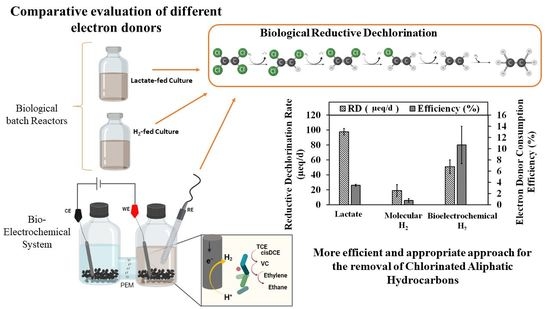
Evaluation of the Bioelectrochemical Approach and Different Electron Donors for Biological Trichloroethylene Reductive Dechlorination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Dehalococcoides Mccartyi (Dhc)-Enriched Culture as Inoculum
2.2. Lactate-Fed and H2-Fed Anaerobic Cultures
2.3. Electrochemical Systems Setup
2.4. Analytical Methods
2.5. Data Elaboration
3. Results and Discussion
3.1. Lactate and H2 Fed Tests
3.2. Performances of Biotic and Abiotic H-Cell Reactors
3.3. Comparative Evaluation of Different Electron Donors
- All lactate added to the culture was entirely fermented with H2.
- The bioavailable hydrogen that was entirely consumed by the RD reaction was the hydrogen dissolved in the liquid phase.
3.4. Outlook and Perspectives of the Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MEC | microbial electrolysis cell |
| BES | bioelectrochemical systems |
| RD | reductive dechlorination |
| TCE | trichloroethylene |
| cis-DCE | cis-dichloroethylene |
| VC | vinyl chloride |
| Eth | ethylene |
| Eta | ethane |
References
- Doherty, R.E. A History of the Production and Use of Carbon Tetrachloride, Tetrachloroethylene, Trichloroethylene and 1,1,1-Trichloroethane in the United States: Part 1—Historical Background; Carbon Tetrachloride and Tetrachloroethylene. Environ. Forensics 2000, 1, 69–81. [Google Scholar] [CrossRef]
- Moran, M.J.; Zogorski, J.S.; Squillace, P.J. Chlorinated solvents in groundwater of the United States. Environ. Sci. Technol. 2007, 41, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Squillace, P.J.; Scott, J.C.; Moran, M.J.; Nolan, B.T.; Kolpin, D.W. VOCs, pesticides, nitrate, and their mixtures in groundwater used for drinking water in the United States. Environ. Sci. Technol. 2002, 36, 1923–1930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aulenta, F.; Tocca, L.; Verdini, R.; Reale, P.; Majone, M. Dechlorination of trichloroethene in a continuous-flow bioelectrochemical reactor: Effect of cathode potential on rate, selectivity, and electron transfer mechanisms. Environ. Sci. Technol. 2011, 45, 8444–8451. [Google Scholar] [CrossRef] [PubMed]
- US Environmental Protection Agency. Sources of Toxic Compounds in Household Wastewater; Office of Research and Development: Washington, DC, USA, 1980; Volume 2, pp. 80–128. [Google Scholar]
- Directive 2006/118/EC of the European Parliament and of the Council of 12 December 2006 on the Protection of Groundwater against Pollution and Deterioration. Available online: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2006:372:0019:0031:EN:PDF (accessed on 9 January 2022).
