Biochar-Supported TiO2-Based Nanocomposites for the Photocatalytic Degradation of Sulfamethoxazole in Water—A Review
Abstract
:1. Introduction
- (i)
- Synthesis methods of biochar-supported TiO2 nanoparticles;
- (ii)
- The effect of the integration of biochar with TiO2 particles in terms of changes in the physicochemical properties and increase in the photocatalytic response under UV-visible light;
- (iii)
- Delineate the photochemistry of SMX in water and its major sources into the environment;
- (iv)
- Delineate the chemical pathways and mechanisms involved during the photocatalytic degradation of SMX in water using the biochar-supported TiO2 nanoparticles.
2. Recent Degradation Techniques of the Common Antibiotics
3. Sulfamethoxazole (SMX) in Water
3.1. Sources of Sulfamethoxazole in Water and Their Environmental Impacts
3.2. Photochemistry of Sulfamethoxazole in Water
4. Synthesis of Biochar-Supported TiO2 Nanocomposites
4.1. Sol–Gel Method
4.2. Ultrasound Method
4.3. Thermal Polycondensation Method
4.4. Solvothermal Method
5. Effect of Biochar Addition on the Chemical and Structural Characteristics of TiO2 Nanoparticles
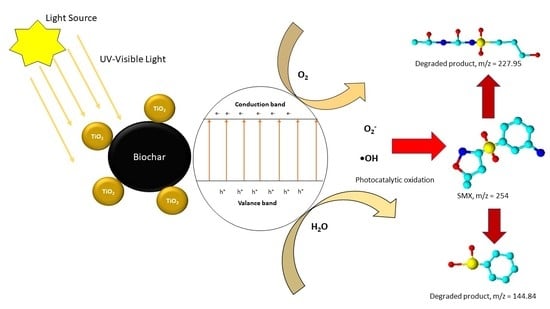
6. Application of Biochar-Supported TiO2 Nanoparticles for the Photocatalytic Degradation of Sulfamethoxazole
7. Conclusions and Future Prospective
- (1)
- The use of a biochar-supported TiO2 composite for the photocatalytic degradation of antibiotics is an attractive method due to its high efficiency and low cost of operation compared to the existing treatment processes.
- (2)
- The integration of biochar with the TiO2 nanoparticles increased photocatalytic degradation of SMX by increasing its photocatalytic response in the UV-visible range (200–700 nm) and the interaction of SMX with the TiO2 through the adsorption onto the biochar/TiO2 composite interface.
- (3)
- The biochar-supported TiO2 composite can remove up to more than 95% of SMX in the aqueous solution within the UV range and up to 75% efficiency in the visible range.
- (4)
- Unlike the doped photocatalyst, the biochar-supported TiO2 nanoparticles degrade the sulfamethoxazole both by adsorption and photocatalysis process and could also be used for the photocatalytic degradation of other antibiotics.
- (5)
- The •OH free radical is the prime key component that degrades the sulfamethoxazole through the oxidation or reduction process.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gebre, S.L.; Cattrysse, D.; Van Orshoven, J. Multi-criteria decision-making methods to address water allocation problems: A systematic review. Water 2021, 13, 125. [Google Scholar] [CrossRef]
- Santy, S.; Mujumdar, P.; Bala, G. Potential Impacts of Climate and Land Use Change on the Water Quality of Ganga River around the Industrialized Kanpur Region. Sci. Rep. 2020, 10, 9107. [Google Scholar] [CrossRef]
- Al Maliki, A.A.; Abbass, Z.D.; Hussain, H.M.; Al-Ansari, N. Assessment of the groundwater suitability for irrigation near Al Kufa City and preparing the final water quality maps using spatial distribution tools. Environ. Earth Sci. 2020, 79, 330. [Google Scholar] [CrossRef]
- Usman, M.; Farooq, M.; Hanna, K. Environmental side effects of the injudicious use of antimicrobials in the era of COVID-19. Sci. Total Environ. 2020, 745, 141053. [Google Scholar] [CrossRef]
- Lucien, M.A.B.; Canarie, M.F.; Kilgore, P.E.; Jean-Denis, G.; Fénélon, N.; Pierre, M.; Cerpa, M.; Joseph, G.A.; Maki, G.; Zervos, M.J.; et al. Antibiotics and antimicrobial resistance in the COVID-19 era: Perspective from resource-limited settings. Int. J. Infect. Dis. 2021, 104, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.K.M.; Ghaly, T.M.; Gillings, M.R. A survey of sub-inhibitory concentrations of antibiotics in the environment. J. Environ. Sci. 2021, 99, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Harrower, J.; McNaughtan, M.; Hunter, C.; Hough, R.; Zhang, Z.; Helwig, K. Chemical Fate and Partitioning Behaviour of Antibiotics in the Aquatic Environment—A Review. Environ. Toxicol. Chem. 2021, 1–24. [Google Scholar] [CrossRef]
