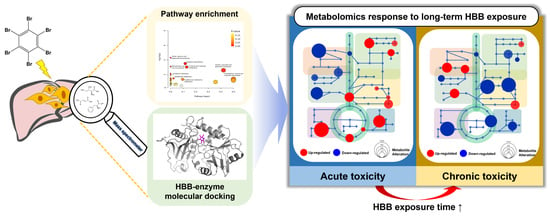
Hepatocellular Metabolic Abnormalities Induced by Long-Term Exposure to Novel Brominated Flame Retardant, Hexabromobenzene
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Cell Culture Conditions and Treatment with HBB
2.3. Cell Viability
2.4. Metabolite Extraction and Derivatization
2.5. Data Processing and Statistical Analyses
2.6. Molecular Docking
3. Results
3.1. Cytotoxicity of Hepatic Cells under HBB Exposure
3.2. Observation of Hepatic Metabolite Alterations Induced by HBB Exposure
3.3. Metabolic Perturbation Analysis of Acute and Chronic Toxicity Induced by HBB Exposure
3.4. Pathway Enrichment Analysis Affected by HBB Exposure
3.5. Molecular Docking
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Wit, C. An overview of brominated flame retardants in the environment. Chemosphere 2022, 46, 583–624. [Google Scholar] [CrossRef] [PubMed]
- Law, R.J.; Allchin, C.R.; de Boer, J.; Covaci, A.; Herzke, D.; Lepom, P.; Morris, S.; Tronczynski, J.; de Wit, C. Levels and trends of brominated flame retardants in the European environment. Chemosphere 2006, 64, 187–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, T.H.; Lee, Y.J.; Lee, E.; Kim, M.S.; Kwack, S.J.; Kim, K.B.; Chung, K.K.; Kang, T.S.; Han, S.Y.; Lee, J.; et al. Effects of Gestational Exposure to Decabromodiphenyl Ether on Reproductive Parameters, Thyroid Hormone Levels, and Neuronal Development in Sprague-Dawley Rats Offspring. J. Toxicol. Environ. Health Part A 2009, 72, 1296–1303. [Google Scholar] [CrossRef]
- Kim, Y.R.; Harden, F.A.; Toms, L.-M.L.; Norman, R.E. Health consequences of exposure to brominated flame retardants: A systematic review. Chemosphere 2014, 106, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Blake, C.A.; McCoy, G.L.; Hui, Y.Y.; LaVoie, H.A. Perinatal exposure to low-dose DE-71 increases serum thyroid hormones and gonadal osteopontin gene expression. Exp. Biol. Med. 2011, 236, 445–455. [Google Scholar] [CrossRef] [Green Version]
- UNEP. The new POPs under the Stockholm Convention, Stockholm Convention on Persistent Organic Pollutants (Decision SC-10/13), Châtelaine, Switzerland. Available online: http://chm.pops.int/TheConvention/ThePOPs/TheNewPOPs/tabid/2511/Default.aspx (accessed on 19 November 2022).
- Gauthier, L.T.; Hebert, C.E.; Weseloh, D.V.C.; Letcher, R.J. Current-Use Flame Retardants in the Eggs of Herring Gulls (Larus argentatus) from the Laurentian Great Lakes. Environ. Sci. Technol. 2007, 41, 4561–4567. [Google Scholar] [CrossRef]
- Xiong, P.; Yan, X.; Zhu, Q.; Qu, G.; Shi, J.; Liao, C.; Jiang, G. A Review of Environmental Occurrence, Fate, and Toxicity of Novel Brominated Flame Retardants. Environ. Sci. Technol. 2019, 53, 13551–13569. [Google Scholar] [CrossRef]
- Ezechiáš, M.; Covino, S.; Cajthaml, T. Ecotoxicity and biodegradability of new brominated flame retardants: A review. Ecotoxicol. Environ. Saf. 2014, 110, 153–167. [Google Scholar] [CrossRef]
- Hou, R.; Lin, L.; Li, H.; Liu, S.; Xu, X.; Xu, Y.; Jin, X.; Yuan, Y.; Wang, Z. Occurrence, bioaccumulation, fate, and risk assessment of novel brominated flame retardants (NBFRs) in aquatic environments—A critical review. Water Res. 2021, 198, 117168. [Google Scholar] [CrossRef]
- Persson, J.; Wang, T.; Hagberg, J. Temporal trends of decabromodiphenyl ether and emerging brominated flame retardants in dust, air and window surfaces of newly built low-energy preschools. Indoor Air 2019, 29, 263–275. [Google Scholar] [CrossRef]
