Inhibitory Effect of Steamed Soybean Wastewater Against DSS-Induced Intestinal Inflammation in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of SSW Extract
2.2. Determination of Radical Scavenging Capability
2.3. Determination of Total Phenolic Content and Total Flavonoid Content in SSW
2.4. Quantification of Isoflavone and Sugar Contents in SSW
2.5. Cell Culture
2.6. Cytotoxicity of SSW
2.7. Detection of Intracellular Reactive Oxygen Species (ROS) Level
2.8. Determination of Nitric Oxide (NO) Production
2.9. Animals
2.10. Animal Experimental Design
2.11. Histological Analysis
2.12. Measurement of Inflammatory Cytokine Levels
2.13. Measurement of Plasma 8-Hydroxy-2′-deoxyguanosine (8-OHdG) Level
2.14. Determination of Lipid Peroxidation in Liver Tissues
2.15. Western Blot Analysis
2.16. Statistical Analysis
3. Results
3.1. Radical Scavenging Activity and Total Phenolic and Total Flavonoid Contents of SSW
3.2. Composition of Isoflavones in SSW
3.3. Oligosaccharide and Sugar Contents of SSW
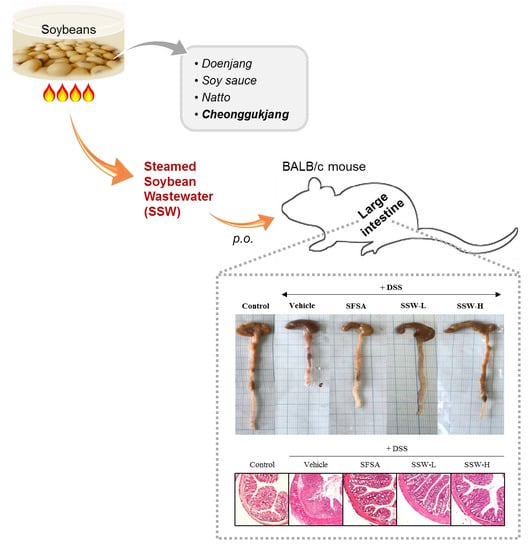
3.4. Oral Supplementation of SSW Prevented DSS-Induced Shortening of the Large Intestine in Mice
3.5. Oral Supplementation of SSW Protected the Large Intestine from DSS-Induced Histological Damage
3.6. Oral Supplementation of SSW Decreased Oxidative Stress Markers in DSS-Treated Mice
3.7. Oral Supplementation of SSW Lowered the Plasma Levels of Pro-Inflammatory Cytokines in DSS-Treated Mice
3.8. Oral Supplementation of SSW Suppressed the Expression of Colonic Inflammatory Markers in DSS-Treated Mice
3.9. SSW Reduced the Intracellular ROS Level in tBHP-Treated RAW264.7 Macrophages
3.10. SSW Inhibited the Production of NO and Pro-Inflammatory Cytokines in LPS-Treated RAW264.7 Macrophages
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Matsubara, Y.; Iwasaki, K.; Nakajima, M.; Nabetani, H.; Nakao, S. Recovery of Oligosaccharides from Steamed Soybean Waste Water in Tofu Processing by Reverse Osmosis and Nanofiltration Membranes. Biosci. Biotechnol. Biochem. 1996, 60, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, H.X.; Wu, Z.L.; Wang, Y.J.; Wang, L.J. Recovery of Isoflavone Aglycones from Soy Whey Wastewater Using Foam Fractionation and Acidic Hydrolysis. J. Agric. Food Chem. 2013, 61, 7366–7372. [Google Scholar] [CrossRef]
- Huang, Y.T.; Wen, C.C.; Chen, Y.H.; Huang, W.C.; Huang, L.T.; Lin, W.C.; Arulselvan, P.; Liao, J.W.; Lin, S.H.; Hsiao, P.W.; et al. Dietary uptake of Wedelia chinensis extract attenuates dextran sulfate sodium-induced colitis in mice. PLoS ONE 2013, 8, e64152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiocchi, C. Inflammatory bowel disease: Etiology and pathogenesis. Gastroenterology 1998, 115, 182–205. [Google Scholar] [CrossRef]
- Hanauer, S.B. Inflammatory bowel disease: Epidemiology, pathogenesis, and therapeutic opportunities. Inflamm. Bowel Dis. 2006, 12 (Suppl. S1), S3–S9. [Google Scholar] [CrossRef]
- Eichele, D.D.; Kharbanda, K.K. Dextran sodium sulfate colitis murine model: An indispensable tool for advancing our understanding of inflammatory bowel diseases pathogenesis. World J. Gastroenterol. 2017, 23, 6016–6029. [Google Scholar] [CrossRef]
- Murano, M.; Maemura, K.; Hirata, I.; Toshina, K.; Nishikawa, T.; Hamamoto, N.; Sasaki, S.; Saitoh, O.; Katsu, K. Therapeutic effect of intracolonically administered nuclear factor kappa B (p65) antisense oligonucleotide on mouse dextran sulphate sodium (DSS)-induced colitis. Clin. Exp. Immunol. 2000, 120, 51–58. [Google Scholar] [CrossRef]
- Melgar, S.; Karlsson, L.; Rehnstrom, E.; Karlsson, A.; Utkovic, H.; Jansson, L.; Michaelsson, E. Validation of murine dextran sulfate sodium-induced colitis using four therapeutic agents for human inflammatory bowel disease. Int. Immunopharmacol. 2008, 8, 836–844. [Google Scholar] [CrossRef]
- Ghattamaneni, N.K.R.; Panchal, S.K.; Brown, L. Nutraceuticals in rodent models as potential treatments for human Inflammatory Bowel Disease. Pharmacol. Res. 2018, 132, 99–107. [Google Scholar] [CrossRef]
- Kovacs-Nolan, J.; Zhang, H.; Ibuki, M.; Nakamori, T.; Yoshiura, K.; Turner, P.V.; Matsui, T.; Mine, Y. The PepT1-transportable soy tripeptide VPY reduces intestinal inflammation. Biochim. Biophys. Acta. 2012, 1820, 1753–1763. [Google Scholar] [CrossRef]
- Kawahara, M.; Nemoto, M.; Nakata, T.; Kondo, S.; Takahashi, H.; Kimura, B.; Kuda, T. Anti-inflammatory properties of fermented soy milk with Lactococcus lactis subsp. lactis S-SU2 in murine macrophage RAW264.7 cells and DSS-induced IBD model mice. Int. Immunopharmacol. 2015, 26, 295–303. [Google Scholar] [CrossRef]
- Wijeratne, S.S.; Cuppett, S.L. Soy isoflavones protect the intestine from lipid hydroperoxide mediated oxidative damage. J. Agric. Food Chem. 2007, 55, 9811–9816. [Google Scholar] [CrossRef]
- Young, D.; Ibuki, M.; Nakamori, T.; Fan, M.; Mine, Y. Soy-Derived Di- and Tripeptides Alleviate Colon and Ileum Inflammation in Pigs with Dextran Sodium Sulfate-Induced Colitis. J. Nutr. 2012, 142, 363–368. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Wu, C. Dietary soy isoflavones alleviate dextran sulfate sodium-induced inflammation and oxidative stress in mice. Exp. Ther. Med. 2017, 14, 276–282. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.J.; Suh, H.J.; Kim, J.H.; Park, S.; Joo, Y.C.; Kim, J.S. Antioxidant activity of glyceollins derived from soybean elicited with Aspergillus sojae. J. Agric. Food Chem. 2010, 58, 11633–11638. [Google Scholar] [CrossRef]
- Wiseman, D.S.; Waterhouse, D.A.; Korver, D.O. The health effects of tea and tea components: Opportunities for standardizing research methods. Crit. Rev. Food Sci. Nutr. 2001, 41, 387–412. [Google Scholar] [CrossRef]
- Gorinstein, S.; Vargas, O.J.M.; Jaramillo, N.O.; Salas, I.A.; Ayala, A.L.M.; Arancibia-Avila, P.; Toledo, F.; Katrich, E.; Trakhtenberg, S. The total polyphenols and the antioxidant potentials of some selected cereals and pseudocereals. Eur. Food Res. Technol. 2007, 225, 321–328. [Google Scholar] [CrossRef]
- Wang, H.J.; Murphy, P.A. Isoflavone Content in Commercial Soybean Foods. J. Agric. Food Chem. 1994, 42, 1666–1673. [Google Scholar] [CrossRef]
- Park, S.M.; Oh, J.; Kim, J.E.; Kim, J.S. Effect of Drying Conditions on Nutritional Quality and In Vitro Antioxidant Activity of Traditional Doenjang. Prev. Nutr. Food. Sci. 2018, 23, 144–151. [Google Scholar] [CrossRef]
- Shin, M.; Kim, J.W.; Ye, S.; Kim, S.; Jeong, D.; Lee, D.Y.; Kim, J.N.; Jin, Y.S.; Kim, K.H.; Kim, S.R. Comparative global metabolite profiling of xylose-fermenting Saccharomyces cerevisiae SR8 and Scheffersomyces stipitis. Appl. Microbiol. Biotechnol. 2019, 103, 5435–5446. [Google Scholar] [CrossRef] [PubMed]
- Woo, Y.; Lee, H.; Jeong, Y.S.; Shin, G.Y.; Oh, J.G.; Kim, J.S.; Oh, J. Antioxidant Potential of Selected Korean Edible Plant Extracts. Biomed Res. Int. 2017, 7695605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Joseph, J.A. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic. Biol. Med. 1999, 27, 612–616. [Google Scholar] [CrossRef]