- Zainab, S.M.; Junaid, M.; Rehman, M.Y.A.; Lv, M.; Yue, L.; Xu, N.; Malik, R.N. First insight into the occurrence, spatial distribution, sources, and risks assessment of antibiotics in groundwater from major urban-rural settings of Pakistan. Sci. Total Environ. 2021, 791, 148298. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Shao, H.; Huo, Z.; Xie, N.; Gu, J.; Xu, G. Typical antibiotics in the receiving rivers of direct-discharge sources of sewage across Shanghai: Occurrence and source analysis. RSC Adv. 2021, 11, 21579–21587. [Google Scholar] [CrossRef]
- Xu, B.; Mao, D.; Luo, Y.; Xu, L. Sulfamethoxazole biodegradation and biotransformation in the water-sediment system of a natural river. Bioresour. Technol. 2011, 102, 7069–7076. [Google Scholar] [CrossRef]
- Drori, Y.; Aizenshtat, Z.; Chefetz, B. Sorption-Desorption Behavior of Atrazine in Soils Irrigated with Reclaimed Wastewater. Soil Sci. Soc. Am. J. 2005, 69, 1703–1710. [Google Scholar] [CrossRef]
- Steven Leeder, J.; Dosch, H.M.; Spielberg, S.P. Cellular toxicity of sulfamethoxazole reactive metabolites-I. Inhibition of intracellular esterase activity prior to cell death. Biochem. Pharmacol. 1991, 41, 567–574. [Google Scholar] [CrossRef]
- Jesús García-Galán, M.; Díaz-Cruz, M.S.; Barceló, D. Identification and determination of metabolites and degradation products of sulfonamide antibiotics. Trends Anal. Chem. 2008, 27, 1008–1022. [Google Scholar] [CrossRef]
- Tamtam, F.; Mercier, F.; Le, B.; Eurin, J.; Tuc, Q.; Clément, M.; Chevreuil, M.; Pierre, U.; Ephe, C.; Sisyphe, U.M.R.; et al. Occurrence and fate of antibiotics in the Seine River in various hydrological conditions. Sci. Total Environ. 2007, 93, 84–95. [Google Scholar] [CrossRef]
- Luo, Y.; Mao, D.; Rysz, M.; Zhou, Q.; Zhang, H.; Xu, L.; Alvarez, P. Trends in antibiotic resistance genes occurrence in the Haihe River, China. Environ. Sci. Technol. 2010, 44, 7220–7225. [Google Scholar] [CrossRef]
- Moreno-González, R.; Rodríguez-Mozaz, S.; Gros, M.; Pérez-Cánovas, E.; Barceló, D.; León, V.M. Input of pharmaceuticals through coastal surface watercourses into a Mediterranean lagoon (Mar Menor, SE Spain): Sources and seasonal variations. Sci. Total Environ. 2014, 490, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Cetecioglu, Z.; Ince, B.; Gros, M.; Rodriguez-Mozaz, S.; Barceló, D.; Ince, O.; Orhon, D. Biodegradation and reversible inhibitory impact of sulfamethoxazole on the utilization of volatile fatty acids during anaerobic treatment of pharmaceutical industry wastewater. Sci. Total Environ. 2015, 536, 667–674. [Google Scholar] [CrossRef]
- Song, L.; Li, L.; Yang, S.; Lan, J.; He, H.; McElmurry, S.P.; Zhao, Y. Sulfamethoxazole, tetracycline and oxytetracycline and related antibiotic resistance genes in a large-scale landfill, China. Sci. Total Environ. 2016, 551–552, 9–15. [Google Scholar] [CrossRef]
- Yang, Q.; Chen, D.; Chu, L.; Wang, J. Enhancement of ionizing radiation-induced catalytic degradation of antibiotics using Fe/C nanomaterials derived from Fe-based MOFs. J. Hazard. Mater. 2020, 389, 122148. [Google Scholar] [CrossRef]
- Kumar Subramani, A.; Rani, P.; Wang, P.H.; Chen, B.Y.; Mohan, S.; Chang, C.T. Performance assessment of the combined treatment for oxytetracycline antibiotics removal by sonocatalysis and degradation using Pseudomonas aeruginosa. J. Environ. Chem. Eng. 2019, 7, 103215. [Google Scholar] [CrossRef]
- Liu, Q.; Li, M.; Liu, X.; Zhang, Q.; Liu, R.; Wang, Z.; Shi, X.; Quan, J. Removal of sulfamethoxazole and trimethoprim from reclaimed water and the biodegradation mechanism. Front. Environ. Sci. Eng. 2018, 12, 6. [Google Scholar] [CrossRef]
- Shang, Y.; Chen, C.; Zhang, P.; Yue, Q.; Li, Y.; Gao, B.; Xu, X. Removal of sulfamethoxazole from water via activation of persulfate by Fe3C@NCNTs including mechanism of radical and nonradical process. Chem. Eng. J. 2019, 375, 122004. [Google Scholar] [CrossRef]
- Tian, Y.; Gao, B.; Morales, V.L.; Chen, H.; Wang, Y.; Li, H. Removal of sulfamethoxazole and sulfapyridine by carbon nanotubes in fixed-bed columns. Chemosphere 2013, 90, 2597–2605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.L.; Wu, Q.Y.; Huang, N.; Xu, Z.B.; Lee, M.Y.; Hu, H.Y. Potential risks from UV/H2O2 oxidation and UV photocatalysis: A review of toxic, assimilable, and sensory-unpleasant transformation products. Water Res. 2018, 141, 109–125. [Google Scholar] [CrossRef] [PubMed]
- Salimi, M.; Esrafili, A.; Gholami, M.; Jonidi Jafari, A.; Rezaei Kalantary, R.; Farzadkia, M.; Kermani, M.; Sobhi, H.R. Contaminants of emerging concern: A review of new approach in AOP technologies. Environ. Monit. Assess. 2017, 189, 414. [Google Scholar] [CrossRef]
- Mirzaei, A.; Yerushalmi, L.; Chen, Z.; Haghighat, F. Photocatalytic degradation of sulfamethoxazole by hierarchical magnetic ZnO@g-C3N4: RSM optimization, kinetic study, reaction pathway and toxicity evaluation. J. Hazard. Mater. 2018, 359, 516–526. [Google Scholar] [CrossRef]
- Bayarri, B.; Gime, J.; Costa, J.; Abella, M.N. Photocatalytic degradation of sulfamethoxazole in aqueous suspension of TiO2. Appl. Catal. B Environ. 2007, 74, 233–241. [Google Scholar] [CrossRef]
- Yuan, R.; Zhu, Y.; Zhou, B.; Hu, J. Photocatalytic oxidation of sulfamethoxazole in the presence of TiO2: Effect of matrix in aqueous solution on decomposition mechanisms. Chem. Eng. J. 2019, 359, 1527–1536. [Google Scholar] [CrossRef]
- Elmolla, E.S.; Chaudhuri, M. Photocatalytic degradation of amoxicillin, ampicillin and cloxacillin antibiotics in aqueous solution using UV/TiO2 and UV/H2O2/TiO2 photocatalysis. Desalination 2010, 252, 46–52. [Google Scholar] [CrossRef]
- Dong, H.; Zeng, G.; Tang, L.; Fan, C. An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasures. Water Res. 2015, 79, 128–146. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, Y.; Xing, M.; Ahmed, S.; Leghari, K.; Sajjad, S. Development of modified N doped TiO2 photocatalyst with metals, nonmetals and metal oxides. Energy Environ. Sci. 2010, 3, 715–726. [Google Scholar] [CrossRef]
- Hao, H.; Zhang, J. Microporous and Mesoporous Materials The study of Iron (III) and nitrogen co-doped mesoporous TiO2 photocatalysts: Synthesis, characterization and activity. Microporous Mesoporous Mater. 2009, 121, 52–57. [Google Scholar] [CrossRef]
- Koo, Y.; Littlejohn, G.; Collins, B.; Yun, Y.; Shanov, V.N.; Schulz, M.; Pai, D.; Sankar, J. Synthesis and characterization of Ag–TiO2–CNT nanoparticle composites with high photocatalytic activity under artificial light. Compos. Part B 2014, 57, 105–111. [Google Scholar] [CrossRef]
- Kasemets, K.; Ivask, A.; Dubourguier, H.; Kahru, A. Toxicology of nanoparticles of ZnO, CuO and TiO2 to yeast Saccharomyces cerevisiae. Toxicol. In Vitro 2009, 23, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Shan, R.; Lu, L.; Gu, J.; Zhang, Y.; Yuan, H. Photocatalytic degradation of methyl orange by Ag/TiO2/biochar composite catalysts in aqueous solutions. Mater. Sci. Semicond. Process. 2020, 114, 105088. [Google Scholar] [CrossRef]
- Chandra, S.; Bhattacharya, J. Influence of temperature and duration of pyrolysis on the property heterogeneity of rice straw biochar and optimization of pyrolysis conditions for its application in soils. J. Clean. Prod. 2019, 215, 1123–1139. [Google Scholar] [CrossRef]
- Kappler, A.; Wuestner, M.L.; Ruecker, A.; Harter, J.; Halama, M.; Behrens, S. Biochar as an Electron Shuttle between Bacteria and Fe (III) Minerals. Environ. Sci. Technol. Lett. 2014, 1, 339–344. [Google Scholar] [CrossRef]
- Kim, J.R.; Kan, E. Heterogeneous photocatalytic degradation of sulfamethoxazole in water using a biochar-supported TiO2 photocatalyst. J. Environ. Manag. 2016, 180, 94–101. [Google Scholar] [CrossRef] [Green Version]
- Xie, X.; Li, S.; Zhang, H.; Wang, Z.; Huang, H. Promoting charge separation of biochar-based Zn-TiO2/pBC in the presence of ZnO for efficient sulfamethoxazole photodegradation under visible light irradiation. Sci. Total Environ. 2019, 659, 529–539. [Google Scholar] [CrossRef]
- Avramiotis, E.; Frontistis, Z.; Manariotis, I.D.; Vakros, J.; Mantzavinos, D. Oxidation of sulfamethoxazole by rice husk biochar-activated persulfate. Catalysts 2021, 11, 850. [Google Scholar] [CrossRef]
- Sun, S.; Yao, H.; Li, X.; Deng, S.; Zhao, S.; Zhang, W. Enhanced degradation of sulfamethoxazole (SMX) in toilet wastewater by photo-fenton reactive membrane filtration. Nanomaterials 2020, 10, 180. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Hu, W.; Zhang, H.; Wang, H.; Sun, P. Enhanced degradation of sulfonamide antibiotics by UV irradiation combined with persulfate. Processes 2021, 9, 226. [Google Scholar] [CrossRef]