- Wu, J.-P.; Guan, Y.-T.; Zhang, Y.; Luo, X.-J.; Zhi, H.; Chen, S.-J.; Mai, B.-X. Trophodynamics of Hexabromocyclododecanes and Several Other Non-PBDE Brominated Flame Retardants in a Freshwater Food Web. Environ. Sci. Technol. 2010, 44, 5490–5495. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, Y.; Liang, B.; Chen, T.; Zheng, D.; Zhao, X.; Jing, L.; Zhou, X.; Sun, Z.; Shi, Z. Hepatotoxicity of decabromodiphenyl ethane (DBDPE) and decabromodiphenyl ether (BDE-209) in 28-day exposed Sprague-Dawley rats. Sci. Total Environ. 2020, 705, 135783. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Yu, D.; Yang, L.; Sui, S.; Lv, S.; Bai, Y.; Sun, W.; Wang, Y.; Chen, L.; Sun, Z.; et al. Thyroid function and decabromodiphenyl ethane (DBDPE) exposure in Chinese adults from a DBDPE manufacturing area. Environ. Int. 2019, 133, 105179. [Google Scholar] [CrossRef] [PubMed]
- Goodchild, C.; Karouna-Renier, N.K.; Henry, P.F.; Letcher, R.J.; Schultz, S.L.; Maddox, C.M.; Bean, T.G.; Peters, L.E.; Palace, V.; Fernie, K.J. Thyroid disruption and oxidative stress in American kestrels following embryonic exposure to the alternative flame retardants, EHTBB and TBPH. Environ. Int. 2021, 157, 106826. [Google Scholar] [CrossRef]
- Dong, L.; Wang, S.; Qu, J.; You, H.; Liu, D. New understanding of novel brominated flame retardants (NBFRs): Neuro(endocrine) toxicity. Ecotoxicol. Environ. Saf. 2021, 208, 111570. [Google Scholar] [CrossRef]
- Zhang, Y.; Luo, X.-J.; Wu, J.-P.; Liu, J.; Wang, J.; Chen, S.-J.; Mai, B.-X. Contaminant pattern and bioaccumulation of legacy and emerging organhalogen pollutants in the aquatic biota from an e-waste recycling region in South China. Environ. Toxicol. Chem. 2010, 29, 852–859. [Google Scholar] [CrossRef]
- Usenko, C.Y.; Abel, E.L.; Hopkins, A.; Martinez, G.; Tijerina, J.; Kudela, M.; Norris, N.; Joudeh, L.; Bruce, E.D. Evaluation of Common Use Brominated Flame Retardant (BFR) Toxicity Using a Zebrafish Embryo Model. Toxics 2016, 4, 21. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Guo, W.; Lei, L.; Guo, Y.; Yang, L.; Han, J.; Zhou, B. Bioconcentration and developmental neurotoxicity of novel brominated flame retardants, hexabromobenzene and pentabromobenzene in zebrafish. Environ. Pollut. 2021, 268, 115895. [Google Scholar] [CrossRef]
- Feng, M.; Qu, R.; Li, Y.; Wei, Z.; Wang, Z. Biochemical biomarkers in liver and gill tissues of freshwater fish Carassius auratus following in vivo exposure to hexabromobenzene. Environ. Toxicol. 2014, 29, 1460–1470. [Google Scholar] [CrossRef]
- Yan, S.; Tian, S.; Meng, Z.; Yan, J.; Jia, M.; Sun, W.; Wang, Q.; Diao, J.; Zhu, W.; Zhou, Z. In utero exposure to decabromodiphenyl ethane causes rapid growth in mice cubs by activating glycogenolysis and lipid synthesis. Toxicol. Lett. 2022, 366, 72–80. [Google Scholar] [CrossRef]
- Ji, C.; Wu, H.; Wei, L.; Zhao, J.; Lu, H.; Yu, J. Proteomic and metabolomic analysis of earthworm Eisenia fetida exposed to different concentrations of 2,2′,4,4′-tetrabromodiphenyl ether. J. Proteom. 2013, 91, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wu, Q.; Miao, X.; Fan, T.; Meng, Z.; Chen, X.; Zhu, W. Study on toxicity effects of environmental pollutants based on metabolomics: A review. Chemosphere 2022, 286, 131815. [Google Scholar] [CrossRef] [PubMed]
- Bouhifd, M.; Hartung, T.; Hogberg, H.T.; Kleensang, A.; Zhao, L. Review: Toxicometabolomics. J. Appl. Toxicol. 2013, 33, 1365–1383. [Google Scholar] [CrossRef]
- Abu Bakar, N.; Ibrahim, W.N.W.; Abdullah, C.A.C.; Ramlan, N.F.; Shaari, K.; Shohaimi, S.; Mediani, A.; Nasruddin, N.S.; Kim, C.-H.; Faudzi, S.M.M. Embryonic Arsenic Exposure Triggers Long-Term Behavioral Impairment with Metabolite Alterations in Zebrafish. Toxics 2022, 10, 493. [Google Scholar] [CrossRef]
