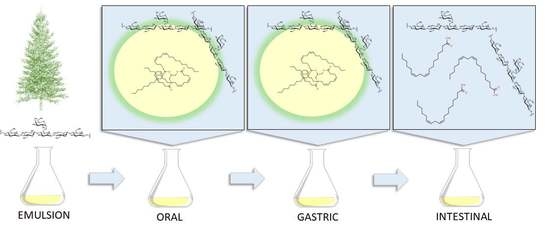
Spruce Galactoglucomannan-Stabilized Emulsions Enhance Bioaccessibility of Bioactive Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Emulsion Preparation
2.3. In Vitro Digestion
2.4. Emulsion Morphology
2.5. Analysis of Triacylglycerols by HPLC-ELSD
2.6. Determination of Molar Mass by HPSEC-MALLS-RI
2.7. Analysis of Monosaccharides by HPAEC-PAD
2.8. Analysis of Phenolic Compounds by UHPLC-DAD-FLD
2.9. Statistical Analyses
3. Results
3.1. Lipid Release from GGM-Stabilized Emulsions
3.2. Physical Stability of GGM Stabilized Emulsions
3.3. Stability of GGM
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A


References
- Sjöström, E. Wood Chemistry Fundamentals and Applications; Academic Press: San Diego, CA, USA, 1993. [Google Scholar]
- Lee, K.N.; Kritchevsky, D.; Parizaa, M.W. Conjugated linoleic acid and atherosclerosis in rabbits. Atherosclerosis 1994, 108, 19–25. [Google Scholar] [CrossRef]
- Marchioli, R.; Barzi, F.; Bomba, E.; Chieffo, C.; Di Gregorio, D.; Di Mascio, R.; Grazia Franzosi, M.; Geraci, E.; Levantesi, G.; Maggioni, A.P.; et al. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: Time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI)-Prevenzione. Circulation 2002, 105, 1897–1903. [Google Scholar] [CrossRef] [Green Version]
- Basu, A.; Imrhan, V. Tomatoes versus lycopene in oxidative stress and carcinogenesis: Conclusions from clinical trials. Eur. J. Clin. Nutr. 2007, 61, 295–303. [Google Scholar] [CrossRef] [Green Version]
- McClements, D.J.; Decker, E.A.; Weiss, J. Emulsion-based delivery systems for lipophilic bioactive components. J. Food Sci. 2007, 72, 109. [Google Scholar] [CrossRef]
- McClements, D.J. Design of nano-laminated coatings to control bioavailability of lipophilic food components. J. Food Sci. 2010, 75, 30–42. [Google Scholar] [CrossRef] [Green Version]
- McClements, D.J.; Rao, J. Food-grade nanoemulsions: Formulation, fabrication, properties, performance, biological fate, and potential toxicity. Crit. Rev. Food Sci. Nutr. 2011, 51, 285–330. [Google Scholar] [CrossRef] [PubMed]
- Dalgleish, D.G. Food emulsions: Their structures and properties. In Food Emulsions, 4th ed.; Friberg, S.E., Larsson, K., Sjoblom, J., Eds.; Marcel Dekker: New York, NY, USA, 2004; pp. 17–44. [Google Scholar]
- Scheller, H.V.; Ulvskov, P. Hemicelluloses. Annu. Rev. Plant Biol. 2010, 61, 263–289. [Google Scholar] [CrossRef] [PubMed]
- Mikkonen, K.S.; Tenkanen, M. Sustainable food-packaging materials based on future biorefinery products: Xylans and mannans. Trends Food Sci. Technol. 2012, 28, 90–102. [Google Scholar] [CrossRef]
- Willför, S.; Sjöholm, R.; Laine, C.; Roslund, M.; Hemming, J.; Holmbom, B. Characterisation of water-soluble galactoglucomannans from Norway spruce wood and thermomechanical pulp. Carbohydr. Polym. 2003, 52, 175–187. [Google Scholar] [CrossRef]
- Willför, S.; Rehn, P.; Sundberg, A.; Sundberg, K.; Holmbom, B. Recovery of water-soluble acetyl-galactoglucomannans from mechanical pulp of spruce. Tappi J. 2003, 2, 27–32. [Google Scholar]
- Willför, S.; Sundberg, K.; Tenkanen, M.; Holmbom, B. Spruce-derived mannans: A potential raw material for hydrocolloids and novel advanced natural materials. Carbohydr. Polym. 2008, 72, 197–210. [Google Scholar] [CrossRef]
- Kilpeläinen, P.O.; Hautala, S.S.; Byman, O.O.; Tanner, L.J.; Korpinen, R.I.; Lillandt, M.K.-J.; Pranovich, A.V.; Kitunen, V.H.; Willför, S.M.; Ilvesniemi, H.S.; et al. Pressurized hot water flow-through extraction system scale up from the laboratory to the pilot scale. Green Chem. 2014, 16, 3186–3194. [Google Scholar] [CrossRef]
- Von Schoultz, S. US20150167234: Method for Extracting Biomass. U.S. Available online: https://patents.google.com/patent/US20150167234 (accessed on 18 June 2015).
- Mikkonen, K.S.; Xu, C.; Berton-Carabin, C.; Schroën, K. Spruce galactoglucomannans in rapeseed oil-in-water emulsions: Efficient stabilization performance and structural partitioning. Food Hydrocoll. 2016, 52, 615–624. [Google Scholar] [CrossRef]
- Mikkonen, K.S.; Merger, D.; Kilpeläinen, P.; Murtomäki, L.; Schmidt, U.S.; Wilhelm, M. Determination of physical emulsion stabilization mechanisms of wood hemicelluloses via rheological and interfacial characterization. Soft Matter 2016, 12, 8690–8700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikkonen, K.S. Strategies for structuring diverse emulsion systems by using wood lignocellulose-derived stabilizers. Green Chem. 2020. [Google Scholar] [CrossRef] [Green Version]
- Lehtonen, M.I.; Teräslahti, S.; Xu, C.; Yadav, M.P.; Lampi, A.-M.; Mikkonen, K.S. Spruce galactoglucomannans inhibit lipid oxidation in rapeseed oil-in-water emulsions. Food Hydrocoll. 2016, 58, 255–266. [Google Scholar] [CrossRef]
- Lehtonen, M.I.; Merinen, M.; Kilpeläinen, P.O.; Xu, C.; Willför, S.M.; Mikkonen, K.S. Phenolic residues in spruce galactoglucomannans improve stabilization of oil-in-water emulsions. J. Colloid Interface Sci. 2018, 512, 536–547. [Google Scholar] [CrossRef] [Green Version]
- Valoppi, F.; Maina, N.; Allen, M.; Miglioli, R.; Kilpelainen, P.O.; Mikkonen, K. Spruce galactoglucomannan-stabilized emulsions as essential fatty acid delivery systems for functionalized drinkable yogurt and oat-based beverage. Eur. Food Res. Technol. 2019, 245, 1387–1398. [Google Scholar] [CrossRef] [Green Version]
- Valoppi, F.; Lahtinen, M.; Bhattarai, M.; Kirjoranta, S.; Juntti, V.; Peltonen, L.; Mikkonen, K.S. Centrifugal fractionation of softwood extracts improves the biorefinery workflow and yields functional emulsifiers. Green Chem. 2019, 21, 4691–4705. [Google Scholar] [CrossRef] [Green Version]
- Lahtinen, M.; Valoppi, F.; Juntti, V.; Heikkinen, S.; Kilpeläinen, P.; Maina, N.; Mikkonen, K.S. Lignin-rich PHWE hemicellulose extracts responsible for extended emulsion stabilization. Front. Chem. 2019, 7, 871. [Google Scholar] [CrossRef]
- Mun, S.; Decker, E.A.; Park, Y.; Weiss, J.; McClements, D.J. Influence of interfacial composition on in vitro digestibility of emulsified lipids: Potential mechanism for Chitosan’s ability to inhibit fat digestion. Food Biophys. 2006, 1, 21–29. [Google Scholar] [CrossRef]
- Klinkesorn, U.; McClements, D.J. Influence of chitosan on stability and lipase digestibility of lecithin-stabilized tuna oil-in-water emulsions. Food Chem. 2019, 114, 1308–1315. [Google Scholar] [CrossRef]
- Ebringerová, A.; Hromádková, Z.; Hříbalová, V.; Xu, C.; Holmbom, B.; Sundberg, A.; Willför, S. Norway spruce galactoglucomannans exhibiting immunomodulating and radical-scavenging activities. Int. J. Biol. Macromol. 2008, 42, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.H.; Lu, J.; Luo, J.P.; Zha, X.Q.; Wang, J.H. Preventive effect of a galactoglucomannan (GGM) from Dendrobium huoshanense on selenium-induced liver injury and fibrosis in rats. Exp. Toxicol. Pathol. 2020, 64, 899–904. [Google Scholar] [CrossRef] [PubMed]