- Vignati, D.A.L.; Lofrano, G.; Libralato, G.; Guida, M.; Siciliano, A.; Carraturo, F.; Carotenuto, M. Photocatalytic ZnO-assisted degradation of spiramycin in urban wastewater: Degradation kinetics and toxicity. Water 2021, 13, 1051. [Google Scholar] [CrossRef]
- Zhu, Z.; Han, S.; Cao, Y.; Jiang, J. Synthesis of a Novel Photocatalyst MVO4/g-C3N4 (M = La, Gd) with Better Photocatalytic Activity for Tetracycline Hydrochloride Degradation under Visible-Light Irradiation. Crystals 2021, 11, 756. [Google Scholar] [CrossRef]
- Anh, H.Q.; Le, T.P.Q.; Da Le, N.; Lu, X.X.; Duong, T.T.; Garnier, J.; Rochelle-Newall, E.; Zhang, S.; Oh, N.H.; Oeurng, C.; et al. Antibiotics in surface water of East and Southeast Asian countries: A focused review on contamination status, pollution sources, potential risks, and future perspectives. Sci. Total Environ. 2021, 764. [Google Scholar] [CrossRef]
- Le, T.X.; Munekage, Y. Residues of selected antibiotics in water and mud from shrimp ponds in mangrove areas in Viet Nam. Mar. Pollut. Bull. 2004, 49, 922–929. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, A.; Takada, H.; Koike, T.; Takeshita, A.; Saha, M.; Rinawati; Nakada, N.; Murata, A.; Suzuki, T.; Suzuki, S.; et al. Ubiquitous occurrence of sulfonamides in tropical Asian waters. Sci. Total Environ. 2013, 452–453, 108–115. [Google Scholar] [CrossRef]
- Tran, N.H.; Hoang, L.; Nghiem, L.D.; Nguyen, N.M.H.; Ngo, H.H.; Guo, W.; Trinh, Q.T.; Mai, N.H.; Chen, H.; Nguyen, D.D.; et al. Occurrence and risk assessment of multiple classes of antibiotics in urban canals and lakes in Hanoi, Vietnam. Sci. Total Environ. 2019, 692, 157–174. [Google Scholar] [CrossRef]
- Sim, W.J.; Lee, J.W.; Lee, E.S.; Shin, S.K.; Hwang, S.R.; Oh, J.E. Occurrence and distribution of pharmaceuticals in wastewater from households, livestock farms, hospitals and pharmaceutical manufactures. Chemosphere 2011, 82, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-P.; Jin, D.R.; Lee, W.; Chae, M.; Park, J. Occurrence and Removal of Veterinary Antibiotics in Livestock Wastewater Treatment Plants, South Korea. Processes 2020, 8, 720. [Google Scholar] [CrossRef]
- Lien, L.T.Q.; Hoa, N.Q.; Chuc, N.T.K.; Thoa, N.T.M.; Phuc, H.D.; Diwan, V.; Dat, N.T.; Tamhankar, A.J.; Lundborg, C.S. Antibiotics in wastewater of a rural and an urban hospital before and after wastewater treatment, and the relationship with antibiotic use-a one year study from Vietnam. Int. J. Environ. Res. Public Health 2016, 13, 588. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.; Zhang, D.; Xiao, S.; Geng, C.; Zhang, X. Occurrence and sources of antibiotics and their metabolites in river water, WWTPs, and swine wastewater in Jiulongjiang River basin, south China. Environ. Sci. Pollut. Res. 2013, 20, 9075–9083. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Huang, Z.; Yang, K.; Graham, D.; Xie, B. Relationships between Antibiotics and Antibiotic Resistance Gene Levels in Municipal Solid Waste Leachates in Shanghai, China. Environ. Sci. Technol. 2015, 49, 4122–4128. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, J.; Zhuo, L.; Yan, X. Antibiotics in wastewater from multiple sources and surface water of the Yangtze River in Chongqing in China. Environ. Monit. Assess. 2020, 192, 1–13. [Google Scholar] [CrossRef] [PubMed]
- City, M.; Vo, T.; Bui, X.; Cao, N.; Luu, V. Investigation of antibiotics in health care wastewater in Ho Chi. Environ. Monit. Assess. 2016, 188, 1–9. [Google Scholar] [CrossRef]
- Anh, H.; Ha, N.; Tung, H.; Thuong, T.; Viet, H.; Pham, V.C.; Berg, M.; Giger, W.; Alder, A.C. Occurrence, fate and antibiotic resistance of fluoroquinolone antibacterials in hospital wastewaters in Hanoi, Vietnam. Chemosphere 2008, 72, 968–973. [Google Scholar] [CrossRef]
- Thai, P.K.; Ky, L.X.; Binh, V.N.; Nhung, P.H.; Nhan, P.T.; Hieu, N.Q.; Dang, N.T.T.; Tam, N.K.B.; Anh, N.T.K. Occurrence of antibiotic residues and antibiotic-resistant bacteria in effluents of pharmaceutical manufacturers and other sources around Hanoi, Vietnam. Sci. Total Environ. 2018, 645, 393–400. [Google Scholar] [CrossRef]
- Avisar, D.; Lester, Y.; Ronen, D. Sulfamethoxazole contamination of a deep phreatic aquifer. Sci. Total Environ. 2009, 407, 4278–4282. [Google Scholar] [CrossRef]
- Çalişkan, E.; Göktürk, S. Adsorption characteristics of sulfamethoxazole and metronidazole on activated carbon. Sep. Sci. Technol. 2010, 45, 244–255. [Google Scholar] [CrossRef]
- Dirany, A.; Efremova Aaron, S.; Oturan, N.; Sirés, I.; Oturan, M.A.; Aaron, J.J. Study of the toxicity of sulfamethoxazole and its degradation products in water by a Thaibioluminescence method during application of the electro-Fenton treatment. Anal. Bioanal. Chem. 2011, 400, 353–360. [Google Scholar] [CrossRef]
- Prasannamedha, G.; Kumar, P.S. A review on contamination and removal of sulfamethoxazole from aqueous solution using cleaner techniques: Present and future perspective. J. Clean. Prod. 2020, 250, 119553. [Google Scholar] [CrossRef]
- Białk-bieli, A.; Stolte, S.; Matzke, M.; Fabia, A.; Maszkowska, J.; Kołodziejska, M.; Liberek, B.; Stepnowski, P.; Kumirska, J. Hydrolysis of sulphonamides in aqueous solutions. J. Hazard. Mater. 2012, 222, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, A.; McArdell, C.S.; Joss, A.; Giger, W. Occurrence and Sorption Behavior of Sulfonamides, Macrolides, and Trimethoprim in Activated Sludge Treatment. Environ. Sci. Technol. 2005, 39, 3981–3989. [Google Scholar]
- Długosz, M.; Zmudzki, P.; Kwiecień, A.; Szczubiałka, K.; Krzek, J.; Nowakowska, M. Photocatalytic degradation of sulfamethoxazole in aqueous solution using a floating TiO2-expanded perlite photocatalyst. J. Hazard. Mater. 2015, 298, 146–153. [Google Scholar] [CrossRef]
- Jing, H.; Li, Y.; Wang, X. Environmental Science Water Research & Technology supported Mg(OH)2/bentonite composite. Environ. Sci. Water Res. Technol. 2019, 5, 931–943. [Google Scholar] [CrossRef]
- Kitano, M.; Matsuoka, M.; Ueshima, M.; Anpo, M. Recent developments in titanium oxide-based photocatalysts. Appl. Catal. A Gen. 2007, 325, 1–14. [Google Scholar] [CrossRef]
- Shi, M.; Shen, J.; Ma, H.; Li, Z.; Lu, X.; Li, N.; Ye, M. Physicochemical and Engineering Aspects Preparation of graphene–TiO2 composite by hydrothermal method from peroxotitanium acid and its photocatalytic properties. Colloids Surf. A Physicochem. Eng. Asp. 2012, 405, 30–37. [Google Scholar] [CrossRef]
- Andriantsiferana, C.; Mohamed, E.F.; Delmas, H. Photocatalytic degradation of an azo-dye on TiO2/activated carbon composite material. Environ. Technol. 2014, 35, 355–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorraj, M.; Alizadeh, M.; Asrina, N.; Jefrey, W. Enhanced visible-light photocatalytic activity of copper-doped titanium oxide–zinc oxide heterojunction for methyl orange degradation. Appl. Surf. Sci. 2017, 414, 251–261. [Google Scholar] [CrossRef]
- Lu, L.; Shan, R.; Shi, Y.; Wang, S.; Yuan, H. A novel TiO2/biochar composite catalysts for photocatalytic degradation of methyl orange. Chemosphere 2019, 222, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Matos, J.; Herna, J.C.; Atienzar, P.; Garcı, H. Nanocrystalline carbon–TiO2 hybrid hollow spheres as possible electrodes for solar cells. Carbon 2013, 53, 169–181. [Google Scholar] [CrossRef]
- Matos, J.; Corma, A. General Selective phenol hydrogenation in aqueous phase on Pd-based catalysts supported on hybrid TiO2-carbon materials. Appl. Catal. A Gen. 2011, 404, 103–112. [Google Scholar] [CrossRef]
- Mian, M.; Liu, G. Recent progress in biochar-supported photocatalysts: Synthesis, role of biochar, and applications. RSC Adv. 2018, 8, 14237–14248. [Google Scholar] [CrossRef] [Green Version]
- Yin, S.; Zhang, Q.; Saito, F.; Sato, T. Preparation of visible light-activated titania photocatalyst by mechanochemical method. Chem. Lett. 2003, 32, 358–359. [Google Scholar] [CrossRef] [Green Version]
- Mian, M.; Liu, G. Sewage sludge-derived TiO2/Fe/Fe3C-biochar composite as an efficient heterogeneous catalyst for degradation of methylene blue. Chemosphere 2019, 215, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, S.; Stefanello, N.; Sulkovski, A.; Luiz, E. Preparation of TiO2 supported on MDF biochar for simultaneous removal of methylene blue by adsorption and photocatalysis. J. Chem. Technol. Biotechnol. 2019, 95, 2723–2729. [Google Scholar] [CrossRef]
- Rismanchian, M.; Golbabaei, F.; Mortazavi, Y.; Pourtaghi, G.; Rahimi Foroushani, A.; Nassiri, P. A Comparative Evaluation of TiO2 Suspension Coating Techniques: A Novel Technique to Achieve Optimal Thickness and Uniformity of Photocatalytic Film. Int. J. Photoenergy 2012, 2012, 634802. [Google Scholar] [CrossRef] [Green Version]
- Lisowski, P.; Colmenares, J.C.; Mašek, O. Dual functionality of TiO2/biochar hybrid materials: Photocatalytic phenol degradation in liquid phase and selective oxidation of methanol in gas phase. ACS Sustain. Chem. Eng. 2017, 5, 6274–6287. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Chen, C.; Gray, E.M.; Boyd, S.E. Effect of feedstock and pyrolysis temperature on properties of biochar governing end-use efficacy. Biomass Bioenergy 2017, 105, 136–146. [Google Scholar] [CrossRef]
- Pamphile, N.; Xuejiao, L.; Guangwei, Y.; Yin, W. Synthesis of a novel core-shell-structure activated carbon material and its application in sulfamethoxazole adsorption. J. Hazard. Mater. 2019, 368, 602–612. [Google Scholar] [CrossRef]
- Nielsen, L.; Biggs, M.J.; Skinner, W.; Bandosz, T.J. The effects of activated carbon surface features on the reactive adsorption of carbamazepine and sulfamethoxazole. Carbon 2014, 80, 419–432. [Google Scholar] [CrossRef] [Green Version]
- Moral-Rodríguez, A.I.; Leyva-Ramos, R.; Ocampo-Pérez, R.; Mendoza-Barron, J.; Serratos-Alvarez, I.N.; Salazar-Rabago, J.J. Removal of ronidazole and sulfamethoxazole from water solutions by adsorption on granular activated carbon: Equilibrium and intraparticle diffusion mechanisms. Adsorption 2016, 22, 89–103. [Google Scholar] [CrossRef]
- Nam, S.W.; Jung, C.; Li, H.; Yu, M.; Flora, J.R.V.; Boateng, L.K.; Her, N.; Zoh, K.D.; Yoon, Y. Adsorption characteristics of diclofenac and sulfamethoxazole to graphene oxide in aqueous solution. Chemosphere 2015, 136, 20–26. [Google Scholar] [CrossRef]
- Rostamian, R.; Behnejad, H. A comparative adsorption study of sulfamethoxazole onto graphene and graphene oxide nanosheets through equilibrium, kinetic and thermodynamic modelling. Process Saf. Environ. Prot. 2016, 102, 20–29. [Google Scholar] [CrossRef]
- Liu, S.; Pan, M.; Feng, Z.; Qin, Y.; Wang, Y.; Tan, L.; Sun, T. Ultra-high adsorption of tetracycline antibiotics on garlic skin-derived porous biomass carbon with high surface area. New J. Chem. 2020, 44, 1097–1106. [Google Scholar] [CrossRef]
- Cuerda-Correa, E.M.; Alexandre-Franco, M.F.; Fern, C. Advanced Oxidation Processes for the Removal of Antibiotics from Water. An Overview. Water 2020, 12, 102. [Google Scholar] [CrossRef] [Green Version]
- Gong, H.; Chu, W. Determination and toxicity evaluation of the generated products in sulfamethoxazole degradation by UV/CoFe2O4/TiO2. J. Hazard. Mater. 2016, 314, 197–203. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Z.; Li, R.; Guo, J.; Li, Y.; Zhu, J.; Xie, X. TiO2 supported on reed straw biochar as an adsorptive and photocatalytic composite for the efficient degradation of sulfamethoxazole in aqueous matrices. Chemosphere 2017, 185, 351–360. [Google Scholar] [CrossRef]
- Schwarz, P.F.; Turro, N.J.; Bossmann, S.H.; Braun, A.M.; Abdel Wahab, A.M.A.; Dürr, H. A new method to determine the generation of hydroxyl radicals in illuminated TiO2 suspensions. J. Phys. Chem. B 1997, 101, 7127–7134. [Google Scholar] [CrossRef]
- Gar Alalm, M.; Tawfik, A.; Ookawara, S. Enhancement of photocatalytic activity of TiO2 by immobilization on activated carbon for degradation of pharmaceuticals. J. Environ. Chem. Eng. 2016, 4, 1929–1937. [Google Scholar] [CrossRef]
- Bems, B.; Jentoft, F.C.; Schlögl, R. Photoinduced decomposition of nitrate in drinking water in the presence of titania and humic acids. Appl. Catal. B Environ. 1999, 20, 155–163. [Google Scholar] [CrossRef] [Green Version]
- Hassani, A.; Khataee, A.; Karaca, S.; Karaca, C.; Gholami, P. Sonocatalytic degradation of ciprofloxacin using synthesized TiO2 nanoparticles on montmorillonite. Ultrason. Sonochem. 2017, 35, 251–262. [Google Scholar] [CrossRef]
- Song, C.; Wang, L.; Ren, J.; Lv, B.; Sun, Z.; Yan, J.; Li, X.; Liu, J. Comparative study of diethyl phthalate degradation by UV/H2O2 and UV/TiO2: Kinetics, mechanism, and effects of operational parameters. Environ. Sci. Pollut. Res. 2016, 23, 2640–2650. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Rao, G.; Rogers, J.; Zhao, C.; Liu, L.; Li, Y. Novel anti-fouling Fe2O3/TiO2 nanowire membranes for humic acid removal from water. Chem. Eng. J. 2015, 271, 180–187. [Google Scholar] [CrossRef]
- Nawaz, M.; Khan, A.A.; Hussain, A.; Jang, J.; Jung, H.Y.; Lee, D.S. Reduced graphene oxide−TiO2/sodium alginate 3-dimensional structure aerogel for enhanced photocatalytic degradation of ibuprofen and sulfamethoxazole. Chemosphere 2020, 261, 127702. [Google Scholar] [CrossRef] [PubMed]
- Awfa, D.; Ateia, M.; Fujii, M.; Yoshimura, C. Novel Magnetic Carbon Nanotube-TiO2 Composites for Solar Light Photocatalytic Degradation of Pharmaceuticals in the Presence of Natural Organic Matter. J. Water Process Eng. 2019, 31, 100836. [Google Scholar] [CrossRef]
- Alfred, M.O.; Omorogie, M.O.; Bodede, O.; Moodley, R.; Ogunlaja, A.; Adeyemi, O.G.; Günter, C.; Taubert, A.; Iermak, I.; Eckert, H.; et al. Solar-active clay-TiO2 nanocomposites prepared via biomass assisted synthesis: Efficient removal of ampicillin, sulfamethoxazole and artemether from water. Chem. Eng. J. 2020, 398, 125544. [Google Scholar] [CrossRef]
- Evgenidou, E.; Chatzisalata, Z.; Tsevis, A.; Bourikas, K.; Torounidou, P.; Sergelidis, D.; Koltsakidou, A.; Lambropoulou, D.A. Photocatalytic degradation of a mixture of eight antibiotics using Cu-modified TiO2 photocatalysts: Kinetics, mineralization, antimicrobial activity elimination and disinfection. J. Environ. Chem. Eng. 2021, 9, 105295. [Google Scholar] [CrossRef]
- Asha, R.C.; Yadav, M.S.P.; Kumar, M. Sulfamethoxazole Removal in Membrane-Photocatalytic Reactor System—Experimentation and Modelling. Environ. Technol. 2019, 40, 1607–1704. [Google Scholar] [CrossRef]
- Ricardo, P.; Pretto, P.; Palácio, S.M.; de Campos, É.A.; Pazini, C.R.; Veit, M.T. Sulfamethoxazole photocatalytic degradation in a continuous flow reactor using artificial radiation. J. Environ. Chem. Eng. 2018, 6, 1926–1933. [Google Scholar] [CrossRef]
- Ling, C.; Yue, C.; Yuan, R.; Qiu, J.; Liu, F.; Zhu, J. Enhanced removal of sulfamethoxazole by a novel composite of TiO2 nanocrystals in situ wrapped-Bi2O4 microrods under simulated solar irradiation. Chem. Eng. J. 2020, 384, 123278. [Google Scholar] [CrossRef]
- Martins, A.C.; Cazetta, A.L.; Pezoti, O.; Souza, J.R.B.; Zhang, T.; Pilau, E.J.; Asefa, T.; Almeida, V.C. Sol-gel synthesis of new TiO2/activated carbon photocatalyst and its application for degradation of tetracycline. Ceram. Int. 2017, 43, 4411–4418. [Google Scholar] [CrossRef]
- Peñas-Garzón, M.; Gómez-Avilés, A.; Bedia, J.; Rodriguez, J.J.; Belver, C. Effect of activating agent on the properties of TiO2/activated carbon heterostructures for solar photocatalytic degradation of acetaminophen. Materials 2019, 12, 378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.Y.; Zheng, Y.F.; Song, X.C. Biomass Assisted Synthesis of 3D Hierarchical Structure BiOX(X Cl, Br)-(CMC) with Enhanced Photocatalytic Activity. J. Nanosci. Nanotechnol. 2019, 19, 5287–5294. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.P.; Pereira, D.; Calisto, V.; Martins, M.A.; Otero, M.; Esteves, V.I.; Lima, D.L.D. Biochar-TiO2 magnetic nanocomposites for photocatalytic solar-driven removal of antibiotics from aquaculture effluents. J. Environ. Manag. 2021, 294, 112937. [Google Scholar] [CrossRef]
- Karaolia, P.; Michael-Kordatou, I.; Hapeshi, E.; Drosou, C.; Bertakis, Y.; Christofilos, D.; Armatas, G.S.; Sygellou, L.; Schwartz, T.; Xekoukoulotakis, N.P.; et al. Removal of antibiotics, antibiotic-resistant bacteria and their associated genes by graphene-based TiO2 composite photocatalysts under solar radiation in urban wastewaters. Appl. Catal. B Environ. 2018, 224, 810–824. [Google Scholar] [CrossRef]
- Zhu, N.; Li, C.; Bu, L.; Tang, C.; Wang, S.; Duan, P.; Yao, L.; Tang, J.; Dionysiou, D.D.; Wu, Y. Bismuth impregnated biochar for efficient estrone degradation: The synergistic effect between biochar and Bi/Bi2O3 for a high photocatalytic performance. J. Hazard. Mater. 2020, 384, 121258. [Google Scholar] [CrossRef]
- Song, W.; Zhao, J.; Xie, X.; Liu, W.; Liu, S.; Chang, H.; Wang, C. Novel BiOBr by compositing low-cost biochar for efficient ciprofloxacin removal: The synergy of adsorption and photocatalysis on the degradation kinetics and mechanism insight. RSC Adv. 2021, 11, 15369–15379. [Google Scholar] [CrossRef]
- Thiruppathi, M.; Leeladevi, K.; Ramalingan, C.; Chen, K.C.; Nagarajan, E.R. Construction of novel biochar supported copper tungstate nanocomposites: A fruitful divergent catalyst for photocatalysis and electrocatalysis. Mater. Sci. Semicond. Process. 2020, 106, 104766. [Google Scholar] [CrossRef]










| S. No. | Type of Photocatalyst | Synthesis Method | pH | Light Irradiation Range | SMX Removal (%) | References |
|---|---|---|---|---|---|---|
| 1 | UV light without photocatalyst | - | 4 | UV range (200–400 nm) | 47.24 | [88] |
| 2 | TiO2 | - | 4 | UV range (200–400 nm) | 58.47 | |
| 3 | TiO2/Biochar | Sol–gel | 4 | UV range (200–400 nm) | 91.27 | |
| 4 | TiO2/Biochar | Sol–gel | 5.95 | UV range (200–400 nm) | 82.24 | |
| 5 | TiO2/Biochar | Sol–gel | 8.53 | UV range (200–400 nm) | 65.17 | |
| 6 | TiO2/Biochar | Sol–gel | 10.77 | UV range (200–400 nm) | 40.58 | |
| 7 | RGOT/SA (Reduced graphene oxide TiO2/Sodium alginate) | - | UV range (200–400 nm) | 77.6 | [95] | |
| 8 | MCNT/TiO2 (Multiwalled carbon nano tube/TiO2) | Acid catalysed Sol–gel | - | UV range (200–400 nm) | 90 | [96] |
| 9 | TiO2/Biochar | Sol–gel | - | UV range (200–400 nm) | 91 | [38] |
| 10 | ZnO-TiO2/Biochar | Modified Sol–gel | 3.95 | UV irradiation (λ < 410 nm) | 78.34 | [39] |
| 11 | ZnO-TiO2/Biochar | 5.03 | 81.21 | |||
| 12 | ZnO-TiO2/Biochar | 6.92 | 75.48 | |||
| 13 | ZnO-TiO2/Biochar | 8.95 | 71.10 | |||
| 14 | Clay-TiO2 composite | Sol–gel | - | UV irradiation | 70.2 | [97] |
| 15 | Cu-TiO2 | Sol–gel | - | UV-visible (300–800 nm) | 94% | [98] |
| 16 | TiO2-GAC-MPR (Activated Carbon-Membrane photobioreactor | Sol–gel | - | UV irradiation | 83.60 | [99] |
| 17 | TiO2-Borosilicate Glass | Solvothermal | - | UV irradiation | 70 | [100] |
| 18 | Bi2O4–TiO2 | Hydrothermal | 5.0 | UV-Visible (190–1100 nm) | 90 | [101] |
| S. No. | Composite Material | Synthesis Method Used | BET Surface Area (m2/g) | Pore Volume (cc/g) | Targeted Antibiotic | Mechanism of Removal | Removal Efficiency | References |
|---|---|---|---|---|---|---|---|---|
| 1 | AC/TiO2 | Sol–gel | 129 | 0.30 | Tetracycline | Photocatalysis | ~97% | [102] |
| 2 | BC/Zn/TiO2 | Solvothermal | 435 | - | acetaminophen | Photocatalysis | 92% | [103] |
| 3 | BC-BiOCl | One step hydrolysis | 3,546 | 0.011 | Tetracycline | Photocatalysis | 60.3% | [104] |
| 4 | BC-TiO2 | Ultrasound promoted wet impregnation | 399 | - | Phenol | Photocatalysis | 64.1% (UV light) 55667733.6% (Visible light) | [78] |
| 5 | Magnetic BC/TiO2 | Solvothermal | - | - | Sulfadiazine | Photocatalysis | ~88% | [105] |
| 6 | TiO2/rGO | Hydrothermal | 48.09 | - | Sulfamethoxazole | Photocatalysis | ~90% | [106] |
| 7 | rGO/TiO2/Na Alginate | Hydrothermal | - | - | Azithromycin | Photocatalysis | ~99% | [81] |
| 8 | Bi/Bi2O3/BC | Thermal method | 338.2 | 0.161 | Estrone | Photocatalysis | ~90% | [107] |
| 9 | BioBr/BC | Solvothermal | - | - | Ciprofloxacin | Photocatalysis | 96.8% | [108] |
| 10 | CuWO4/BC | Hydrothermal | 6.8104 | - | Ciprofloxacin | Photocatalysis | 97% | [109] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chandra, S.; Jagdale, P.; Medha, I.; Tiwari, A.K.; Bartoli, M.; Nino, A.D.; Olivito, F. Biochar-Supported TiO2-Based Nanocomposites for the Photocatalytic Degradation of Sulfamethoxazole in Water—A Review. Toxics 2021, 9, 313. https://doi.org/10.3390/toxics9110313
Chandra S, Jagdale P, Medha I, Tiwari AK, Bartoli M, Nino AD, Olivito F. Biochar-Supported TiO2-Based Nanocomposites for the Photocatalytic Degradation of Sulfamethoxazole in Water—A Review. Toxics. 2021; 9(11):313. https://doi.org/10.3390/toxics9110313
Chicago/Turabian StyleChandra, Subhash, Pravin Jagdale, Isha Medha, Ashwani Kumar Tiwari, Mattia Bartoli, Antonio De Nino, and Fabrizio Olivito. 2021. "Biochar-Supported TiO2-Based Nanocomposites for the Photocatalytic Degradation of Sulfamethoxazole in Water—A Review" Toxics 9, no. 11: 313. https://doi.org/10.3390/toxics9110313
APA StyleChandra, S., Jagdale, P., Medha, I., Tiwari, A. K., Bartoli, M., Nino, A. D., & Olivito, F. (2021). Biochar-Supported TiO2-Based Nanocomposites for the Photocatalytic Degradation of Sulfamethoxazole in Water—A Review. Toxics, 9(11), 313. https://doi.org/10.3390/toxics9110313