prospective Investigation into Cancer and Nutrition study. BMJ 2012, 344, e363. [Google Scholar] [CrossRef] [PubMed]
- Holgado, F.; Ruiz-Mendez, M.V.; Velasco, J.; Marquez-Ruiz, G. Performance of Olive-Pomace Oils in Discontinuous and Continuous Frying. Comparative Behavior with Sunflower Oils and High-Oleic Sunflower Oils. Foods 2021, 10, 3081. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Chen, H.; Yin, C.; Song, S.; Zhang, Y.; Liu, X.; Hu, Z. Research on mechanical-structure properties during sunflower seed extrusion-oil extraction. J. Food Process. Preserv. 2022, 46, e16158. [Google Scholar] [CrossRef]
- Gultekin Subasi, B.; Vahapoglu, B.; Capanoglu, E.; Mohammadifar, M.A. A review on protein extracts from sunflower cake: Techno-functional properties and promising modification methods. Crit. Rev. Food Sci. Nutr. 2022, 62, 6682–6697. [Google Scholar] [CrossRef] [PubMed]
- Ahmar, S.; Gill, R.A.; Jung, K.; Faheem, A.; Qasim, M.U.; Mubeen, M.; Zhou, W. Conventional and Molecular Techniques from Simple Breeding to Speed Breeding in Crop Plants: Recent Advances and Future Outlook. Int. J. Mol. Sci. 2020, 21, 2590. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Shen, X.; Peng, H.; Zhou, Q.; Yun, J.; Sun, Y.; Ho, C.; Cai, H.; Hou, R. Identification of rancidity markers in roasted sunflower seeds produced from raw materials stored for different periods of time. LWT-Food Sci. Technol. 2020, 118, 108721. [Google Scholar] [CrossRef]
- Hadidi, M.; Aghababaei, F.; McClements, D.J. Sunflower meal/cake as a sustainable protein source for global food demand: Towards a zero-hunger world. Food Hydrocoll. 2024, 147, 109329. [Google Scholar] [CrossRef]
- Luzaic, T.; Romanic, R.; Grahovac, N.; Jocic, S.; Cvejic, S.; Hladni, N.; Pezo, L. Prediction of mechanical extraction oil yield of new sunflower hybrids: Artificial neural network model. J. Sci. Food Agric. 2021, 101, 5827–5833. [Google Scholar] [CrossRef] [PubMed]
- Xiang, F.; Xia, Y.; Wang, Y.; Wang, Y.; Wu, K.; Ni, X. Preparation of konjac glucomannan based films reinforced with nanoparticles and its effect on cherry tomatoes preservation. Food Packag. Shelf Life 2021, 29, 100701. [Google Scholar] [CrossRef]
- Liu, Z.; Shi, A.; Wu, C.; Hei, X.; Li, S.; Liu, H.; Jiao, B.; Adhikari, B.; Wang, Q. Natural Amphiphilic Shellac Nanoparticle-Stabilized Novel Pickering Emulsions with Droplets and Bi-continuous Structures. Acs Appl. Mater. Interfaces 2022, 14, 57350–57361. [Google Scholar] [CrossRef]
- Huang, Z.; Du, M.; Qian, X.; Cui, H.; Tong, P.; Jin, H.; Feng, Y.; Zhang, J.; Wu, Y.; Zhou, S.; et al. Oxidative stability, shelf-life and stir-frying application of Torreya grandis seed oil. Int. J. Food Sci. Technol. 2022, 57, 1836–1845. [Google Scholar] [CrossRef]
- Guo, Q.; Li, T.; Qu, Y.; Wang, X.; Liu, L.; Liu, H.; Wang, Q. Action of phytosterols on thermally induced trans fatty acids in peanut oil. Food Chem. 2021, 344, 128637. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Guo, Q.; Liang, M.; Qu, Y.; Zhang, Y.; Wang, Q. Impact of additives on the formation of thermally induced trans linoleic acid in peanut oil. Int. J. Food Sci. Technol. 2023, 58, 2498–2504. [Google Scholar] [CrossRef]
- Li, T.; Guo, Q.; Qu, Y.; Liu, H.; Liu, L.; Zhang, Y.; Wang, Q. Inhibition mechanism of trans-resveratrol on thermally induced trans fatty acids in peanut oil. Food Chem. 2023, 406, 134863. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Liu, H.; Erasmus, S.W.; Zhao, S.; Wang, Q.; van Ruth, S.M. Rapid high-throughput determination of major components and amino acids in a single peanut kernel based on portable near-infrared spectroscopy combined with chemometrics. Ind. Crops Prod. 2020, 158, 112956. [Google Scholar] [CrossRef]
- Li, J.; Shi, A.; Liu, H.; Hu, H.; Wang, Q.; Adhikari, B.; Jiao, B.; Pignitter, M. Effect of Hydrothermal Cooking Combined with High-Pressure Homogenization and Enzymatic Hydrolysis on the Solubility and Stability of Peanut Protein at Low pH. Foods 2022, 11, 1289. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, S.-B. Structural and functional properties of self-assembled peanut protein nanoparticles prepared by ultrasonic treatment: Effects of ultrasound intensity and protein concentration. Food Chem. 2023, 413, 135626. [Google Scholar] [CrossRef]
- Li, S.; Liu, Z.; Hei, X.; Wu, C.; Ma, X.; Hu, H.; Jiao, B.; Zhu, J.; Adhikari, B.; Wang, Q.; et al. Effect of Physical Modifications on Physicochemical and Functional Properties of Walnut Protein. Foods 2023, 12, 3709. [Google Scholar] [CrossRef]
- Goszkiewicz, A.; Kolodziejczyk, E.; Ratajczyk, F. Comparison of microwave and convection method of roasting sunflower seeds and its effect on sensory quality, texture and physicochemical characteristics. Food Struct. 2020, 25, 100144. [Google Scholar] [CrossRef]
- Muresan, V.; Danthine, S.; Muresan, A.E.; Racolta, E.; Blecker, C.; Muste, S.; Socaciu, C.; Baeten, V. In situ analysis of lipid oxidation in oilseed-based food products using near-infrared spectroscopy and chemometrics: The sunflower kernel paste (tahini) example. Talanta 2016, 155, 336–346. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, O.; Cai, S.; Zhao, L.; Zhao, L. Composition, functional properties, health benefits and applications of oilseed proteins: A systematic review. Food Res. Int. 2023, 171, 11306. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Chang, L.; Xu, Y.; Rao, J.; Chen, B. Water-soluble fraction of pea protein isolate is critical for the functionality of protein-glucose conjugates obtained via wet-heating Maillard reaction. Food Res. Int. 2023, 174, 113503. [Google Scholar] [CrossRef] [PubMed]