- Ramirez, T.; Strigun, A.; Verlohner, A.; Huener, H.-A.; Peter, E.; Herold, M.; Bordag, N.; Mellert, W.; Walk, T.; Spitzer, M.; et al. Prediction of liver toxicity and mode of action using metabolomics in vitro in HepG2 cells. Arch. Toxicol. 2018, 92, 893–906. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Aracama, A.; Peijnenburg, A.; Kleinjans, J.; Jennen, D.; van Delft, J.; Hellfrisch, C.; Lommen, A. An untargeted multi-technique metabolomics approach to studying intracellular metabolites of HepG2 cells exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. BMC Genom. 2011, 12, 251. [Google Scholar] [CrossRef] [Green Version]
- Valdiviezo, A.; Kato, Y.; Baker, E.S.; Chiu, W.A.; Rusyn, I. Evaluation of Metabolism of a Defined Pesticide Mixture through Multiple In Vitro Liver Models. Toxics 2022, 10, 566. [Google Scholar] [CrossRef]
- Elje, E.; Mariussen, E.; Moriones, O.H.; Bastús, N.G.; Puntes, V.; Kohl, Y.; Dusinska, M.; Rundén-Pran, E. Hepato(Geno)Toxicity Assessment of Nanoparticles in a HepG2 Liver Spheroid Model. Nanomaterials 2020, 10, 545. [Google Scholar] [CrossRef] [Green Version]
- Cuykx, M.; Rodrigues, R.; Laukens, K.; Vanhaecke, T.; Covaci, A. In vitro assessment of hepatotoxicity by metabolomics: A review. Arch. Toxicol. 2018, 92, 3007–3029. [Google Scholar] [CrossRef]
- Lee, D.-K.; Na, E.; Park, S.; Park, J.H.; Lim, J.; Kwon, S.W. In Vitro Tracking of Intracellular Metabolism-Derived Cancer Volatiles via Isotope Labeling. ACS Central Sci. 2018, 4, 1037–1044. [Google Scholar] [CrossRef]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef]
- Kuwahata, M.; Yoshimura, T.; Sawai, Y.; Amano, S.; Tomoe, Y.; Segawa, H.; Tatsumi, S.; Ito, M.; Ishizaki, S.; Ijichi, C.; et al. Localization of polypyrimidine-tract-binding protein is involved in the regulation of albumin synthesis by branched-chain amino acids in HepG2 cells. J. Nutr. Biochem. 2008, 19, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, T.; Izumi, N.; Charlton, M.R.; Sata, M. Branched-chain amino acids as pharmacological nutrients in chronic liver disease. Hepatology 2011, 54, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Okuno, M.; Moriwaki, H.; Kato, M.; Muto, Y.; Kojima, S. Changes in the Ratio of Branched-Chain to Aromatic Amino Acids Affect the Secretion of Albumin in Cultured Rat Hepatocytes. Biochem. Biophys. Res. Commun. 1995, 214, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Holecek, M. Three targets of branched-chain amino acid supplementation in the treatment of liver disease. Nutrition 2010, 26, 482–490. [Google Scholar] [CrossRef]
- Duff, C.; Baruteau, J. Modelling urea cycle disorders using iPSCs. npj Regen. Med. 2022, 7, 1–9. [Google Scholar] [CrossRef]
- Skowrońska, M.; Albrecht, J. Oxidative and nitrosative stress in ammonia neurotoxicity. Neurochem. Int. 2013, 62, 731–737. [Google Scholar] [CrossRef]
- Parmeggiani, B.; Vargas, C.R. Oxidative stress in urea cycle disorders: Findings from clinical and basic research. Clin. Chim. Acta 2018, 477, 121–126. [Google Scholar] [CrossRef]
- Tessari, P.; Vettore, M.; Millioni, R.; Puricelli, L.; Orlando, R. Effect of liver cirrhosis on phenylalanine and tyrosine metabolism. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 81–86. [Google Scholar] [CrossRef]
- Srinivasan, S.; Torres, A.; de Pouplana, L.R. Inosine in Biology and Disease. Genes 2021, 12, 600. [Google Scholar] [CrossRef]
