Effects of Simultaneous Application of Double Chelating Agents to Pb-Contaminated Soil on the Phytoremediation Efficiency of Indocalamus decorus Q. H. Dai and the Soil Environment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Preparation and Experimental Materials
2.2. Experimental Setup
2.3. Plant Analysis
2.3.1. Plant Biomass
2.3.2. Pb Level in Plant Organs
2.3.3. BCF and TF Analysis
2.4. Soil Analysis
2.4.1. Water-Soluble Pb
2.4.2. Morphological Pb Analysis
2.4.3. Determination of Soil-Enzyme Activities
2.5. Statistical Analysis
3. Results
3.1. Plant Biomass
3.2. Pb Level in Plants
3.3. Bioaccumulation and Transfer of Pb in Plants
3.4. Water-Soluble Pb in Soil
3.5. Morphological Distribution of Pb in the Soil
3.6. Soil-Enzyme Activity
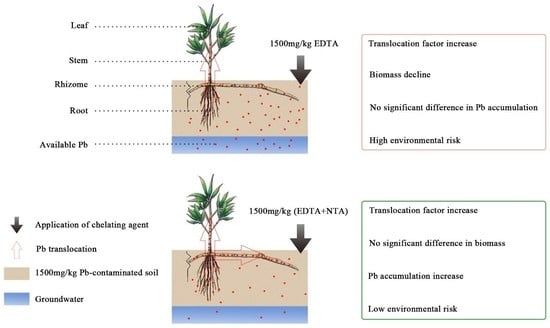
4. Discussion
4.1. Effects of Applying Chelating Agents on Plant Growth
4.2. Effects of Chelating Agents on Pb Uptake in Plants and Phytoremediation Efficiency
4.3. Effect of Chelating Agents on the Soil Environment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elturk, M.; Abdullah, R.; Rozainah, M.; Abu Bakar, N.K. Evaluation of heavy metals and environmental risk assessment in the Mangrove Forest of Kuala Selangor estuary, Malaysia. Mar. Pollut. Bull. 2018, 136, 1–9. [Google Scholar] [CrossRef]
- Jiang, B.; Adebayo, A.; Jia, J.; Xing, Y.; Deng, S.; Guo, L.; Liang, Y.; Zhang, D. Impacts of heavy metals and soil properties at a Nigerian e-waste site on soil microbial community. J. Hazard. Mater. 2018, 362, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Li, Z.; Liu, J.; Bi, X.; Ning, Y.; Yang, S.; Yang, X. Apportionment of sources of heavy metals to agricultural soils using isotope fingerprints and multivariate statistical analyses. Environ. Pollut. 2019, 249, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Datko-Williams, L.; Wilkie, A.; Richmond-Bryant, J. Analysis of U.S. soil lead (Pb) studies from 1970 to 2012. Sci. Total Environ. 2013, 468–469, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, A.; Hans, N.; Kumar, S.; Rani, R. A critical review on speciation, mobilization and toxicity of lead in soil-microbe-plant system and bioremediation strategies. Ecotoxicol. Environ. Saf. 2018, 147, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Wang, X.; Tong, W.; Gurajala, H.K.; Lu, M.; Hamid, Y.; Feng, Y.; He, Z.; Yang, X. Distribution, availability and translocation of heavy metals in soil-oilseed rape (Brassica napus L.) system related to soil properties. Environ. Pollut. 2019, 252, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Gabriele, I.; Race, M.; Papirio, S.; Esposito, G. Phytoremediation of pyrene-contaminated soils: A critical review of the key factors affecting the fate of pyrene. J. Environ. Manag. 2021, 293, 112805. [Google Scholar] [CrossRef] [PubMed]
- Mahar, A.; Wang, P.; Ali, A.; Awasthi, M.K.; Lahori, A.H.; Wang, Q.; Li, R.; Zhang, Z. Challenges and opportunities in the phytoremediation of heavy metals contaminated soils: A review. Ecotoxicol. Environ. Saf. 2016, 126, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Bian, F.; Zhong, Z.; Zhang, X.; Yang, C.; Gai, X. Bamboo–An untapped plant resource for the phytoremediation of heavy metal contaminated soils. Chemosphere 2019, 246, 125750. [Google Scholar] [CrossRef] [PubMed]
- Zhong, B.; Chen, J.; Shafi, M.; Guo, J.; Wang, Y.; Wu, J.; Ye, Z.; He, L.; Liu, D. Effect of lead (Pb) on antioxidation system and accumulation ability of Moso bamboo (Phyllostachys pubescens). Ecotoxicol. Environ. Saf. 2017, 138, 71–77. [Google Scholar] [CrossRef]
- Jiang, M.; Liu, S.; Li, Y.; Li, X.; Luo, Z.; Song, H.; Chen, Q. EDTA-facilitated toxic tolerance, absorption and translocation and phytoremediation of lead by dwarf bamboos. Ecotoxicol. Environ. Saf. 2019, 170, 502–512. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Jiang, M.; Liao, J.; Yang, Y.; Li, N.; Cheng, Q.; Li, X.; Song, H.; Luo, Z.; Liu, S. Biomass allocation strategies and Pb-enrichment characteristics of six dwarf bamboos under soil Pb stress. Ecotoxicol. Environ. Saf. 2020, 207, 111500. [Google Scholar] [CrossRef]
- Cai, X.; Liao, J.; Yang, Y.; Li, N.; Xu, M.; Jiang, M.; Chen, Q.; Li, X.; Liu, S.; Luo, Z.; et al. Physiological resistance of Sasa argenteostriata (Regel) E.G. Camus in response to high-concentration soil Pb stress. Acta Physiol. Plant. 2021, 43, 21. [Google Scholar] [CrossRef]
- Zhang, X.; Zhong, B.; Shafi, M.; Guo, J.; Liu, C.; Guo, H.; Peng, D.; Wang, Y.; Liu, D. Effect of EDTA and citric acid on absorption of heavy metals and growth of Moso bamboo. Environ. Sci. Pollut. Res. 2018, 25, 18846–18852. [Google Scholar] [CrossRef]
- Gul, I.; Manzoor, M.; Kallerhoff, J.; Arshad, M. Enhanced phytoremediation of lead by soil applied organic and inorganic amendments: Pb phytoavailability, accumulation and metal recovery. Chemosphere 2020, 258, 127405. [Google Scholar] [CrossRef] [PubMed]
- Gul, I.; Manzoor, M.; Hashmi, I.; Bhatti, M.F.; Kallerhoff, J.; Arshad, M. Plant uptake and leaching potential upon application of amendments in soils spiked with heavy metals (Cd and Pb). J. Environ. Manag. 2019, 249, 109408. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, Q.; Cui, Y.; Dong, Y.; Christie, P. Slow release chelate enhancement of lead phytoextraction by corn (Zea mays L.) from contaminated soil—A preliminary study. Sci. Total Environ. 2005, 339, 179–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Guo, Q.; Yang, J.; Ma, J.; Chen, G.; Chen, T.; Zhu, G.; Wang, J.; Zhang, G.; Wang, X.; et al. Comparison of chelates for enhancing Ricinus communis L. phytoremediation of Cd and Pb contaminated soil. Ecotoxicol. Environ. Saf. 2016, 133, 57–62. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Wu, Q.; He, Z. Synergetic effects of DA-6/GA 3 with EDTA on plant growth, extraction and detoxification of Cd by Lolium perenne. Chemosphere 2014, 117, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Attinti, R.; Barrett, K.R.; Datta, R.; Sarkar, D. Ethylenediaminedisuccinic acid (EDDS) enhances phytoextraction of lead by vetiver grass from contaminated residential soils in a panel study in the field. Environ. Pollut. 2017, 225, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Ali, A.; Ren, C.; Du, J.; Li, R.; Lahori, A.H.; Xiao, R.; Zhang, Z.; Zhang, Z. EDTA and organic acids assisted phytoextraction of Cd and Zn from a smelter contaminated soil by potherb mustard (Brassica juncea, Coss) and evaluation of its bioindicators. Ecotoxicol. Environ. Saf. 2018, 167, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Hseu, Z.-Y.; Jien, S.-H.; Wang, S.-H.; Deng, H.-W. Using EDDS and NTA for enhanced phytoextraction of Cd by water spinach. J. Environ. Manag. 2013, 117, 58–64. [Google Scholar] [CrossRef]
- Zhao, L.; Li, T.; Yu, H.; Zhang, X.; Zheng, Z. Effects of [S,S]-ethylenediaminedisuccinic acid and nitrilotriacetic acid on the efficiency of Pb phytostabilization by Athyrium wardii (Hook.) grown in Pb-contaminated soils. J. Environ. Manag. 2016, 182, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Liu, X.; Zhang, X.; Cao, L.; Chen, J.; Yu, H. Increased accumulation of Pb and Cd from contaminated soil with Scirpus triqueter by the combined application of NTA and APG. Chemosphere 2017, 188, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Cui, Y.; Li, Q.; Sun, J. Effective removal of heavy metals from industrial sludge with the aid of a biodegradable chelating ligand GLDA. J. Hazard. Mater. 2015, 283, 748–754. [Google Scholar] [CrossRef]
- Guo, X.; Zhao, G.; Zhang, G.; He, Q.; Wei, Z.; Zheng, W.; Qian, T.; Wu, Q. Effect of mixed chelators of EDTA, GLDA, and citric acid on bioavailability of residual heavy metals in soils and soil properties. Chemosphere 2018, 209, 776–782. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, S.; Zhong, Q.; Peijnenburg, W.J.; Vijver, M.G. Feasibility of Chinese cabbage (Brassica bara) and lettuce (Lactuca sativa) cultivation in heavily metals−contaminated soil after washing with biodegradable chelators. J. Clean. Prod. 2018, 197, 479–490. [Google Scholar] [CrossRef] [Green Version]
- Edwards, J.; Johnson, C.; Santos-Medellín, C.; Lurie, E.; Podishetty, N.K.; Bhatnagar, S.; Eisen, J.A.; Sundaresan, V. Structure, variation, and assembly of the root-associated microbiomes of rice. Proc. Natl. Acad. Sci. USA 2015, 112, E911–E920. [Google Scholar] [CrossRef] [Green Version]
- Anning, A.K.; Akoto, R. Assisted phytoremediation of heavy metal contaminated soil from a mined site with Typha latifolia and Chrysopogon zizanioides. Ecotoxicol. Environ. Saf. 2018, 148, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Y.; Mahmood, Q.; Islam, E.; Jin, X.; Li, T.; Yang, X.; Liu, D. The effect of EDDS addition on the phytoextraction efficiency from Pb contaminated soil by Sedum alfredii Hance. J. Hazard. Mater. 2009, 168, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Rauret, G.; López-Sánchez, J.F.; Sahuquillo, A.; Rubio, R.; Davidson, C.; Ure, A.; Quevauviller, P. Improvement of the BCR three step sequential extraction procedure prior to the certification of new sediment and soil reference materials. J. Environ. Monit. 1999, 1, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Willis, R.B.; Montgomery, M.E.; Allen, P.R. Improved Method for Manual, Colorimetric Determination of Total Kjeldahl Nitrogen Using Salicylate. J. Agric. Food Chem. 1996, 44, 1804–1807. [Google Scholar] [CrossRef]
- Gonçalves, C.; Rodriguez-Jasso, R.M.; Gomes, N.; Teixeira, J.A.; Belo, I. Adaptation of dinitrosalicylic acid method to microtiter plates. Anal. Methods 2010, 2, 2046–2048. [Google Scholar] [CrossRef] [Green Version]
- Shang, Z.; Wu, Z.; Li, D.; Zhu, P.; Gao, H.; Zhang, L.; Gong, P. The activity and kinetic parameters of oxidoreductases in phaeozem in response to long-term fertiliser management. J. Soil Sci. Plant Nutr. 2012, 12, 597–607. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Zhang, L.; Gu, J.; Zhao, J.; Fu, J. Citric acid and EDTA on the growth, photosynthetic properties and heavy metal accumulation of Iris halophila Pall. cultivated in Pb mine tailings. Int. Biodeterior. Biodegradation 2018, 128, 15–21. [Google Scholar] [CrossRef]
- Ruley, A.T.; Sharma, N.C.; Sahi, S.V.; Singh, S.R.; Sajwan, K.S. Effects of lead and chelators on growth, photosynthetic activity and Pb uptake in Sesbania drummondii grown in soil. Environ. Pollut. 2006, 144, 11–18. [Google Scholar] [CrossRef]
- Borowiec, M.; Huculak, M.; Hoffmann, K.; Hoffmann, J. Biodegradation of selected substances used in liquid fertilizers as an element of Life Cycle Assessment. Pol. J. Chem. Technol. 2009, 11, 1–3. [Google Scholar] [CrossRef]
- Wang, K.; Liu, Y.; Song, Z.; Wang, D.; Qiu, W. Chelator complexes enhanced Amaranthus hypochondriacus L. phytoremediation efficiency in Cd-contaminated soils. Chemosphere 2019, 237, 124480. [Google Scholar] [CrossRef]
- Brunet, J.; Varrault, G.; Zuily-Fodil, Y.; Repellin, A. Accumulation of lead in the roots of grass pea (Lathyrus sativus L.) plants triggers systemic variation in gene expression in the shoots. Chemosphere 2009, 77, 1113–1120. [Google Scholar] [CrossRef]
- Gupta, D.; Nicoloso, F.; Schetinger, M.; Rossato, L.; Pereira, L.; Castro, G.; Srivastava, S.; Tripathi, R. Antioxidant defense mechanism in hydroponically grown Zea mays seedlings under moderate lead stress. J. Hazard. Mater. 2009, 172, 479–484. [Google Scholar] [CrossRef]
- Jiang, W.; Liu, D. Pb-induced cellular defense system in the root meristematic cells of Allium sativum L. BMC Plant Biol. 2010, 10, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonçalves, A.C.; Schwantes, D.; de Sousa, R.F.B.; da Silva, T.R.B.; Guimarães, V.F.; Campagnolo, M.A.; de Vasconcelos, E.S.; Zimmermann, J. Phytoremediation capacity, growth and physiological responses of Crambe abyssinica Hochst on soil contaminated with Cd and Pb. J. Environ. Manag. 2020, 262, 110342. [Google Scholar] [CrossRef] [PubMed]
- Neugschwandtner, R.W.; Tlustoš, P.; Komárek, M.; Száková, J. Phytoextraction of Pb and Cd from a contaminated agricultural soil using different EDTA application regimes: Laboratory versus field scale measures of efficiency. Geoderma 2008, 144, 446–454. [Google Scholar] [CrossRef]
- Liu, D.; Islam, E.; Li, T.; Yang, X.; Jin, X.; Mahmood, Q. Comparison of synthetic chelators and low molecular weight organic acids in enhancing phytoextraction of heavy metals by two ecotypes of Sedum alfredii Hance. J. Hazard. Mater. 2008, 153, 114–122. [Google Scholar] [CrossRef]
- Barrutia, O.; Garbisu, C.; Hernández-Allica, J.; García-Plazaola, J.I.; Becerril, J.M. Differences in EDTA-assisted metal phytoextraction between metallicolous and non-metallicolous accessions of Rumex acetosa L. Environ. Pollut. 2010, 158, 1710–1715. [Google Scholar] [CrossRef]
