Occurrence and Health Effects of Hexabromocyclododecane: An Updated Review
Abstract
:1. Introduction
2. Approach to the Review
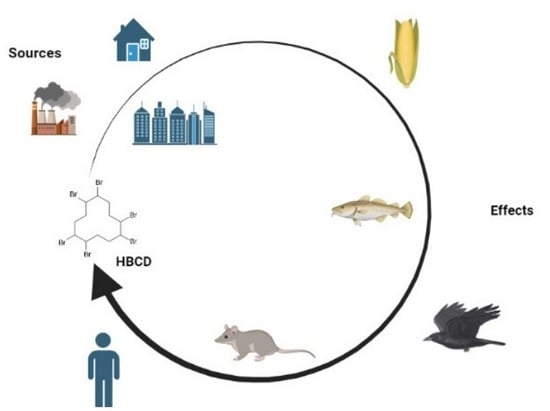
3. Occurrence and Exposure to HBCD
4. Studies in Plants
5. Studies in Animal Models
| Study | Study Organism | Exposure | Effects | Reference |
|---|---|---|---|---|
| In vitro | Juvenile tilapia (Oreochromis mossambicus) | The enzyme supernatant was exposed to HBCD (1.6 × 10−13–1.6 × 10−7 mol/L) | Decreased the EROD activity. | [98] |
| Pseudomonas aeruginosa Strain HS9 | 1.6 × 10−6 mol/L HBCDs mineral salt medium (MSM). | Changed. 277 proteins expression. Upregulated some genes related to heavy metals were. Upregulated cytochrome P450 coding gene cyp168A1. | [99] | |
| The murine cell line 3T3-L1 | Exposure 24 h with HBCD for short-term exposure and exposure with 2.5 × 10−7 mol of HBCD for at least 4 weeks for chronic exposure. | Increased lipid accumulation and monounsaturated fatty acids. No alteration of the PPARα expression and the CIDEA expression. Increased PPARγ expression. | [100] | |
| In vitro and in vivo | Wistar Rats | Primary culture of Leydig cells incubated with HBCD in the presence or absence of hCG (1.95 × 10−10 mol/L), or with only HBCD (0, 1 × 10−6, 5 × 10−6 and 1 × 10−5 mol/L). | Decreased ATP levels. Inhibition of cAMP accumulation. Decreased transcription of some of the genes involved in steroidogenesis. | [73] |
| Earthworms | Cultivation with exposure to HBCD (0, 0.05, 0.1, 0.2 and 0.4 g/Kg dry soil) | Upregulated of SOD and Hsp70. No alteration in CAT levels. No inhibition of growth. Increased oxidative stress in earthworms. | [74] | |
| Zebrafish embryos and rat cardiomyocyte cell line H9C2 | Exposure of the zebrafish to HBCD (0.2 × 10−9, 2 × 10−8, and 2 × 10−7 mol/L). Exposure of the cell line H9C2 to the same concentrations of HBCD. | Increased ventricular wall thickness in the hearts. Increased collagen deposition in the extracellular matrix. Increased atrial and brain natriuretic peptide levels. Cardiac hypertrophy. Disturbed calcium handling. | [75] | |
| C57BL/6J mice | Orally dose with 0.025 g/Kg of HBCD for 30 days. Ventral mesencephalic neurons cultures with HBCD (0, 1.75 × 10−6, 2.5 × 10−6, 5 × 10−6, 7.5 × 10−6 and 1 × 10−5 mol/L). | Reduced cell viability. Decreased total number of TH+ neurons and reduction in neurite branching and neurite length of the TH+ neurons. Reduction in the expression of plasmalemmal DAT and VMAT2. | [76] | |
| Zebrafish | Specific concentrations of specific HBCD diastereoisomers (α- HBCD, β-HBCD and γ-HBCD at 1.6 × 10−9, 1.6 × 10−8, and 1.6 × 10−7 mol/L. | Different expression patterns of the AHRs. α-HBCD and β-HBCD increased the expressions of ahr 1a and ahr1b. γ-HBCD decreased the expressions of ahr1a and ahr1b. Changed EROD activity. | [77] | |
| Zebrafish | Exposure to HBCD (1.9 × 10−6, 3.9 × 10−6, 7.8 × 10−6, 1.6 × 10−5, and 3.12 × 10−5 mol/L). | Increased curved body malformations. Decreased rate of spontaneous movement. Increased level of GST activity. | [78] | |
| Winstar wu Rats | The HBCD was dissolved in corn oil (0.0001 L corn oil added to 0.0009 L custard pudding), and the rats were fed with 0.0005 L of custard pudding. After 4 days of exposure, the rats were orally administered 0, 0.003, and 0.03 g/Kg body weight of HBCD. | Modulation of the proteins involved in metabolic processes and connected with oxidative stress reactions. Decreased hydroxymethylglutaryl-CoA synthase levels. No alteration on gluconeogenesis/glycolysis and the metabolism of several amino acids. No alteration in oxidative stress-related proteins, such as BDH, AL1L1, and ETFB. | [79] | |
| BALB/c mice | Two different concentrations of α-HBCD (0.0001 and 0.1 g/Kg Body weight per day) | Modulation of the hepatic TAG levels. Increased of the AST levels. Reduced of eiosadienoic acid. Increased of total saturated FAs. Increased the expression of peroxisome proliferator-activated receptor alpha. | [80] | |
| Rats | Male euthyroid rats (mET) and hypothyroid (mHT) rats were fed with 0, 0.003, and 0.03 g/Kg body weight of HBCD. | Increased corticosterone in mHT. In mET two proteins changes. In mHT 6 proteins changes. In mET male the accumulation of HBCD in adipose tissue was lower than in females. Alterations in lipid metabolism, glycolysis/gluconeogenesis, redox and CYP protein-related responses. | [81] | |
| Earthworms | Exposure to HBCD (0, 0.05, 0.1, 0.2, 0.4, and 0.6 g/Kg dry soil) | Modulation of the SOD and GST levels. Increased anaerobic respiration. Increased ATP production. Increased of amino acids release. Changes in osmotic pressure. | [82] | |
| BALB/c mice | Exposure to 0.199 g/Kg body weight per day. | 83 genes were altered, 10 upregulated, and 73 downregulated. Decreased in glutamate-dependent Ca2+ signaling. | [83] | |
| BALB/c mice | Exposure to 6 × 10−9 g/Kg in the diet. | Increased vacuolation in hepatocytes, lymphocytic infiltration, and hyperaemic vessels. Increased stress in thymus. Increased the reduced density of endometrial glands in uterus. Modulation of four proteins in the brain. Modulation of some proteins, such as MAPK14 and HSP A8. | [84] | |
| Carassius auratus | Exposure to 3.12 × 10−9, 3.12 × 10−8, and 3.12 × 10−7 mol/L of HBCD. | Decreased TT4 content. Increased AChE activity in brain. Decreased the swimming activity. | [85] | |
| Fischer Rats | Exposure to 0, 0.25, 1.25, and 5 g/Kg of HBCD. | Modulation of genes expression (involved in metabolism of xenobiotic compounds, steroids and hormones, nuclear receptor activity, cell proliferation, metabolism of glucose and lipids, disruption of the hormonal balance, and oxidative stress). | [86] | |
| nematode Caenorhabditis elegans | Exposure to 0, 2 × 10−10, 2 × 10−9, 2 × 10−8, and 2 × 10−7 mol/L of HBCD. | Transferred effects from the parental generation (F0) to the next (F1). Increased stress-related gene expression in F0. Increased apoptosis and oxidative stress. | [87] | |
| Liver Zebrafish and zebrafish | After 56 days of exposure: decreased, T3 and T4 in the liver. Decreases the ratio of T3/T4 in the liver first and then increases with the increase in exposure concentration under long-term exposure. Inhibited the malondialdehyde (MDA) activity in low exposure levels and increased in high exposure groups. Increased SOD activity first, then decreases with higher concentrations. | [88] | ||
| 3T3-L1 preadipocytes and Hek293 and male C57BL/6 mice | Exposure to HBCD (1 × 10−7–1 × 10−5 mol/L) of the Hek293 cells. 3T3-L1 preadipocytes were exposed to 1 × 10−5 mol/L. Male C57BL/6 mice received oral HBCD (5 × 10−5 g/Kg per week) | Adipogenic effect. Increased the expression of adipocyte marker genes Fabp4, PPARγ, Adipoq, and LPL. | [89] | |
| Liver sections of fathead minnow | Exposure to 4.9 × 10−10, 2.5 × 10−9, 1.3 × 10−8, 6.2 × 10−8, 3.12 × 10−7, 1.6 × 10−6, 7.8 × 10−6, 3.9 × 10−5, and 1.95 × 10−4 mol/L of HBCD. | Down-regulation of caspase2 and apopOn after 6h of exposure. Decreased gene expression of GST, CAT, PI3K and Akt. Decreased enzymes levels involved in xenobiotics metabolism. | [90] | |
| M. japonicus crabs | Exposure to 1.6 × 10−9, 1.6 × 10−8, and 1.6 × 10−7 mol/L of HBCD. | Increased Catalase expression. Increased Mjp53 expression. | [91] | |
| In vivo | Sprague−Dawley Rats | HBCD was administered orally (0.00725 g/Kg) | The highest concentrations were in lipophilic tissues, adipose tissue, adrenals, skin, and GI tract (>500 ng/g). A total of 50% of the 3 diastereoisomers doses were excreted within 4 days. Urine was the most important excretion pathway. β-HBCD was 80% metabolized, γ-HBCD was 65%, and α-HBCD was 51%. α-HBCD is the most dominant diastereoisomer in biological tissues. | [92] |
| Mesocosms | Exposition to 68, 8.5, and 1.1 g/Kg dw of HBCD. To have nine suspensions with a gradient of HBCD, was added the three initial suspensions to obtain various nominal amounts of HBCD per mesocosm (1.3 × 10−6, 2.7 × 10−6, 5.3 × 10−6, 1.06 × 10−5, 2.13 × 10−5, 4.25 × 10−5, 8.5 × 10−5, 0.00017 and 0.00034 Kg) | Negative relation between HBCD concentration and the animal biomass Changes in phytoplankton and zooplankton distribution. Dysregulation of the coastal ecosystems. | [93] | |
| CD-1 mice | Mice were administrated by oral dose with 0.01 and 0.05 g/Kg body weight. | Induced endogenous variations on metabolites, such as increased citrate, 2-ketoglutarate, with decreased alanine, acetate, formate, TMA, 3-hydroxybutyrate, and malonic acid. Modulation of lysine, alanine, and phenylalanine levels. | [94] | |
| Laying hens | A diet containing 1 × 10−6 g/Kg γ-HBCD with laid eggs containing 4 × 10–7 g/Kg lw of HBCD. | Increased HBCD levels in tissues. Alcohols, aldehydes and ketones were identified as exposure markers. | [95] | |
| PND10 mice | Exposure to 0.003, 0.01, and 0.03 g/Kg of α-HBCD, 0.003, and 0.03 g/Kg of γ-HBCD and 0.03 g/Kg of HBCD. | Decreased levels of phenylalanine, glutamate, and arginine. Increased levels of O-phosphocholine and choline. Increased levels of ketone bodies, acetoacetate, and acetone. | [96] | |
| Carassius Carassius Tent | Exposure to 3.12 × 10−9, 3.12 × 10−8, and 3.12 × 10−7 mol/L of HBCD. | Induced SOD activity. Increased CAT activity. ROS accumulation. | [97] |
6. Studies in Humans
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alaee, M. An overview of commercially used brominated flame retardants, their applications, their use patterns in different countries/regions and possible modes of release. Environ. Int. 2003, 29, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Q.; Lu, Y.; Jones, K.; Sweetman, A.J. Hexabromocyclododecanes (HBCDDs) in surface soils from coastal cities in North China: Correlation between diastereoisomer profiles and industrial activities. Chemosphere 2016, 148, 504–510. [Google Scholar] [CrossRef]
- Koch, C.; Schmidt-Kötters, T.; Rupp, R.; Sures, B. Review of hexabromocyclododecane (HBCD) with a focus on legislation and recent publications concerning toxicokinetics and -dynamics. Environ. Pollut. 2015, 199, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Lu, Y.; Zhang, Y.; Khan, K.; Wang, C.; Baninla, Y. An overview of hexabromocyclododecane (HBCDs) in environmental media with focus on their potential risk and management in China. Environ. Pollut. 2018, 236, 283–295. [Google Scholar] [CrossRef]
- Marvin, C.H.; Tomy, G.T.; Armitage, J.M.; Arnot, J.A.; McCarty, L.; Covaci, A.; Palace, V. Hexabromocyclododecane: Current Understanding of Chemistry, Environmental Fate and Toxicology and Implications for Global Management. Environ. Sci. Technol. 2011, 45, 8613–8623. [Google Scholar] [CrossRef] [PubMed]
- Feiteiro, J.; Mariana, M.; Cairrão, E. Health toxicity effects of brominated flame retardants: From environmental to human exposure. Environ. Pollut. 2021, 285, 117475. [Google Scholar] [CrossRef]
- Heeb, N.V.; Schweizer, W.B.; Kohler, M.; Gerecke, A.C. Structure elucidation of hexabromocyclododecanes—A class of compounds with a complex stereochemistry. Chemosphere 2005, 61, 65–73. [Google Scholar] [CrossRef]
- Lyman, W.J.; Reehl, W.F.; Rosenblatt, D.H. Handbook of Chemical Property Estimation Methods; American Chemical Society: Washington, DC, USA, 1990. [Google Scholar]
- Covaci, A.; Gerecke, A.C.; Law, R.J.; Voorspoels, S.; Kohler, M.; Heeb, N.V.; Leslie, H.; Allchin, C.R.; de Boer, J. Hexabromocyclododecanes (HBCDs) in the Environment and Humans: A Review. Environ. Sci. Technol. 2006, 40, 3679–3688. [Google Scholar] [CrossRef]
- Barontini, F.; Cozzani, V.; Petarca, L. Thermal Stability and Decomposition Products of Hexabromocyclododecane. Ind. Eng. Chem. Res. 2001, 40, 3270–3280. [Google Scholar] [CrossRef]
- Sharkey, M.; Harrad, S.; Abdallah, M.A.-E.; Drage, D.S.; Berresheim, H. Phasing-out of legacy brominated flame retardants: The UNEP Stockholm Convention and other legislative action worldwide. Environ. Int. 2020, 144, 106041. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.; Zhang, Y.; Chen, Y.; Wang, X.; Sun, Y. Bioaccumulation and Biomagnification of Hexabromocyclododecane in Marine Biota from China: A Review. Toxics 2022, 10, 620. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Qu, R.; Wang, C.; Wang, L.; Wang, Z. Comparative antioxidant status in freshwater fish Carassius auratus exposed to six current-use brominated flame retardants: A combined experimental and theoretical study. Aquat. Toxicol. 2013, 140-141, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Van Der Ven, L.T.M.; Verhoef, A.; Van De Kuil, T.; Slob, W.; Leonards, P.E.G.; Visser, T.J.; Hamers, T.; Herlin, M.; Håkansson, H.; Olausson, H.; et al. A 28-Day Oral Dose Toxicity Study Enhanced to Detect Endocrine Effects of Hexabromocyclododecane in Wistar Rats. Toxicol. Sci. 2006, 94, 281–292. [Google Scholar] [CrossRef]
- van der Ven, L.T.; van de Kuil, T.; Leonards, P.E.; Slob, W.; Lilienthal, H.; Litens, S.; Herlin, M.; Håkansson, H.; Cantón, R.F.; van den Berg, M.; et al. Endocrine effects of hexabromocyclododecane (HBCD) in a one-generation reproduction study in Wistar rats. Toxicol. Lett. 2009, 185, 51–62. [Google Scholar] [CrossRef]
- Abrantes-Soares, F.; Lorigo, M.; Cairrao, E. Effects of BPA substitutes on the prenatal and cardiovascular systems. Crit. Rev. Toxicol. 2022, 52, 469–498. [Google Scholar] [CrossRef] [PubMed]
- Sellström, U.; Kierkegaard, A.; Wit, C.D.; Jansson, B.O. Polybrominated diphenyl ethers and hexabromocyclododecane in sediment and fish from a Swedish River. Environ. Toxicol Chem. 1998, 17, 1065–1072. [Google Scholar] [CrossRef]
- Law, K.; Palace, V.P.; Halldorson, T.; Danell, R.; Wautier, K.; Evans, B.; Alaee, M.; Marvin, C.; Tomy, G.T. Dietary accumulation of hexabromocyclododecane diastereoisomers in juvenile rainbow trout (Oncorhynchus mykiss) I: Bioaccumulation parameters and evidence of bioisomerization. Environ. Toxicol. Chem. 2006, 25, 1757–1761. [Google Scholar] [CrossRef]
- Xu, C.; Lin, X.; Yin, S.; Zhao, L.; Liu, Y.; Liu, K.; Li, F.; Yang, F.; Liu, W. Enantioselectivity in biotransformation and bioaccumulation processes of typical chiral contaminants. Environ. Pollut. 2018, 243, 1274–1286. [Google Scholar] [CrossRef]
- de Wit, C.A.; Herzke, D.; Vorkamp, K. Brominated flame retardants in the Arctic environment—Trends and new candidates. Sci. Total Environ. 2010, 408, 2885–2918. [Google Scholar] [CrossRef]
- Guerra, P.; Eljarrat, E.; Barceló, D. Enantiomeric specific determination of hexabromocyclododecane by liquid chromatography–quadrupole linear ion trap mass spectrometry in sediment samples. J. Chromatogr. A 2008, 1203, 81–87. [Google Scholar] [CrossRef]
- Guerra, P.; Eljarrat, E.; Barceló, D. Simultaneous determination of hexabromocyclododecane, tetrabromobisphenol A, and related compounds in sewage sludge and sediment samples from Ebro River basin (Spain). Anal. Bioanal. Chem. 2010, 397, 2817–2824. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ruan, Y.; Sun, H.; Zhao, L.; Gan, Z. Hexabromocyclododecanes in surface sediments and a sediment core from Rivers and Harbor in the northern Chinese city of Tianjin. Chemosphere 2013, 90, 1610–1616. [Google Scholar] [CrossRef]
- Vorkamp, K.; Thomsen, M.; Falk, K.; Leslie, H.; Møller, S.; Sørensen, P.B. Temporal Development of Brominated Flame Retardants in Peregrine Falcon (Falco peregrinus) Eggs from South Greenland (1986−2003). Environ. Sci. Technol. 2005, 39, 8199–8206. [Google Scholar] [CrossRef]
- Ismail, N.; Gewurtz, S.B.; Pleskach, K.; Whittle, D.M.; Helm, P.A.; Marvin, C.H.; Tomy, G.T. Brominated and chlorinated flame retardants in Lake Ontario, Canada, lake trout (Salvelinus namaycush) between 1979 and 2004 and possible influences of food-web changes. Environ. Toxicol. Chem. 2009, 28, 910–920. [Google Scholar] [CrossRef] [PubMed]
- Fängström, B.; Athanassiadis, I.; Odsjö, T.; Norén, K.; Bergman, Å. Temporal trends of polybrominated diphenyl ethers and hexabromocyclododecane in milk from Stockholm mothers, 1980–2004. Mol. Nutr. Food Res. 2008, 52, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Knutsen, H.K.; Kvalem, H.E.; Thomsen, C.; Frøshaug, M.; Haugen, M.; Becher, G.; Alexander, J.; Meltzer, H.M. Dietary exposure to brominated flame retardants correlates with male blood levels in a selected group of Norwegians with a wide range of seafood consumption. Mol. Nutr. Food Res. 2008, 52, 217–227. [Google Scholar] [CrossRef]
- Li, H.; Mo, L.; Yu, Z.; Sheng, G.; Fu, J. Levels, isomer profiles and chiral signatures of particle-bound hexabromocyclododecanes in ambient air around Shanghai, China. Environ. Pollut. 2012, 165, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Janák, K.; Covaci, A.; Voorspoels, S.; Becher, G. Hexabromocyclododecane in Marine Species from the Western Scheldt Estuary: Diastereoisomer- and Enantiomer-Specific Accumulation. Environ. Sci. Technol. 2005, 39, 1987–1994. [Google Scholar] [CrossRef]
- Janák, K.; Sellström, U.; Johansson, A.-K.; Becher, G.; de Wit, C.; Lindberg, P.; Helander, B. Enantiomer-specific accumulation of hexabromocyclododecanes in eggs of predatory birds. Chemosphere 2008, 73, S193–S200. [Google Scholar] [CrossRef]
- Harrad, S.; Abdallah, M.A.-E.; Rose, N.L.; Turner, S.D.; Davidson, T.A. Current-Use Brominated Flame Retardants in Water, Sediment, and Fish from English Lakes. Environ. Sci. Technol. 2009, 43, 9077–9083. [Google Scholar] [CrossRef]
- Wu, T.; Wang, S.; Huang, H.; Zhang, S. Diastereomer-Specific Uptake, Translocation, and Toxicity of Hexabromocyclododecane Diastereoisomers to Maize. J. Agric. Food Chem. 2012, 60, 8528–8534. [Google Scholar] [CrossRef] [PubMed]
- Heeb, N.V.; Schweizer, W.B.; Mattrel, P.; Haag, R.; Gerecke, A.C.; Kohler, M.; Schmid, P.; Zennegg, M.; Wolfensberger, M. Solid-state conformations and absolute configurations of (+) and (−) α-, β-, and γ-hexabromocyclododecanes (HBCDs). Chemosphere 2007, 68, 940–950. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.-Z.; Xiang, N.; Duan, Y.-P.; Chen, L.; Zeng, E.Y. Hexabromocyclododecane in consumer fish from South China: Implications for human exposure via dietary intake. Environ. Toxicol. Chem. 2012, 31, 1424–1430. [Google Scholar] [CrossRef]
- Law, R.J.; Covaci, A.; Harrad, S.; Herzke, D.; Abdallah, M.A.-E.; Fernie, K.; Toms, L.-M.L.; Takigami, H. Levels and trends of PBDEs and HBCDs in the global environment: Status at the end of 2012. Environ. Int. 2014, 65, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Lin, L.; Yan, C.; Zhang, X. Diastereoisomer- and Enantiomer-Specific Accumulation, Depuration, and Bioisomerization of Hexabromocyclododecanes in Zebrafish (Danio rerio). Environ. Sci. Technol. 2012, 46, 11040–11046. [Google Scholar] [CrossRef]
- Tomy, G.T.; Palace, V.; Marvin, C.; Stapleton, H.M.; Covaci, A.; Harrad, S. Biotransformation of HBCD in Biological Systems Can Confound Temporal-Trend Studies. Environ. Sci. Technol. 2011, 45, 364–365. [Google Scholar] [CrossRef]
- Badea, S.-L.; Niculescu, V.C.; Ionete, R.-E.; Eljarrat, E. Advances in enantioselective analysis of chiral brominated flame retardants. Current status, limitations and future perspectives. Sci. Total Environ. 2016, 566–567, 1120–1130. [Google Scholar] [CrossRef]
- Harrad, S.; Goosey, E.; Desborough, J.; Abdallah, M.A.-E.; Roosens, L.; Covaci, A. Dust from U.K. Primary School Classrooms and Daycare Centers: The Significance of Dust as a Pathway of Exposure of Young U.K. Children to Brominated Flame Retardants and Polychlorinated Biphenyls. