Development of Bacterial Spore Pouches as a Tool to Evaluate the Sterilization Efficiency—A Case Study with Microwave Sterilization Using Clostridium sporogenes and Geobacillus stearothermophilus
Abstract
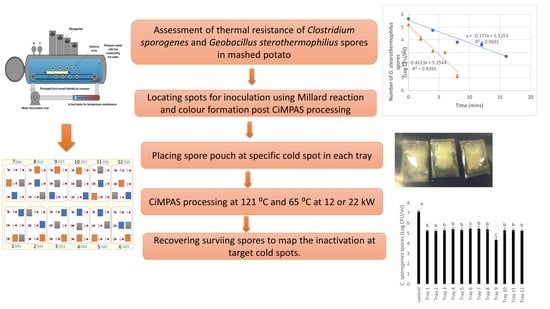
:1. Introduction
2. Materials and Methods
2.1. Preparation of Spores
2.2. Product/Food Model Formulation
2.3. CiMPAS Processing
2.4. Identification of Cold Spots for Inoculation by Colorimetric Analysis and High-Pressure Liquid Chromatography (HPLC) Analysis of Chemical Marker 4-hydroxy-5-methyl-3(2H)-furanone (M2)
2.5. Estimation of Thermal Resistance at 121 °C (D Values) of C. sporogenes and G. stearothermophilus Spores
2.5.1. Estimation of Decimal Reduction Time for C. sporogenes and G. stearothermophilus Spores in Milli-Q Water
2.5.2. Estimation of Decimal Reduction Time for C. sporogenes and G. stearothermophilus Spores in Mashed Potato
2.6. Inoculation of Bacterial Spores in the Food Model
2.6.1. Spot Inoculation of G. stearothermophilus Spores in Pouches
2.6.2. Inoculation of G. stearothermophilus Spores in the Whole Tray of Mashed Potato (Food Model)
2.6.3. Spot Inoculation of C. sporogenes Spores
2.7. Enumeration of Surviving Spores
2.8. Statistical Analysis
3. Results and Discussion
3.1. Determination of Cold Spots for Microbial Innoculation
3.2. Thermal Resistance of C. sporogenes and G. stearothermophilus Spores in Mashed Potato and Milli-Q Water
3.3. Effect of CiMPAS on Inactivation of Spores
3.3.1. Inactivation of G. stearothermophilus Spores
3.3.2. Inactivation of C. sporogenes Spores by CiMPAS
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Soni, A.; Smith, J.; Thompson, A.; Brightwell, G. Microwave-induced thermal sterilization- A review on history, technical progress, advantages and challenges as compared to the conventional methods. Trends Food Sci. Technol. 2020, 97, 433–442. [Google Scholar] [CrossRef]
- Tang, J. Unlocking Potentials of Microwaves for Food Safety and Quality. J. Food. Sci. 2015, 80, E1776–E1793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, J.; Liu, F.; Pathak, S.K.; Eves, I.E.E. Apparatus and method for heating objects with microwaves. U.S. Patent 7119313B2, 10 October 2006. [Google Scholar]
- Barbosa-Cánovas, G.V.; Medina-Meza, I.; Candoǧan, K.; Bermúdez-Aguirre, D. Advanced retorting, microwave assisted thermal sterilization (MATS), and pressure assisted thermal sterilization (PATS) to process meat products. Meat Sci. 2014, 98, 420–434. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Sun, D.-W.; Cheng, J.-H.; Han, Z. Non-destructive detection and screening of non-uniformity in microwave sterilization using hyperspectral imaging analysis. Food Anal. Methods 2018, 11, 1568–1580. [Google Scholar] [CrossRef]
- Guo, Q.; Sun, D.W.; Cheng, J.H.; Han, Z. Microwave processing techniques and their recent applications in the food industry. Trends Food Sci. Technol. 2017, 67, 236–247. [Google Scholar] [CrossRef]
- Pandit, R.B.; Tang, J.; Liu, F.; Mikhaylenko, G. A computer vision method to locate cold spots in foods in microwave sterilization processes. Pattern Recogn. 2007, 40, 3667–3676. [Google Scholar] [CrossRef]
- Soni, A.; Al-Sarayreh, M.; Reis, M.M.; Smith, J.; Tong, K.; Brightwell, G. Identification of Cold Spots Using Non-Destructive Hyperspectral Imaging Technology in Model Food Processed by Coaxially Induced Microwave Pasteurization and Sterilization. Foods 2020, 9, 837. [Google Scholar] [CrossRef]
- Pandit, R.B.; Tang, J.; Mikhaylenko, G.; Liu, F. Kinetics of chemical marker M-2 formation in mashed potato—A tool to locate cold spots under microwave sterilization. J. Food Eng. 2006, 76, 353–361. [Google Scholar] [CrossRef]
- Martins, S.I.; Jongen, W.M.; Van Boekel, M.A. A review of Maillard reaction in food and implications to kinetic modelling. Trends Food Sci. Technol. 2000, 11, 364–373. [Google Scholar] [CrossRef]
- Boca, B.; Pretorius, E.; Gochin, R.; Chapoullie, R.; Apostolides, Z. An overview of the validation approach for moist heat sterilization, part I. Pharm. Technol. 2002, 26, 62–71. [Google Scholar]
- Holdsworth, S.D. Principles of thermal processing: Sterilization. Eng. Asp. Therm. Food Process. 2009, 1, 3–12. [Google Scholar]
- Selkon, J.; Sisson, P.R.; Ingham, H. The use of spore strips for monitoring the sterilization of bottled fluids. Epidemiol. Infect. 1979, 83, 121–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koutchma, T.; Guo, B.; Patazca, E.; Parisi, B. High pressure–high temperature sterilization: From kinetic analysis to process verification†. J. Food Process. Eng. 2005, 28, 610–629. [Google Scholar] [CrossRef]
- Soni, A.; Oey, I.; Silcock, P.; Bremer, P. Bacillus Spores in the Food Industry: A Review on Resistance and Response to Novel Inactivation Technologies. Compr. Rev. Food Sci. Food Saf. 2016, 15, 1139–1148. [Google Scholar] [CrossRef] [Green Version]
- Basaran-Akgul, N. Comparative study of thermal kinetics for Clostridium sporogenes PA 3679 inactivation using glass capillary tube and aluminum tube methods in carrot juice and phosphate buffer. J. Pure Appl. Microbiol. 2013, 7, 117–124. [Google Scholar]
- Guizelini, B.P.; Vandenberghe, L.P.; Sella, S.R.B.; Soccol, C.R. Study of the influence of sporulation conditions on heat resistance of Geobacillus stearothermophilus used in the development of biological indicators for steam sterilization. Arch. Microbiol. 2012, 194, 991–999. [Google Scholar] [CrossRef]