- Polari, L.; Ojansivu, P.; Mäkelä, S.; Eckerman, C.; Holmbom, B.; Salminen, S. Galactoglucomannan extracted from spruce (Picea abies) as a carbohydrate source for probiotic bacteria. J. Agric. Food Chem. 2012, 60, 11037–11043. [Google Scholar] [CrossRef]
- Deloule, V.; Boisset, C.; Hannani, D.; Suau, A.; Le Gouellec, A.; Chroboczek, J.; Botté, C.; Yamaryo-Botté, Y.; Chirat, C.; Toussaint, B.; et al. Prebiotic role of softwood hemicellulose in healthy mice model. J. Funct. Foods 2020, 64, 103688. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food: An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Zhang, Z.; Zhang, H.; Decker, E.A.; McClements, D.J. Influence of emulsifier type on gastrointestinal fate of oil-in-water emulsions containing anionic dietary fiber (pectin). Food Hydrocoll. 2015, 45, 175–185. [Google Scholar] [CrossRef]
- Sarkar, A.; Goh, K.K.T.; Singh, H. Colloidal stability and interactions of milk-protein-stabilized emulsions in an artificial saliva. Food Hydrocoll. 2009, 23, 1270–1278. [Google Scholar] [CrossRef]
- Lampi, A.-N.; Damerau, A.; Li, J.; Moisio, T.; Partanen, R.; Forssell, P.; Piironen, V. Changes in lipids and volatile compounds of oat flours and extrudates during processing and storage. J. Cereal Sci. 2015, 62, 102–109. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Y.; Coda, R.; Säde, E.; Tuomainen, P.; Tenkanen, M.; Katina, K. In situ synthesis of exopolysaccharides by Leuconostoc spp. and Weissella spp. and their rheological impacts in fava bean flour. Int. J. Food Microbiol. 2017, 248, 63–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kylli, P.; Nohynek, L.; Puupponen-Pimiä, R.; Westerlund-Wikström, B.; Leppänen, T.; Welling, J.; Moilanen, E.; Heinonen, M. Lingonberry (Vaccinium vitis-idaea) and European cranberry (Vaccinium microcarpon) proanthocyanidins: Isolation, identification, and bioactivities. J. Agric. Food Chem. 2011, 59, 3373–3384. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, M.; Pitkänen, L.M.; Kitunen, V.; Korpinen, R.; Ilvesniemi, H.; Kilpeläinen, P.O.; Mikkonen, K.S. Functionality of spruce galactoglucomannans in oil-in-water emulsions. Food Hydrocoll. 2019, 86, 154–161. [Google Scholar] [CrossRef]
- Moore, P.B.; Langley, K.; Wilde, P.J.; Fillery-Travis, A.; Mela, D.J. Effect of emulsifier type on sensory properties of oil-in-water emulsions. J. Sci. Food Agric. 1998, 76, 469–476. [Google Scholar] [CrossRef]
- Malone, M.E.; Appleqvist, I.A.M.; Norton, I.T. Oral behaviour of food hydrocolloids and emulsions—Part 1: Lubrication and deposition considerations. Food Hydrocoll. 2003, 17, 763–773. [Google Scholar] [CrossRef]
- Chung, C.; Smith, G.; Degner, B.; McClements, D.J. Reduced fat food emulsions: Physicochemical, sensory, and biological aspects. Crit. Rev. Food Sci. Nutr. 2016, 56, 650–685. [Google Scholar] [CrossRef]
- Xu, C.; Pranovich, A.; Vähäsalo, L.; Hemming, J.; Holmbom, B.; Schols, H.A.; Willför, S. Kinetics of acid hydrolysis of water-soluble spruce O-acetyl galactoglucomannans. J. Agric. Food Chem. 2008, 56, 2429–2435. [Google Scholar] [CrossRef]
- Bhattarai, M.; Valoppi, F.; Hirvonen, S.-P.; Hietala, S.; Kilpelainen, P.; Aseyev, V.; Mikkonen, K.S. Time-dependent self-association of spruce galactoglucomannans depends on pH and mechanical shearing. Food Hydrocoll. 2020, 102, 105607. [Google Scholar] [CrossRef]