- Neves, D.A.; Schmiele, M.; Pallone, J.A.L.; Orlando, E.A.; Risso, E.M.; Emidio Cunha, E.C.; Godoy, H.T. Chemical and nutritional characterization of raw and hydrothermal processed jambu (Acmella oleracea (L.) RK Jansen). Food Res. Int. 2019, 116, 1144–1152. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.; Merrien, A.; Decocq, G.; Fine, F. Stearic sunflower oil as a sustainable and healthy alternative to palm oil. A review. Agron. Sustain. Dev. 2018, 38, 43. [Google Scholar] [CrossRef]
- Ganesan, K.; Sukalingam, K.; Xu, B. Impact of consumption and cooking manners of vegetable oils on cardiovascular diseases- A critical review. Trends Food Sci. Technol. 2018, 71, 132–154. [Google Scholar] [CrossRef]
- Metcalf, R.G.; James, M.J.; Gibson, R.A.; Edwards, J.R.M.; Stubberfield, J.; Stuklis, R.; Roberts-Thomson, K.; Young, G.D.; Cleland, L.G. Effects of fish-oil supplementation on myocardial fatty acids in humans. Am. J. Clin. Nutr. 2007, 85, 1222–1228. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Xiao, P.; Tao, X.; Qin, J.; He, Q.; Zhang, C.; Guo, S.; Du, Y.; Chen, L.; Shen, D.; et al. Unsaturated bond recognition leads to biased signal in a fatty acid receptor. Science 2023, 380, eadd6220. [Google Scholar] [CrossRef] [PubMed]
- Endo, J.; Arita, M. Cardioprotective mechanism of omega-3 polyunsaturated fatty acids. J. Cardiol. 2016, 67, 22–27. [Google Scholar] [CrossRef]
- Wei, C.; Wang, X.; Ai, Y.; Zhang, S.; Zhang, X.; Zhou, Y.; Lu, S.; Gu, H.; Zhang, X.; Luo, X.; et al. Effect of oleic/linoleic acid ratio on simultaneous formation of epoxy fatty acids and polar compounds in deep frying oils. LWT-Food Sci. Technol. 2023, 187, 115389. [Google Scholar] [CrossRef]
- Salas, J.J.; Bootello, M.A.; Martinez-Force, E.; Venegas Caleron, M.; Garces, R. High stearic sunflower oil: Latest advances and 694 applications. Ocl-Oilseeds Fats Crops Lipids 2021, 28, 35. [Google Scholar] [CrossRef]
- Vandenbeuch, A.; Kinnamon, S.C. Glutamate: Tastant and Neuromodulator in Taste Buds. Adv. Nutr. 2016, 7, 823S–827S. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Duan, J.; Liu, X.; Zhang, M.; Bao, X. Preparation of sunflower seed-derived umami protein hydrolysates and their synergistic effect with monosodium glutamate and disodium inosine-5′-monophosphate. J. Food Sci. 2023, 88, 3332–3340. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.W.; Bunkelmann, J.; Towill, L.; Kleff, S.; Trelease, R.N. Identification of Peroxisome Membrane Proteins (PMPs) in Sunflower (Helianthus annuus L.) Cotyledons and Influence of Light on the PMP Developmental Pattern. Plant Physiol. 1994, 106, 293–302. [Google Scholar] [CrossRef] [PubMed]
- González-Pérez, S.; Vereijken, J.M.; Merck, K.B.; Van Koningsveld, G.A.; Gruppen, H.; Voragen, A.G.J. Conformational states of sunflower (Helianthus annuus) helianthinin: Effect of heat and pH. J. Agric. Food Chem. 2004, 52, 6770–6778. [Google Scholar] [CrossRef] [PubMed]
- Wildermuth, S.R.; Young, E.E.; Were, L.M. Chlorogenic Acid Oxidation and Its Reaction with Sunflower Proteins to Form Green-Colored Complexes. Compr. Rev. Food Sci. Food Saf. 2016, 15, 829–843. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Perez, S.; Vereijken, J.M. Sunflower proteins: Overview of their physicochemical, structural and functional properties. J. Sci. Food Agric. 2007, 87, 2173–2191. [Google Scholar] [CrossRef]
- Hei, X.; Liu, Z.; Li, S.; Wu, C.; Jiao, B.; Hu, H.; Ma, X.; Zhu, J.; Adhikari, B.; Wang, Q.; et al. Freeze-thaw stability of Pickering emulsion stabilized by modified soy protein particles and its application in plant-based ice cream. Int. J. Biol. Macromol. 2023, 257, 128183. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wu B-c Jiang, Y.; Zhang, Y.; Jiao, B.; Wang, Q. Improving enzyme accessibility in the aqueous enzymatic extraction process by microwave-induced porous cell walls to increase oil body and protein yields. Food Hydrocoll. 2024, 147, 109407. [Google Scholar] [CrossRef]
- Qu, Y.; Guo, Q.; Li, T.; Zhang, Y.; Gao, Q.; Liu, H.; Wang, Q. A novel environmentally friendly hot-pressed peanut meal protein adhesive. J. Clean. Prod. 2021, 327. [Google Scholar] [CrossRef]
- Qu, Y.; Guo, Q.; Li, T.; Liu, H.; Wang, Q. Effects of Different Denaturants on the Properties of a Hot-Pressed Peanut Meal-Based Adhesive. Molecules 2022, 27, 4878. [Google Scholar] [CrossRef]
- Wu, M.; Li, Z.; Wei, R.; Luan, Y.; Hu, J.; Wang, Q.; Liu, R.; Ge, Q.; Yu, H. Role of Disulfide Bonds and Sulfhydryl Blocked by N-Ethylmaleimide on the Properties of Different Protein-Stabilized Emulsions. Foods 2021, 10, 3079. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-L.; Xie, Q.-T.; Liu, W.-J.; Xu, B.-C.; Zhang, B. Self-Assembled Pea Protein Isolate Nanoparticles with Various Sizes: Explore the Formation Mechanism. J. Agric. Food Chem. 2021, 69, 9905–9914. [Google Scholar] [CrossRef] [PubMed]
- Martins, V.B.; Netto, F.M. Physicochemical and functional properties of soy protein isolate as a function of water activity and storage. Food Res. Int. 2006, 39, 145–153. [Google Scholar] [CrossRef]
- Beaubier, S.; Albe-Slabi, S.; Aymes, A.; Bianeis, M.; Galet, O.; Kapel, R. A Rational Approach for the Production of Highly Soluble and Functional Sunflower Protein Hydrolysates. Foods 2021, 10, 664. [Google Scholar] [CrossRef] [PubMed]
- Denkov, N.; Tcholakova, S.; Politova-Brinkova, N. Physicochemical control of foam properties. Curr. Opin. Colloid Interface Sci. 2020, 50, 101376. [Google Scholar] [CrossRef]