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr. Protoc. Immunol. 2014, 104, 15–25. [Google Scholar] [CrossRef]
- Seo, H.; Oh, J.; Hahn, D.; Kwon, C.S.; Lee, J.S.; Kim, J.S. Protective Effect of Glyceollins in a Mouse Model of Dextran Sulfate Sodium-Induced Colitis. J. Med. Food 2017, 20, 1055–1062. [Google Scholar] [CrossRef]
- Seril, D.N.; Liao, J.; Ho, K.L.; Warsi, A.; Yang, C.S.; Yang, G.Y. Dietary iron supplementation enhances DSS-induced colitis and associated colorectal carcinoma development in mice. Dig. Dis. Sci. 2002, 47, 1266–1278. [Google Scholar] [CrossRef]
- Laroui, H.; Ingersoll, S.A.; Liu, H.C.; Baker, M.T.; Ayyadurai, S.; Charania, M.A.; Laroui, F.; Yan, Y.; Sitaraman, S.V.; Merlin, D. Dextran sodium sulfate (DSS) induces colitis in mice by forming nano-lipocomplexes with medium-chain-length fatty acids in the colon. PLoS ONE 2012, 7, e32084. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Damiani, C.R.; Benetton, C.A.; Stoffel, C.; Bardini, K.C.; Cardoso, V.H.; Di Giunta, G.; Pinho, R.A.; Dal-Pizzol, F.; Streck, E.L. Oxidative stress and metabolism in animal model of colitis induced by dextran sulfate sodium. J. Gastroenterol. Hepatol. 2007, 22, 1846–1851. [Google Scholar] [CrossRef]
- Perse, M.; Cerar, A. Dextran sodium sulphate colitis mouse model: Traps and tricks. J. Biomed. Biotechnol. 2012, 2012, 718617. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [Green Version]
- Li, M.C.; He, S.H. IL-10 and its related cytokines for treatment of inflammatory bowel disease. World J. Gastroenterol. 2004, 10, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Feng, J. Signaling pathways associated with inflammatory bowel disease. Recent Pat. Inflamm. Allergy Drug Discov. 2010, 4, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Atreya, I.; Atreya, R.; Neurath, M.F. NF-kappaB in inflammatory bowel disease. J. Intern. Med. 2008, 263, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Hokamura, A.; Yunoue, Y.; Goto, S.; Matsusaki, H. Biosynthesis of Polyhydroxyalkanoate from Steamed Soybean Wastewater by a Recombinant Strain of Pseudomonas sp. 61-3. Bioengineering (Basel) 2017, 4, 68. [Google Scholar] [CrossRef] [Green Version]
- Kwak, E.J.; Lim, S.I. The effect of sugar, amino acid, metal ion, and NaCl on model Maillard reaction under pH control. Amino Acids 2004, 27, 85–90. [Google Scholar] [CrossRef]
- Dai, Z.; Feng, S.; Liu, A.; Wang, H.; Zeng, X.; Yang, C.S. Anti-inflammatory effects of newly synthesized alpha-galacto-oligosaccharides on dextran sulfate sodium-induced colitis in C57BL/6J mice. Food Res. Int. 2018, 109, 350–357. [Google Scholar] [CrossRef]
- Yousef, M.; Pichyangkura, R.; Soodvilai, S.; Chatsudthipong, V.; Muanprasat, C. Chitosan oligosaccharide as potential therapy of inflammatory bowel disease: Therapeutic efficacy and possible mechanisms of action. Pharmacol. Res. 2012, 66, 66–79. [Google Scholar] [CrossRef]
- Winkler, J.; Butler, R.; Symonds, E. Fructo-oligosaccharide reduces inflammation in a dextran sodium sulphate mouse model of colitis. Digest. Dis. Sci. 2007, 52, 52–58. [Google Scholar] [CrossRef]
- Wada, K.; Watabe, J.; Mizutani, J.; Tomoda, M.; Suzuki, H.; Saitoh, Y. Effects of Soybean Oligosaccharides in a Beverage on Human Fecal Flora and Metabolites. Nippon Nogeikagaku Kaishi J. Jpn. Soc. Biosci. Biotechnol. Agrochem. 1992, 66, 127–135. [Google Scholar]