- Casasso, A.; Tosco, T.; Bianco, C.; Bucci, A.; Sethi, R. How can we make pump and treat systems more energetically sustainable? Water 2020, 12, 67. [Google Scholar] [CrossRef] [Green Version]
- Rossi, M.M.; Dell’Armi, E.; Lorini, L.; Amanat, N.; Zeppilli, M.; Villano, M.; Papini, M.P. Combined Strategies to Prompt the Biological Reduction of Chlorinated Aliphatic Hydrocarbons: New Sustainable Options for Bioremediation Application. Bioengineering 2021, 8, 109. [Google Scholar] [CrossRef]
- Brooks, M.C.; Yarney, E.; Huang, J. Strategies for Managing Risk due to Back Diffusion. Groundw. Monit. Remediat. 2021, 41, 76–98. [Google Scholar] [CrossRef]
- Silva-Castro, G.A.; Rodríguez-Calvo, A.; Robledo-Mahón, T.; Aranda, E.; González-López, J.; Calvo, C. Design of bio-absorbent systems for the removal of hydrocarbons from industrial wastewater: Pilot-plant scale. Toxics 2021, 9, 162. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.P.; Almeida, C.M.R.; Salgado, M.A.; Carvalho, M.F.; Mucha, A.P. Pharmaceutical compounds in aquatic environments—Occurrence, fate and bioremediation prospective. Toxics 2021, 9, 257. [Google Scholar] [CrossRef] [PubMed]
- El Fantroussi, S.; Naveau, H.; Agathos, S.N. Anaerobic dechlorinating bacteria. Biotechnol. Prog. 1998, 14, 167–188. [Google Scholar] [CrossRef]
- Field, J.A.; Sierra-Alvarez, R. Biodegradability of chlorinated solvents and related chlorinated aliphatic compounds. Rev. Environ. Sci. Biotechnol. 2004, 3, 185–254. [Google Scholar] [CrossRef]
- Fennell, D.E.; Gossett, J.M.; Zinder, S.H. Comparison of butyric acid, ethanol, lactic acid, and propionic acid as hydrogen donors for the reductive dechlorination of tetrachloroethene. Environ. Sci. Technol. 1997, 31, 918–926. [Google Scholar] [CrossRef]
- Gerritse, J.; Drzyzga, O.; Kloetstra, G.; Keijmel, M.; Wiersum, L.P.; Hutson, R.; Collins, M.D.; Gottschal, J.C. Influence of different electron donors and acceptors on dehalorespiration of tetrachloroethene by Desulfitobacterium frappieri TCE1. Appl. Environ. Microbiol. 1999, 65, 5212–5221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baric, M.; Majone, M.; Beccari, M.; Papini, M.P. Coupling of polyhydroxybutyrate (PHB) and zero valent iron (ZVI) for enhanced treatment of chlorinated ethanes in permeable reactive barriers (PRBs). Chem. Eng. J. 2012, 195–196, 22–30. [Google Scholar] [CrossRef]
- Papini, M.P.; Majone, M.; Arjmand, F.; Silvestri, D.; Sagliaschi, M.; Sucato, S.; Alesi, E.; Barstch, E.; Pierro, L. First pilot test on the integration of GCW (groundwater circulation well) with ENA (enhanced natural attenuation) for chlorinated solvents source remediation. Chem. Eng. Trans. 2016, 49, 91–96. [Google Scholar] [CrossRef]
- Aulenta, F.; Fuoco, M.; Canosa, A.; Papini, M.P.; Majone, M. Use of poly-β-hydroxy-butyrate as a slow-release electron donor for the microbial reductive dechlorination of TCE. Water Sci. Technol. 2008, 57, 921–925. [Google Scholar] [CrossRef]
- Amanat, N.; Matturro, B.; Rossi, M.M.; Valentino, F.; Villano, M.; Papini, M.P. Assessment of Long—Term Fermentability of PHA—Based Materials from Pure and Mixed Microbial Cultures for Potential Environmental Applications. Water 2021, 13, 897. [Google Scholar] [CrossRef]
- Valentino, F.; Lorini, L.; Pavan, P.; Bolzonella, D.; Majone, M. Organic fraction of municipal solid waste conversion into polyhydroxyalkanoates (PHA) in a pilot scale anaerobic/aerobic process. Chem. Eng. Trans. 2019, 74, 265–270. [Google Scholar] [CrossRef]
- Aulenta, F.; Reale, P.; Catervi, A.; Panero, S.; Majone, M. Kinetics of trichloroethene dechlorination and methane formation by a mixed anaerobic culture in a bio-electrochemical system. Electrochim. Acta 2008, 53, 5300–5305. [Google Scholar] [CrossRef]
- Zhao, F.; Heidrich, E.S.; Curtis, T.P.; Dolfing, J. Understanding the complexity of wastewater: The combined impacts of carbohydrates and sulphate on the performance of bioelectrochemical systems. Water Res. 2020, 176, 1–10. [Google Scholar] [CrossRef]