- Okada, Y.; Limure, T.; Takoi, K.; Kaneko, T.; Kihara, M.; Hayashi, K.; Ito, K.; Sato, K.; Takeda, K. The influence of barley malt protein modification on beer foam stability and their relationship to the barley dimeric α-amylase inhibitor-1 (BDAI-1) as a possible foam-promoting protein. J. Agric. Food Chem. 2008, 56, 1458–1464. [Google Scholar] [CrossRef] [PubMed]
- Pei, E.X.; Schmidt, K.A. Ice Cream: Foam Formation and Stabilization—A Review. Food Rev. Int. 2010, 26, 122–137. [Google Scholar]
- Zhang, M.; Liu, H.; Wang, Q. Characterization of β-Glucan-Peanut Protein Isolate/Soy Protein Isolate Conjugates and Their 732 Application on Low-Fat Sausage. Molecules 2022, 27, 3037. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Q.; Liu, Z.; Zhi, L.; Jiao, B.; Hu, H.; Ma, X.; Agyei, D.; Shi, A. Plant protein-based emulsifiers: Mechanisms, techniques for emulsification enhancement and applications. Food Hydrocoll. 2023, 144, 109008. [Google Scholar] [CrossRef]
- Liu, W.; Lanier, T.C. Rapid (microwave) heating rate effects on texture, fat/water holding, and microstructure of cooked comminuted meat batters. Food Res. Int. 2016, 81, 108–113. [Google Scholar] [CrossRef]
- Singh, R.; Koksel, F. Effects of particle size distribution and processing conditions on the techno-functional properties of extruded soybean meal. LWT-Food Sci. Technol. 2021, 152, 112321. [Google Scholar] [CrossRef]
- Zamora, R.S.; Baldelli, A.; Pratap-Singh, A. Characterization of selected dietary fibers microparticles and application of the optimized formulation as a fat replacer in hazelnut spreads. Food Res. Int. 2023, 165, 112466. [Google Scholar] [CrossRef] [PubMed]






| Type | Edible Sunflower Seeds | Oil Sunflower Seeds | ||||||
|---|---|---|---|---|---|---|---|---|
| KBK | F9 | F10 | 601 | NLY1 | NLY2 | 562 | S06 | |
| Lee | 24.33 ± 1.19 a | 24.57 ± 1.19 a | 24.39 ± 1.40 a | 21.88 ± 1.32 b | 11.30 ± 0.44 d | 11.41 ± 0.40 d | 12.65 ± 0.44 c | 10.23 ± 0.59 e |
| W/mm | 7.87 ± 0.58 b | 9.36 ± 0.51 a | 9.19 ± 0.83 a | 8.30 ± 0.73 b | 5.05 ± 0.38 e | 6.30 ± 0.34 c | 5.64 ± 0.39 d | 4.70 ± 0.28 e |
| T/mm | 4.12 ± 0.25 cd | 4.89 ± 0.66 a | 4.47 ± 0.45 b | 4.22 ± 0.53 bc | 3.43 ± 0.24 f | 3.80 ± 0.26 de | 3.59 ± 0.33 ef | 3.05 ± 0.24 g |
| L/W | 3.09 ± 0.20 a | 2.62 ± 0.14 b | 2.67 ± 0.24 b | 2.64 ± 0.24 b | 2.24 ± 0.17 c | 1.81 ± 0.10 d | 2.24 ± 0.34 c | 2.18 ± 0.20 c |
| W/T | 1.91 ± 0.13 b | 1.92 ± 0.25 ab | 2.07 ± 0.22 a | 1.97 ± 0.17 ab | 1.47 ± 0.14 d | 1.66 ± 0.11 c | 1.57 ± 0.13 cd | 1.54 ± 0.14 cd |
| L/T | 5.91 ± 0.47 a | 5.11 ± 0.64 c | 5.50 ± 0.54 b | 5.26 ± 0.65 bc | 3.29 ± 0.30 de | 3.00 ± 0.25 e | 3.52 ± 0.34 d | 3.35 ± 0.38 de |
| Dr/mm | 5.99 ± 0.37 b | 7.12 ± 0.50 a | 6.83 ± 0.55 a | 6.26 ± 0.59 b | 4.24 ± 0.31 e | 5.05 ± 0.25 c | 4.61 ± 0.32 d | 3.88 ± 0.20 f |
| Dα/mm | 12.11 ± 0.54 b | 12.94 ± 0.62 a | 12.68 ± 0.66 a | 11.46 ± 0.70 c | 6.59 ± 0.35 e | 7.17 ± 0.23 d | 7.29 ± 0.33 d | 6.00 ± 0.20 f |