- Kim, S.E.; Kawaguchi, K.; Hayashi, H.; Furusho, K.; Maruyama, M. Remission Effects of Dietary Soybean Isoflavones on DSS-Induced Murine Colitis and an LPS-Activated Macrophage Cell Line. Nutrients 2019, 11, 1746. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Bi, X.; Yu, B.; Chen, D. Isoflavones: Anti-Inflammatory Benefit and Possible Caveats. Nutrients 2016, 8, 361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verdrengh, M.; Jonsson, I.M.; Holmdahl, R.; Tarkowski, A. Genistein as an anti-inflammatory agent. Inflamm. Res. 2003, 52, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Sadowska-Krowicka, H.; Mannick, E.E.; Oliver, P.D.; Sandoval, M.; Zhang, X.J.; Eloby-Childess, S.; Clark, D.A.; Miller, M.J. Genistein and gut inflammation: Role of nitric oxide. Proc. Soc. Exp. Biol. Med. 1998, 217, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Furoku, S.; Nakamoto, M.; Shuto, E.; Hosaka, T.; Nishioka, Y.; Sone, S. Soy Isoflavone Equol Perpetuates Dextran Sulfate Sodium-Induced Acute Colitis in Mice. Biosci. Biotech. Bioch. 2011, 75, 593–595. [Google Scholar] [CrossRef] [Green Version]
- Vezza, T.; Rodriguez-Nogales, A.; Algieri, F.; Utrilla, M.P.; Rodriguez-Cabezas, M.E.; Galvez, J. Flavonoids in Inflammatory Bowel Disease: A Review. Nutrients 2016, 8, 211. [Google Scholar] [CrossRef] [Green Version]
- Hoensch, H.P.; Weigmann, B. Regulation of the intestinal immune system by flavonoids and its utility in chronic inflammatory bowel disease. World J. Gastroenterol. 2018, 24, 877–881. [Google Scholar] [CrossRef]








| Isoflavones | Contents (μg/g DW *) | ||
|---|---|---|---|
| Soybean | SSW | ||
| Aglycone | Daidzein | 1651.96 ± 35.06 b | 74.06 ± 11.24 cd |
| Genistein | 9.31 ± 0.23 d | 54.10 ± 9.79 cd | |
| Glycitein | 15.79 ± 0.67 d | 15.12 ± 0.01 d | |
| Glycosides | Daidzin | 245.68 ± 10.10 e | 6502.67 ± 197.16 b |
| Genistin | 206.85 ± 4.44 e | 2634.93 ± 162.69 b | |
| Glycitin | 544.96 ± 27.67 d | 2585.28 ± 150.25 b | |
| Acetyl glycosides | A-daidzin | 25.21 ± 8.55 d | 34.28 ± 5.74 cd |
| A-genisitn | ND ** | 53.63 ± 4.89 cd | |
| A-glycitin | 600.73 ± 170.22 d | 50.93 ± 17.81 a | |
| Malonyl glycosides | M-daidzin | 3503.81 ± 70.31 a | 148.12 ± 32.72 cd |
| M-genistin | 1112.39 ± 22.51 c | 99.34 ± 7.22 cd | |
| M-glycitin | 242.26 ± 21.15 e | 193.05 ± 44.95 c | |
| Sugar | Contents (mg/g DW *) | |
|---|---|---|
| Soybean | SSW | |
| Stachyose | 9.23 ± 0.18 | 10.09 ± 0.03 |
| Raffinose | 3.59 ± 0.12 | 5.97 ± 0.02 |
| Sucrose | 12.92 ± 0.85 | ND ** |
| Glucose | ND | ND |
| Fructose | 1.06 ± 0.01 | 6.97 ± 0.06 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, S.; Oh, J.; Lim, J.S.; Kim, S.; Jeong, D.; Kim, S.R.; Kim, J.-S. Inhibitory Effect of Steamed Soybean Wastewater Against DSS-Induced Intestinal Inflammation in Mice. Foods 2020, 9, 954. https://doi.org/10.3390/foods9070954
Jeong S, Oh J, Lim JS, Kim S, Jeong D, Kim SR, Kim J-S. Inhibitory Effect of Steamed Soybean Wastewater Against DSS-Induced Intestinal Inflammation in Mice. Foods. 2020; 9(7):954. https://doi.org/10.3390/foods9070954
Chicago/Turabian StyleJeong, Soojung, Jisun Oh, Ji Sun Lim, Sunghee Kim, Deokyeol Jeong, Soo Rin Kim, and Jong-Sang Kim. 2020. "Inhibitory Effect of Steamed Soybean Wastewater Against DSS-Induced Intestinal Inflammation in Mice" Foods 9, no. 7: 954. https://doi.org/10.3390/foods9070954
APA StyleJeong, S., Oh, J., Lim, J. S., Kim, S., Jeong, D., Kim, S. R., & Kim, J. -S. (2020). Inhibitory Effect of Steamed Soybean Wastewater Against DSS-Induced Intestinal Inflammation in Mice. Foods, 9(7), 954. https://doi.org/10.3390/foods9070954