- Yan, L.; Li, C.; Zhang, J.; Moodley, O.; Liu, S.; Lan, C.; Gao, Q.; Zhang, W. Enhanced Phytoextraction of Lead from Artificially Contaminated Soil by Mirabilis jalapa with Chelating Agents. Bull. Environ. Contam. Toxicol. 2017, 99, 208–212. [Google Scholar] [CrossRef]
- Saifullah; Meers, E.; Qadir, M.; de Caritat, P.; Tack, F.; Du Laing, G.; Zia, M. EDTA-assisted Pb phytoextraction. Chemosphere 2009, 74, 1279–1291. [Google Scholar] [CrossRef]
- Tandy, S.; Schulin, R.; Nowack, B. The influence of EDDS on the uptake of heavy metals in hydroponically grown sunflowers. Chemosphere 2006, 62, 1454–1463. [Google Scholar] [CrossRef]
- Hernández-Allica, J.; Garbisu, C.; Barrutia, O.; Becerril, J.M. EDTA-induced heavy metal accumulation and phytotoxicity in cardoon plants. Environ. Exp. Bot. 2007, 60, 26–32. [Google Scholar] [CrossRef]
- Bell, P.F.; Mclaughlin, M.J.; Cozens, G.; Stevens, D.P.; Owens, G.; South, H. Plant Uptake of 14 C-EDTA, 14 C-Citrate, and 14 C-Histidine from Chelator-Buffered and Conventional Hydroponic Solutions. Plant Soil 2003, 253, 311–319. [Google Scholar] [CrossRef]
- Zhan, J.; Zhang, Q.; Li, T.; Yu, H.; Zhang, X.; Huang, H. Effects of NTA on Pb phytostabilization efficiency of Athyrium wardii (Hook.) grown in a Pb-contaminated soil. J. Soils Sediments 2019, 19, 3576–3584. [Google Scholar] [CrossRef]
- Yu, H.; Zhan, J.; Zhang, Q.; Huang, H.; Zhang, X.; Wang, Y.; Li, T. NTA-enhanced Pb remediation efficiency by the phytostabilizer Athyrium wardii (Hook.) and associated Pb leaching risk. Chemosphere 2020, 246, 125815. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Zhou, Q.-X.; Wei, S.-H.; Zhang, W.; Cao, L.; Ren, L.-P. Effects of exogenous chelators on phytoavailability and toxicity of Pb in Zinnia elegans Jacq. J. Hazard. Mater. 2007, 146, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Evangelou, M.W.; Ebel, M.; Schaeffer, A. Chelate assisted phytoextraction of heavy metals from soil. Effect, mechanism, toxicity, and fate of chelating agents. Chemosphere 2007, 68, 989–1003. [Google Scholar] [CrossRef]
- Saifullah; Shahid, M.; Zia-Ur-Rehman, M.; Sabir, M.; Ahmad, H.R. Phytoremediation of Pb-Contaminated Soils Using Synthetic Chelates. In Soil Remediation and Plants: Prospects and Challenges; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 397–414. ISBN 9780127999135. [Google Scholar]
- Begum, Z.A.; Rahman, I.M.M.; Tate, Y.; Egawa, Y.; Maki, T.; Hasegawa, H. Formation and Stability of Binary Complexes of Divalent Ecotoxic Ions (Ni, Cu, Zn, Cd, Pb) with Biodegradable Aminopolycarboxylate Chelants (dl-2-(2-Carboxymethyl)Nitrilotriacetic Acid, GLDA, and 3-Hydroxy-2,2′-Iminodisuccinic Acid, HIDS) in Aqueous Solutions. J. Solut. Chem. 2012, 41, 1713–1728. [Google Scholar] [CrossRef]
- Schwab, A.; Zhu, D.; Banks, M. Influence of organic acids on the transport of heavy metals in soil. Chemosphere 2008, 72, 986–994. [Google Scholar] [CrossRef]
- Udovic, M.; Lestan, D. Pb, Zn and Cd mobility, availability and fractionation in aged soil remediated by EDTA leaching. Chemosphere 2009, 74, 1367–1373. [Google Scholar] [CrossRef]
- Duan, C.; Fang, L.; Yang, C.; Chen, W.; Cui, Y.; Li, S. Reveal the response of enzyme activities to heavy metals through in situ zymography. Ecotoxicol. Environ. Saf. 2018, 156, 106–115. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Razavi, B.S. Rhizosphere size and shape: Temporal dynamics and spatial stationarity. Soil Biol. Biochem. 2019, 135, 343–360. [Google Scholar] [CrossRef]




| Treatment | Soil Pb Concentration (mg/kg) | Type and Concentration of Chelating Agent (mg/kg) |
|---|---|---|
| CK | 1500 | - |
| E | 1500 | EDTA 1500 |
| N | 1500 | NTA 1500 |
| G | 1500 | GLDA 1500 |
| EN | 1500 | EDTA 750 + NTA 750 |
| EG | 1500 | EDTA 750 + GLDA 750 |
| Treatment | BCF | TF | |
|---|---|---|---|
| Underground Part | Aerial Part | ||
| CK | 0.28 ± 0.01 c | 0.02 ± 0.00 d | 0.06 ± 0.00 d |
| E | 0.28 ± 0.01 c | 0.09 ± 0.00 b | 0.33 ± 0.01 a |
| N | 0.34 ± 0.01 b | 0.02 ± 0.00 d | 0.05 ± 0.00 d |
| G | 0.34 ± 0.01 b | 0.02 ± 0.00 d | 0.05 ± 0.00 d |
| EN | 0.42 ± 0.01 a | 0.12 ± 0.00 a | 0.28 ± 0.01 b |
| EG | 0.27 ± 0.01 c | 0.06 ± 0.01 c | 0.21 ± 0.02 c |
| Treatment | Soil-Enzyme Species (Rhizosphere) | Soil-Enzyme Species (Non-Rhizosphere) | ||||
|---|---|---|---|---|---|---|
| Urease (mg/g) | Catalase (mg/g) | Invertase (mg/g) | Urease (mg/g) | Catalase (mg/g) | Invertase (mg/g) | |
| CK | 0.67 ± 0.05 b | 5.15 ± 0.27 c | 45.61 ± 0.94 ab | 0.25 ± 0.01 ab | 4.52 ± 0.12 a | 39.01 ± 1.38 b |
| E | 0.85 ± 0.04 a | 4.14 ± 0.12 d | 42.15 ± 0.89 bc | 0.26 ± 0.01 a | 1.65 ± 0.25 d | 33.21 ± 1.26 d |
| N | 0.87 ± 0.04 a | 6.17 ± 0.32 b | 44.60 ± 2.89 ab | 0.19 ± 0.01 c | 4.79 ± 0.33 a | 36.91 ± 2.01 bc |
| G | 0.71 ± 0.07 b | 5.78 ± 0.19 b | 46.62 ± 2.03 a | 0.18 ± 0.02 cd | 4.86 ± 0.22 a | 43.47 ± 1.28 a |
| EN | 0.86 ± 0.03 a | 5.85 ± 0.12 b | 39.61 ± 1.44 c | 0.23 ± 0.01 b | 3.94 ± 0.25 b | 35.31 ± 0.41 cd |
| EG | 0.67 ± 0.03 b | 7.64 ± 0.23 a | 42.11 ± 3.80 bc | 0.17 ± 0.01 d | 3.51 ± 0.06 c | 36.24 ± 2.54 bc |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Jiang, M.; Liao, J.; Luo, Z.; Gao, Y.; Yu, W.; He, R.; Feng, S. Effects of Simultaneous Application of Double Chelating Agents to Pb-Contaminated Soil on the Phytoremediation Efficiency of Indocalamus decorus Q. H. Dai and the Soil Environment. Toxics 2022, 10, 713. https://doi.org/10.3390/toxics10120713
Yang Y, Jiang M, Liao J, Luo Z, Gao Y, Yu W, He R, Feng S. Effects of Simultaneous Application of Double Chelating Agents to Pb-Contaminated Soil on the Phytoremediation Efficiency of Indocalamus decorus Q. H. Dai and the Soil Environment. Toxics. 2022; 10(12):713. https://doi.org/10.3390/toxics10120713
Chicago/Turabian StyleYang, Yixiong, Mingyan Jiang, Jiarong Liao, Zhenghua Luo, Yedan Gao, Weiqian Yu, Rui He, and Shihan Feng. 2022. "Effects of Simultaneous Application of Double Chelating Agents to Pb-Contaminated Soil on the Phytoremediation Efficiency of Indocalamus decorus Q. H. Dai and the Soil Environment" Toxics 10, no. 12: 713. https://doi.org/10.3390/toxics10120713
APA StyleYang, Y., Jiang, M., Liao, J., Luo, Z., Gao, Y., Yu, W., He, R., & Feng, S. (2022). Effects of Simultaneous Application of Double Chelating Agents to Pb-Contaminated Soil on the Phytoremediation Efficiency of Indocalamus decorus Q. H. Dai and the Soil Environment. Toxics, 10(12), 713. https://doi.org/10.3390/toxics10120713