- Sadiq, F.A.; Li, Y.; Liu, T.; Flint, S.; Zhang, G.; Yuan, L.; Pei, Z.; He, G. The heat resistance and spoilage potential of aerobic mesophilic and thermophilic spore forming bacteria isolated from Chinese milk powders. Int. J. Food Microbiol. 2016, 238, 193–201. [Google Scholar] [CrossRef]
- Bornhorst, E.R.; Tang, J.; Sablani, S.S.; Barbosa-Cánovas, G.V. Development of model food systems for thermal pasteurization applications based on Maillard reaction products. LWT 2017, 75, 417–424. [Google Scholar] [CrossRef] [Green Version]
- Pedreschi, F.; Moyano, P.; Kaack, K.; Granby, K. Color changes and acrylamide formation in fried potato slices. FOOD Res. Int. 2005, 38, 1–9. [Google Scholar] [CrossRef]
- Bornhorst, E.R.; Tang, J.; Sablani, S.S.; Barbosa-Cánovas, G.V. Thermal pasteurization process evaluation using mashed potato model food with Maillard reaction products. LWT Food Sci. Technol. 2017, 82, 454–463. [Google Scholar] [CrossRef]
- Perni, S. 5—Microbial control and safety in inhalation devices. In Inhaler Devices; Prokopovich, P., Ed.; Woodhead Publishing: Sawston, Cambridge, UK, 2013; pp. 51–74. [Google Scholar] [CrossRef]
- Coroller, L.; Leguérinel, I.; Mafart, P. Effect of Water Activities of Heating and Recovery Media on Apparent Heat Resistance of Bacillus cereus Spores. Appl. Environ. Microbiol. 2001, 67, 317–322. [Google Scholar] [CrossRef] [Green Version]
- Kort, R.; O’brien, A.C.; Van Stokkum, I.H.; Oomes, S.J.; Crielaard, W.; Hellingwerf, K.J.; Brul, S. Assessment of heat resistance of bacterial spores from food product isolates by fluorescence monitoring of dipicolinic acid release. Appl. Environ. Microbiol. 2005, 71, 3556–3564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soni, A.; Oey, I.; Silcock, P.; Permina, E.; Bremer, P.J. Effect of cold storage and different ions on the thermal resistance of B. cereus NZAS01 spores-analysis of differential gene expression and ion exchange. Food Res. Int. 2019, 116, 578–585. [Google Scholar] [CrossRef]
- Tang, Z.; Mikhaylenko, G.; Liu, F.; Mah, J.-H.; Pandit, R.; Younce, F.; Tang, J. Microwave sterilization of sliced beef in gravy in 7-oz trays. J. Food Eng. 2008, 89, 375–383. [Google Scholar] [CrossRef]
- Wells-Bennik, M.H.; Janssen, P.W.; Klaus, V.; Yang, C.; Zwietering, M.H.; Den Besten, H.M. Heat resistance of spores of 18 strains of Geobacillus stearothermophilus and impact of culturing conditions. Int. J. Food Microbiol. 2019, 291, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Mah, J.-H.; Kang, D.-H.; Tang, J. Effects of minerals on sporulation and heat resistance of Clostridium sporogenes. Int. J. Food Microbiol. 2008, 128, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Silla Santos, M.H.; Nuñez Kalasic, H.; Casado Goti, A.; Rodrigo Enguidanos, M. The effect of pH on the thermal resistance of Clostridium sporogenes (PA 3679) in asparagus purée acidified with citric acid and glucono-δ-lactone. Int. J. Food Microbiol. 1992, 16, 275–281. [Google Scholar] [CrossRef]