| Dg/mm | 9.23 ± 0.43 b | 10.38 ± 0.67 a | 9.99 ± 0.62 a | 9.13 ± 0.71 b | 5.81 ± 0.34 d | 6.48 ± 0.24 c | 6.34 ± 0.36 c | 5.27 ± 0.18 e |
| ϕ | 0.38 ± 0.02 d | 0.42 ± 0.02 c | 0.41 ± 0.02 c | 0.42 ± 0.03 c | 0.51 ± 0.77 b | 0.57 ± 0.02 a | 0.50 ± 0.02 b | 0.52 ± 0.03 b |
| Whf/g | 19.53 ± 0.19 c | 27.02 ± 0.25 a | 24.29 ± 0.34 b | 19.81 ± 0.14 c | 7.46 ± 0.11 e | 8.23 ± 0.18 d | 6.45 ± 0.11 f | 5.69 ± 0.28 g |
| Whk/g | 8.47 ± 0.28 d | 13.49 ± 0.42 a | 11.31 ± 0.25 b | 9.68 ± 0.12 c | 5.52 ± 0.04 f | 6.19 ± 0.14 e | 4.13 ± 0.11 g | 4.65 ± 0.21 g |
| KY | 43.38 ± 1.02 f | 49.89 ± 1.10 d | 46.58 ± 0.54 e | 48.86 ± 0.26 d | 73.96 ± 0.62 b | 74.62 ± 0.70 b | 63.97 ± 0.65 c | 81.79 ± 0.25 a |
| Hd/g | 15,179 ± 3285 c | 18,186 ± 2291 b | 20,366 ± 2309 a | 14,444 ± 1618 c | 7303 ± 1227 d | 6896 ± 1103 d | 6882 ± 832 d | 5218 ± 745 e |
| Bt/g | 8657 ± 1600 b | 13,333 ± 318 | 13,453 ± 997 a | 8809 ± 1170 b | 4139 ± 764 c | 3857 ± 734 cd | 3684 ± 505 cd | 2759 ± 473 d |
| Ch | 0.69 ± 0.07 a | 0.69 ± 0.07 a | 0.69 ± 0.06 a | 0.57 ± 0.06 b | 0.48 ± 0.07 c | 0.42 ± 0.06 d | 0.33 ± 0.03 e | 0.28 ± 0.03 f |
| Crude fat/% | 41.21 ± 0.41 e | 45.24 ± 0.09 cd | 46.76 ± 0.09 c | 42.69 ± 0.64 de | 56.97 ± 1.30 a | 59.40 ± 1.33 a | 51.90 ± 0.46 b | 58.05 ± 0.41 a |
| Crude protein/% | 28.53 ± 0.08 a | 26.82 ± 0.18 a | 26.96 ± 0.77 b | 25.53 ± 0.14 b | 18.46 ± 0.14 d | 18.54 ± 0.27 d | 22.27 ± 0.73 c | 18.56 ± 0.33 d |
| Moisture/% | 5.08 ± 0.12 a | 4.40 ± 0.26 b | 4.41 ± 0.19 b | 4.49 ± 0.22 ab | 3.60 ± 0.31 c | 3.26 ± 0.09 cd | 2.72 ± 0.37 d | 3.21 ± 0.28 cd |
| Ash/% | 3.62 ± 0.07 ab | 3.52 ± 0.10 ab | 3.70 ± 0.06 a | 3.71 ± 0.13 a | 3.20 ± 0.03 bc | 2.96 ± 0.04 cd | 2.74 ± 0.24 d | 3.85 ± 0.15 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Xiang, F.; Huang, X.; Liang, M.; Ma, S.; Gafurov, K.; Gu, F.; Guo, Q.; Wang, Q. Properties and Characterization of Sunflower Seeds from Different Varieties of Edible and Oil Sunflower Seeds. Foods 2024, 13, 1188. https://doi.org/10.3390/foods13081188
Li Z, Xiang F, Huang X, Liang M, Ma S, Gafurov K, Gu F, Guo Q, Wang Q. Properties and Characterization of Sunflower Seeds from Different Varieties of Edible and Oil Sunflower Seeds. Foods. 2024; 13(8):1188. https://doi.org/10.3390/foods13081188
Chicago/Turabian StyleLi, Zhenyuan, Fei Xiang, Xuegang Huang, Manzhu Liang, Sarina Ma, Karim Gafurov, Fengying Gu, Qin Guo, and Qiang Wang. 2024. "Properties and Characterization of Sunflower Seeds from Different Varieties of Edible and Oil Sunflower Seeds" Foods 13, no. 8: 1188. https://doi.org/10.3390/foods13081188
APA StyleLi, Z., Xiang, F., Huang, X., Liang, M., Ma, S., Gafurov, K., Gu, F., Guo, Q., & Wang, Q. (2024). Properties and Characterization of Sunflower Seeds from Different Varieties of Edible and Oil Sunflower Seeds. Foods, 13(8), 1188. https://doi.org/10.3390/foods13081188