- Ji, F.; Sreenivasmurthy, S.G.; Wei, J.; Shao, X.; Luan, H.; Zhu, L.; Song, J.; Liu, L.; Li, M.; Cai, Z. Study of BDE-47 induced Parkinson’s disease-like metabolic changes in C57BL/6 mice by integrated metabolomic, lipidomic and proteomic analysis. J. Hazard. Mater. 2019, 378, 120738. [Google Scholar] [CrossRef] [PubMed]
- Noor, K.K.; Ijaz, M.U.; Ehsan, N.; Tahir, A.; Yeni, D.K.; Zihad, S.N.K.; Uddin, S.J.; Ashraf, A.; Simal-Gandara, J. Hepatoprotective role of vitexin against cadmium-induced liver damage in male rats: A biochemical, inflammatory, apoptotic and histopathological investigation. Biomed. Pharmacother. 2022, 150, 112934. [Google Scholar] [CrossRef] [PubMed]
- Tada, Y.; Fujitani, T.; Ogata, A.; Kamimura, H. Flame retardant tetrabromobisphenol A induced hepatic changes in ICR male mice. Environ. Toxicol. Pharmacol. 2007, 23, 174–178. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, X.; Zhang, H.; Li, J.; Chen, J.; Yu, M.; Li, G.; Zhang, R.; Ge, M. Oxidative stress and ferroptosis involved in 2-ethylhexyl diphenyl phosphate -induced hepatotoxicity in chicken. Chem. Interactions 2022, 368, 110216. [Google Scholar] [CrossRef]
- Yang, W.; Wei, S.; Liu, H.; Yu, H. Insights into the structural and conformational requirements of polybrominated diphenyl ethers and metabolites as potential estrogens based on molecular docking. Chemosphere 2011, 84, 328–335. [Google Scholar] [CrossRef]





| No. | Metabolites | VIP Value | p-Value | Fold Change |
|---|---|---|---|---|
| 1 | Valine | 1.258 | 0.0012 | 0.5314 |
| 2 | Ribose | 1.363 | 0.0020 | 0.5026 |
| 3 | Phenylalanine | 1.266 | 0.0045 | 0.5692 |
| 4 | Alanine | 1.188 | 0.0063 | 0.5354 |
| 5 | Urea | 1.254 | 0.0063 | 0.6518 |
| 6 | Mannose | 1.183 | 0.0073 | 0.6026 |
| 7 | Inosine | 1.176 | 0.0076 | 0.6079 |
| 8 | Cysteine | 1.217 | 0.0140 | 0.6463 |
| 9 | Aspartic acid | 1.142 | 0.0175 | 0.5953 |
| 10 | 3-Phosphoglyceric acid | 1.254 | 0.0225 | 0.3386 |
| 11 | Glutamic acid | 1.181 | 0.0239 | 0.6844 |
| 12 | Fructose | 1.106 | 0.0240 | 0.7314 |
| 13 | Malic acid | 1.090 | 0.0272 | 0.7595 |
| 14 | Isoleucine | 1.095 | 0.0293 | 0.6942 |
| 15 | d-Erythrotetrofuranose | 1.093 | 0.0308 | 1.3647 |
| 16 | Leucine | 1.038 | 0.0398 | 0.6883 |
| 17 | Fructose 1-phosphate | 1.051 | 0.0435 | 0.6184 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, B.; Hong, S.H.; Seo, S.; Jeong, C.H.; Kim, J.; Bae, E.; Lee, D.; Shin, J.H.; Shim, M.; Han, S.B.; et al. Hepatocellular Metabolic Abnormalities Induced by Long-Term Exposure to Novel Brominated Flame Retardant, Hexabromobenzene. Toxics 2023, 11, 101. https://doi.org/10.3390/toxics11020101
Shin B, Hong SH, Seo S, Jeong CH, Kim J, Bae E, Lee D, Shin JH, Shim M, Han SB, et al. Hepatocellular Metabolic Abnormalities Induced by Long-Term Exposure to Novel Brominated Flame Retardant, Hexabromobenzene. Toxics. 2023; 11(2):101. https://doi.org/10.3390/toxics11020101
Chicago/Turabian StyleShin, Bohyun, Se Hee Hong, Sumin Seo, Cho Hee Jeong, Jiyu Kim, Eunbin Bae, Donghee Lee, Jung Hoon Shin, Minki Shim, Sang Beom Han, and et al. 2023. "Hepatocellular Metabolic Abnormalities Induced by Long-Term Exposure to Novel Brominated Flame Retardant, Hexabromobenzene" Toxics 11, no. 2: 101. https://doi.org/10.3390/toxics11020101
APA StyleShin, B., Hong, S. H., Seo, S., Jeong, C. H., Kim, J., Bae, E., Lee, D., Shin, J. H., Shim, M., Han, S. B., & Lee, D. -K. (2023). Hepatocellular Metabolic Abnormalities Induced by Long-Term Exposure to Novel Brominated Flame Retardant, Hexabromobenzene. Toxics, 11(2), 101. https://doi.org/10.3390/toxics11020101