- Marciani, L.; Faulks, R.; Wickham, M.S.J.; Bush, D.; Pick, B.; Wright, J.; Cox, E.F.; Fillery-Travis, A.; Gowland, P.A.; Spiller, R.C.; et al. Effect of intragastric acid stability of fat emulsions on gastric emptying, plasma lipid profile and postprandial satiety. Br. J. Nutr. 2009, 101, 919–928. [Google Scholar] [CrossRef] [Green Version]
- Atgié, M.; Masbernat, O.; Roger, K. Emulsions stabilized by gum arabic: Composition and packing within interfacial films. Langmuir 2019, 35, 962–972. [Google Scholar] [CrossRef]
- Chu, B.S.; Rich, G.T.; Ridout, M.J.; Faulks, R.M.; Wickham, M.S.J.; Wilde, P.J. Modulating pancreatic lipase activity with galactolipids: Effects of emulsion interfacial composition. Langmuir 2009, 25, 9352–9360. [Google Scholar] [CrossRef] [PubMed]
- Torcello-Gómez, A.; Foster, T.J. Influence of interfacial and bulk properties of cellulose ethers on lipolysis of oil-in-water emulsions. Carbohydr. Polym. 2016, 144, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Espinal-Ruiz, M.; Parada-Alfonso, F.; Restrepo-Sánchez, L.P.; Narváez-Cuenca, C.E.; McClements, D.J. Impact of dietary fibers [methyl cellulose, chitosan, and pectin] on digestion of lipids under simulated gastrointestinal conditions. Food Funct. 2014, 5, 3083–3095. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, S.L.; Leth, M.L.; Michalak, L.; Hansen, M.E.; Pudlo, N.A.; Glowacki, R.; Pereira, G.; Workman, C.T.; Arntzen, M.Ø.; Pope, P.B.; et al. The human gut firmicute roseburia intestinalis is a primary degrader of dietary β-mannans. Nat. Commun. 2019, 10, 905. [Google Scholar] [CrossRef]
- Pitkänen, L.; Heinonen, M.; Mikkonen, K.S. Safety considerations of plant polysaccharides for food use: A case study on phenolic-rich softwood galactoglucomannan extract. Food Funct. 2018, 9, 1931–1943. [Google Scholar] [CrossRef] [Green Version]


| TAG % | Spdr-GGM | EtOH-GGM | GA |
|---|---|---|---|
| Initial emulsion | 100 Aa | 100 Aa | 100 Aa |
| Oral phase | 88 Ab ± 2 | 92 Aa ± 7 | 96 Aa ± 1 |
| Gastric phase | 87 Aab ± 5 | 94 Aa ± 7 | 85 Aa ± 8 |
| Intestinal phase | nd | nd | 7 c ± 3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; Mikkonen, K.S.; Kilpeläinen, P.O.; Lehtonen, M.I. Spruce Galactoglucomannan-Stabilized Emulsions Enhance Bioaccessibility of Bioactive Compounds. Foods 2020, 9, 672. https://doi.org/10.3390/foods9050672
Zhao H, Mikkonen KS, Kilpeläinen PO, Lehtonen MI. Spruce Galactoglucomannan-Stabilized Emulsions Enhance Bioaccessibility of Bioactive Compounds. Foods. 2020; 9(5):672. https://doi.org/10.3390/foods9050672
Chicago/Turabian StyleZhao, Hongbo, Kirsi S. Mikkonen, Petri O. Kilpeläinen, and Mari I. Lehtonen. 2020. "Spruce Galactoglucomannan-Stabilized Emulsions Enhance Bioaccessibility of Bioactive Compounds" Foods 9, no. 5: 672. https://doi.org/10.3390/foods9050672
APA StyleZhao, H., Mikkonen, K. S., Kilpeläinen, P. O., & Lehtonen, M. I. (2020). Spruce Galactoglucomannan-Stabilized Emulsions Enhance Bioaccessibility of Bioactive Compounds. Foods, 9(5), 672. https://doi.org/10.3390/foods9050672