- Diao, M.M.; André, S.; Membré, J.-M. Meta-analysis of D-values of proteolytic Clostridium botulinum and its surrogate strain Clostridium sporogenes PA 3679. Int. J. Food Microbiol. 2014, 174, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Molin, N.; Snygg, B.G. Effect of lipid materials on heat resistance of bacterial spores. Appl. Microbiol. 1967, 15, 1422–1426. [Google Scholar] [CrossRef] [Green Version]
- Senhaji, A.; Loncin, M. The protective effect of fat on the heat resistance of bacteria (I). Int. J. Food Sci. Technol. 1977, 12, 203–216. [Google Scholar] [CrossRef]







| Step Number | Processing Step | Time (s) | The Temperature of the Vessel (°C) | Transport Speed (cm/min) | Number of Passes | Microwave Power (kW) |
|---|---|---|---|---|---|---|
| 1 | Pre-Pressurise | ~70 | na | na | na | na |
| 2 | Preheat water in | ~70 | 30 | na | na | na |
| 3 | Preheat hold | 1800 | 30 | na | na | na |
| 4 | Preheat water out | ~70 | 30 | na | na | na |
| 5 | Hot water in and | ~70 | 121 | na | na | na |
| Microwave on | 10 | 121 | na | na | 12 | |
| 6 | Microwave + carrier drive | 250 | 121 | 130 | 6 | 12 |
| 7 | Hold time | 330 | 121 | na | na | na |
| 8 | Hot water out | ~70 | na | na | na | |
| 9 | Cooling water in | ~70 | 30 | na | na | na |
| 10 | Cooling water hold | 900 | 30 | na | na | na |
| 11 | Cooling water out | ~70 | na | na | na | na |
| 12 | Venting | ~70 | na | na | na | na |
| Step Number | Name of the Processing Step | Time (s) | Temperature of the Vessel (°C) | Transport Speed (cm/min) | Number of Passes | Microwave Power (kW) |
|---|---|---|---|---|---|---|
| 1 | Pre-Pressurize | ~70 | na | na | na | na |
| 2 | Preheat water in | ~70 | 30 | na | na | na |
| 3 | Preheat hold | 1800 | 30 | na | na | na |
| 4 | Preheat water out | ~70 | na | na | na | |
| 5 | Hot water in | ~70 | 65 | na | na | na |
| Microwave on | 10 | 65 | na | na | 22 | |
| 6 | Microwave +carrier drive | 500 | 65 | 130 | 12 | 22 |
| 7 | Hold time | 330 | 65 | na | na | na |
| 8 | Hot water out | ~70 | na | na | na | |
| 9 | Cooling water in | ~70 | 30 | na | na | na |
| 10 | Cooling water holding | 900 | 30 | na | na | na |
| 11 | Cooling water out | ~70 | na | na | na | na |
| 12 | Venting | ~70 | na | na | na | na |
| Tray No | *L Values | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Spot 1 | Spot 2 | Spot 3 | Spot 4 | Spot 5 | Spot 6 | Spot 7 | Spot 8 | Spot 9 | |
| a | |||||||||
| 1 | 57.6 ± 0.3 b | 54.8 ± 0.2 ab | 60.9 ± 2.1 ab | 59.5 ± 1.0 ab | 60.3 ± 1.6 ab | 62.0 ± 1.2 a | 58.9 ± 2.9 ab | 60.1 ± 3.1 ab | 60.9 ± 3.1 ab |
| 2 | 61.0 ± 6.4 a | 60.54 ± 4.7 a | 62.4 ± 3.6 a | 62.3 ± 3.2 a | 62 ± 3.5 a | 62.8 ± 2.0 a | 61.8 ± 1.2 a | 62.7 ± 0.7 a | 63.5 ± 1.7 a |
| 3 | 59.7 ± 1.8 abc | 56.1 ± 3.9 d | 61.3 ± 1.0 ab | 61.1 ± 1.3 abc | 58.1 ± 3.5 cd | 62.8 ± 1.0 a | 60.5 ± 1.4 abc | 59.3 ± 1.1 bc | 62.8 ± 0.7 a |
| 4 | 61.8 ± 3.4 ab | 58.06 ± 3.7 b | 61.1 ± 4.8 ab | 61.08 ± 4.8 ab | 61.2 ± 1.3 ab | 59.72 ± 0.8 ab | 63.2 ± 0.8 a | 61.5 ± 2.5 ab | 61.6 ± 2.5 ab |
| 5 | 61.3 ± 3.3 a | 58.8 ± 1.9 a | 60.1 ± 3.5 a | 62.4 ± 2.7 a | 61.7 ± 2.7 a | 61.8 ± 2.6 a | 60.5 ± 2.4 a | 60.4 ± 3.2 a | 61.2 ± 2.4 a |
| 6 | 58.7 ± 3.6 a | 56.5 ± 4.2 a | 57.4 ± 3.5 a | 60.0 ± 3.2 a | 57.3 ± 3.3 a | 58.0 ± 2.1 a | 58.9 ± 4.1 a | 60.6 ± 3.0 a | 57.8 ± 3.0 a |
| 7 | 65.5 ± 2.2 a | 62.9 ± 0.6 bcd | 65.2 ± 0.9 a | 65.4 ± 0.8 a | 62.9 ± 1.2 bcd | 63.8 ± 0.4 abc | 64.0 ± 1.8 ab | 61.8 ± 1.3 cd | 61.4 ± 1.8 d |
| 8 | 65.8 ± 1.5 a | 62.0 ± 1.6 bc | 63.0 ± 2.4 abc | 64.9 ± 2.7 ab | 61.0 ± 0.5 c | 62.3 ± 3.2 bc | 63.4 ± 3.7 abc | 61.2 ± 1.1 bc | 62.2 ± 2.7 bc |
| 9 | 62.6 ± 1.7 a | 62.5 ± 3.5 a | 62.1 ± 3.6 a | 64.0 ± 1.6 a | 62.4 ± 3.1 a | 63.4 ± 3.5 a | 63.7 ± 1.3 a | 63.4 ± 1.8 | 63.6 ± 2.9 a |
| 10 | 63.3 ± 1.5 a | 61.9 ± 3.0 a | 62.1 ± 4.3 a | 63.6 ± 1.4 a | 61.8 ± 3.2 a | 61.5 ± 4.7 a | 63.9 ± 2.5 a | 62.0 ± 2.9 a | 62.5 ± 4.0 a |
| 11 | 63.5 ± 0.8 ab | 61.4 ± 2.7 bc | 60.3 ± 4.5 a | 64.7 ± 0.8 a | 62.3 ± 2.0 a | 60.6 ± 2.8 a | 63.1 ± 0.4 a | 63.0 ± 1.4 a | 61.5 ± 2.4 a |
| 12 | 65.7 ± 1.4 ab | 61.7 ± 2.8 bc | 62.6 ± 3.9 abc | 66.2 ± 1.2 a | 61.0 ± 3.5 c | 63.8 ± 2.7 abc | 66.4 ± 1.1 a | 62.7 ± 2.7 abc | 63.9 ± 3.0 abc |
| b | |||||||||
| 1 | 55.1 ± 9.9 a | 51.7 ± 5.8 a | 50.4 ± 1.7 a | 56.0 ± 9.3 a | 54.1 ± 7.0 a | 52.3 ± 1.9 a | 51.0 ± 6.8 a | 51.2 ± 6.7 a | 50.2 ± 4.5 a |
| 2 | 44.3 ± 1.4 a | 45.3 ± 2.9 a | 46.5 ± 3.8 a | 45.7 ± 0.5 a | 48.6 ± 1.6 a | 47.7 ± 2.5 a | 43.7 ± 1.2 a | 44.6 ± 1.2 a | 46.9 ± 2.7 a |
| 3 | 50.1 ± 6.2 a | 52.2 ± 3.6 a | 53.1 ± 9.5 a | 51.3 ± 5.3 a | 54.5 ± 5.8 a | 55.5 ± 7.4 a | 49.3 ± 5.8 a | 52.7 ± 6.9 a | 53.8 ± 7.1 a |
| 4 | 55.5 ± 5.6 a | 51.0 ± 7.6 a | 54.2 ± 3.7 a | 55.8 ± 8.4 a | 52.9 ± 4.6 a | 53.0 ± 6.7 a | 52.0 ± 8.6 a | 50.9 ± 6.7 a | 49.35 ± 4.9 |
| 5 | 54.8 ± 6.6 a | 51.8 ± 5.6 a | 48.3 ± 6.6 a | 56.7 ± 9.3 a | 55.8 ± 6.8 a | 56.2 ± 7.5 a | 52.8 ± 9.6 a | 53.3 ± 6.1 a | 52.1 ± 8.3 a |
| 6 | 51.2 ± 4.0 a | 51.1 ± 9.0 a | 53.2 ± 9.4 a | 50.8 ± 2.5 a | 54.2 ± 4.3 | 55 ± 1.5 a | 46.7 ± 5.3 a | 50.5 ± 6.2 a | 51.5 ± 5.1 a |
| 7 | 55.4 ± 7.4 a | 51.4 ± 4.3 a | 52.9 ± 7.3 a | 56.0 ± 6.1 a | 53.9 ± 4.2 a | 53.1 ± 7.4 a | 54.1 ± 5.7 a | 50.2 ± 4.7 a | 53.3 ± 6.7 a |
| 8 | 52.1 ± 5.3 a | 53.4 ± 4.0 a | 52.4 ± 6.2 a | 53.8 ± 6.6 a | 54.5 ± 6.6 a | 55.4 ± 8.6 a | 51.0 ± 7.5 a | 53.3 ± 7.5 a | 54.2 ± 9.2 a |
| 9 | 57.2 ± 8.5 a | 53.3 ± 5.6 a | 50.8 ± 3.7 a | 55.3 ± 9.4 a | 54.5 ± 4.4 a | 52.7 ± 5.0 a | 54.9 ± 6.4 a | 51.0 ± 5.5 a | 50.2 ± 6.0 a |
| 10 | 54.4 ± 5.8 a | 52.9 ± 4.6 a | 48.5 ± 5.7 a | 57.2 ± 5.6 a | 53.6 ± 4.0 a | 50.8 ± 4.9 a | 54.4 ± 3.8 a | 49.1 ± 3.0 a | 50.0 ± 4.6 a |
| 11 | 53.4 ± 3.5 a | 53.1 ± 5.8 a | 55.6 ± 7.2 a | 51.2 ± 3.9 a | 53.9 ± 3.5 a | 51.0 ± 3.2 a | 50.0 ± 1.7 a | 52.0 ± 4.6 a | 48.8 ± 1.8 a |
| 12 | 48.7 ± 3.2 a | 52.6 ± 4.1 a | 53.0 ± 4.6 a | 51.9 ± 4.0 a | 55.4 ± 5.2 a | 55.2 ± 3.8 a | 50.9 ± 5.4 a | 50.0 ± 3.9 a | 51.8 ± 4.7 a |
| Tray Number | Three Locations of Inoculation from TABLE 3a | Number of C. sporogenes Spores (Log CFU/g) | ||
|---|---|---|---|---|
| First Location | Second Location | Third Location | ||
| Control | 1, 5, 9 | 7.2 ± 0.1 | 7.3 ± 0.1 | 7.5 ± 0.1 |
| Tray 1 | 1, 3, 6 | nd | nd | nd |
| Tray 2 | 1, 2, 8 | nd | nd | nd |
| Tray 3 | 3, 5, 8 | nd | nd | nd |
| Tray 4 | 4, 6, 9 | nd | nd | nd |
| Tray 5 | 2, 4, 9 | nd | nd | nd |
| Tray 6 | 2, 5, 9 | nd | nd | nd |
| Tray 7 | 3, 5, 6 | nd | nd | nd |
| Tray 8 | 4, 6, 9 | nd | nd | nd |
| Tray 9 | 4, 6, 9 | nd | nd | nd |
| Tray 10 | 2, 5, 9 | nd | nd | nd |
| Tray 11 | 2, 5, 9 | nd | nd | nd |
| Tray 12 | 2, 3, 5 | nd | nd | nd |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soni, A.; Smith, J.; Archer, R.; Gardner, A.; Tong, K.; Brightwell, G. Development of Bacterial Spore Pouches as a Tool to Evaluate the Sterilization Efficiency—A Case Study with Microwave Sterilization Using Clostridium sporogenes and Geobacillus stearothermophilus. Foods 2020, 9, 1342. https://doi.org/10.3390/foods9101342
Soni A, Smith J, Archer R, Gardner A, Tong K, Brightwell G. Development of Bacterial Spore Pouches as a Tool to Evaluate the Sterilization Efficiency—A Case Study with Microwave Sterilization Using Clostridium sporogenes and Geobacillus stearothermophilus. Foods. 2020; 9(10):1342. https://doi.org/10.3390/foods9101342
Chicago/Turabian StyleSoni, Aswathi, Jeremy Smith, Richard Archer, Amanda Gardner, Kris Tong, and Gale Brightwell. 2020. "Development of Bacterial Spore Pouches as a Tool to Evaluate the Sterilization Efficiency—A Case Study with Microwave Sterilization Using Clostridium sporogenes and Geobacillus stearothermophilus" Foods 9, no. 10: 1342. https://doi.org/10.3390/foods9101342
APA StyleSoni, A., Smith, J., Archer, R., Gardner, A., Tong, K., & Brightwell, G. (2020). Development of Bacterial Spore Pouches as a Tool to Evaluate the Sterilization Efficiency—A Case Study with Microwave Sterilization Using Clostridium sporogenes and Geobacillus stearothermophilus. Foods, 9(10), 1342. https://doi.org/10.3390/foods9101342