- Lai, A.; Aulenta, F.; Mingazzini, M.; Palumbo, M.T.; Papini, M.P.; Verdini, R.; Majone, M. Bioelectrochemical approach for reductive and oxidative dechlorination of chlorinated aliphatic hydrocarbons (CAHs). Chemosphere 2017, 169, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Zeppilli, M.; Dell’Armi, E.; Cristiani, L.; Papini, M.P.; Majone, M. Reductive/oxidative sequential bioelectrochemical process for perchloroethylene removal. Water 2019, 11, 2579. [Google Scholar] [CrossRef] [Green Version]
- Aulenta, F.; Catervi, A.; Majone, M.; Panero, S.; Reale, P.; Rossetti, S. Electron transfer from a solid-state electrode assisted by methyl viologen sustains efficient microbial reductive dechlorination of TCE. Environ. Sci. Technol. 2007, 41, 2554–2559. [Google Scholar] [CrossRef] [PubMed]
- Zeppilli, M.; Dell’Armi, E.; Petrangeli Papini, M.; Majone, M. Sequential reductive/oxidative bioelectrochemical processfor groundwater perchloroethylene removal. Chem. Eng. Trans. 2021, 86, 373–378. [Google Scholar] [CrossRef]
- Rosenbaum, M.; Aulenta, F.; Villano, M.; Angenent, L.T. Cathodes as electron donors for microbial metabolism: Which extracellular electron transfer mechanisms are involved? Bioresour. Technol. 2011, 102, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Cristiani, L.; Ferretti, J.; Zeppilli, M. Electrons recycle concept in a microbial electrolysis cell for biogas upgrading. Chem. Eng. Technol. 2021, 45, 1–8. [Google Scholar] [CrossRef]
- Zeppilli, M.; Paiano, P.; Torres, C.; Pant, D. A critical evaluation of the pH split and associated effects in bioelectrochemical processes. Chem. Eng. J. 2021, 422, 130155. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, X.; Li, N.; Wang, Q.; Liao, C. Bioelectrochemical system for dehalogenation: A review. Environ. Pollut. 2022, 293, 118519. [Google Scholar] [CrossRef]
- Dell’Armi, E.; Zeppilli, M.; Matturro, B.; Rossetti, S.; Papini, M.P.; Majone, M. Effects of the feeding solution composition on a reductive/oxidative sequential bioelectrochemical process for perchloroethylene removal. Processes 2021, 9, 405. [Google Scholar] [CrossRef]
- Zeppilli, M.; Matturro, B.; Dell’Armi, E.; Cristiani, L.; Papini, M.P.; Rossetti, S.; Majone, M. Reductive/oxidative sequential bioelectrochemical process for Perchloroethylene (PCE) removal: Effect of the applied reductive potential and microbial community characterization. J. Environ. Chem. Eng. 2021, 9, 104657. [Google Scholar] [CrossRef]
- Zeppilli, M.; Cristiani, L.; Dell’Armi, E.; Majone, M. Bioelectromethanogenesis reaction in a tubular Microbial Electrolysis Cell (MEC) for biogas upgrading. Renew. Energy 2019, 158, 23–31. [Google Scholar] [CrossRef]
- Wang, X.; Aulenta, F.; Puig, S.; Esteve-Núñez, A.; He, Y.; Mu, Y.; Rabaey, K. Microbial electrochemistry for bioremediation. Environ. Sci. Ecotechnol. 2020, 1, 100013. [Google Scholar] [CrossRef]
- Aulenta, F.; Gossett, J.M.; Papini, M.P.; Rossetti, S.; Majone, M. Comparative study of methanol, butyrate, and hydrogen as electron donors for long-term dechlorination of tetrachloroethene in mixed anerobic cultures. Biotechnol. Bioeng. 2005, 91, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Zeppilli, M.; Chouchane, H.; Scardigno, L.; Mahjoubi, M.; Gacitua, M.; Askri, R.; Cherif, A.; Majone, M. Bioelectrochemical vs hydrogenophilic approach for CO2 reduction into methane and acetate. Chem. Eng. J. 2020, 396, 125243. [Google Scholar] [CrossRef]
- Chen, F.; Freedman, D.L.; Falta, R.W.; Murdoch, L.C. Henry’s law constants of chlorinated solvents at elevated temperatures. Chemosphere 2012, 86, 156–165. [Google Scholar] [CrossRef] [PubMed]
- DiStefano, T.D.; Gossett, J.M.; Zinder, S.H. Hydrogen as an electron donor for dechlorination of tetrachloroethene by an anaerobic mixed culture. Appl. Environ. Microbiol. 1992, 58, 3622–3629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chau, A.T.T.; Lee, M.; Adrian, L.; Manefield, M.J. Syntrophic partners enhance growth and respiratory dehalogenation of hexachlorobenzene by Dehalococcoides mccartyi strain CBDB1. Front. Microbiol. 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Principles and Practices of Enhanced Anaerobic Bioremediation of Chlorinated Solvents; Technical Report TR-2250-ENV; Parsons Corporation: Pasadena, CA, USA, 2004.
- Wang, M.; Wang, Z.; Gong, X.; Guo, Z. The intensification technologies to water electrolysis for hydrogen production—A review. Renew. Sustain. Energy Rev. 2014, 29, 573–588. [Google Scholar] [CrossRef]
- Palma, E.; Daghio, M.; Franzetti, A.; Papini, M.P.; Aulenta, F. The bioelectric well: A novel approach for in situ treatment of hydrocarbon-contaminated groundwater. Microb. Biotechnol. 2018, 11, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Liang, B.; Li, Z.L.; Yang, J.Q.; Huang, C.; Lyu, M.; Yuan, Y.; Nan, J.; Wang, A.J. Bioelectrochemical assisted dechlorination of tetrachloroethylene and 1,2-dichloroethane by acclimation of anaerobic sludge. Chemosphere 2019, 227, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Lai, A.; Verdini, R.; Aulenta, F.; Majone, M. Influence of nitrate and sulfate reduction in the bioelectrochemically assisted dechlorination of cis-DCE. Chemosphere 2015, 125, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Lai, A.; Astolfi, M.L.; Bertelli, V.; Agostinelli, V.G.; Zeppilli, M.; Majone, M. Chromate fate and effect in bioelectrochemical systems for remediation of chlorinated solvents. New Biotechnol. 2021, 60, 27–35. [Google Scholar] [CrossRef] [PubMed]







| Bioremediation by Injection of Lactate 1 | |
|---|---|
| TCE and cis-DCE source zone concentration (µeq/L) | 22 |
| H2 from lactate fermentation (molH2/mol OA) | 6 |
| Lactate injected kg/m3 | 0.12 |
| Lactate efficiency (%) | 0.14 |
| Hydrogen Bioremediation Evaluation | |
|---|---|
| TCE and cis-DCE source zone concentration (µeq/L) | 22 |
| H2 energetic cost (electrolysis) (kWh/m3 H2) | 4.5 |
| H2 for complete RD (m3H2/m3GW) | 0.0003 |
| Minimal energetic cost of the remediation (kWh/m3GW) | 0.001 |
| Efficiency factor for H2 sparging | 0.1–0.01 |
| Estimated energetic cost of the remediation (kWh/m3GW) | 0.01–0.1 |
| Target Compound | BES Configuration | RD Coulombic Efficiency (%) | Ref. |
|---|---|---|---|
| PCE | Tubular membrane-less | 22 | [29] |
| PCE—1,2 DCA | Two Chamber/CEM | 80.4–90 | [43] |
| cis-DCE | Two Chamber/Nafion® | 60–90 | [44] |
| TCE-Cr (VI) | Two Chamber/Nafion® | 4.66 | [45] |
| TCE | Two Chamber/Nafion® | 4.73 | [23] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dell’Armi, E.; Rossi, M.M.; Taverna, L.; Petrangeli Papini, M.; Zeppilli, M. Evaluation of the Bioelectrochemical Approach and Different Electron Donors for Biological Trichloroethylene Reductive Dechlorination. Toxics 2022, 10, 37. https://doi.org/10.3390/toxics10010037
Dell’Armi E, Rossi MM, Taverna L, Petrangeli Papini M, Zeppilli M. Evaluation of the Bioelectrochemical Approach and Different Electron Donors for Biological Trichloroethylene Reductive Dechlorination. Toxics. 2022; 10(1):37. https://doi.org/10.3390/toxics10010037
Chicago/Turabian StyleDell’Armi, Edoardo, Marta Maria Rossi, Lucia Taverna, Marco Petrangeli Papini, and Marco Zeppilli. 2022. "Evaluation of the Bioelectrochemical Approach and Different Electron Donors for Biological Trichloroethylene Reductive Dechlorination" Toxics 10, no. 1: 37. https://doi.org/10.3390/toxics10010037
APA StyleDell’Armi, E., Rossi, M. M., Taverna, L., Petrangeli Papini, M., & Zeppilli, M. (2022). Evaluation of the Bioelectrochemical Approach and Different Electron Donors for Biological Trichloroethylene Reductive Dechlorination. Toxics, 10(1), 37. https://doi.org/10.3390/toxics10010037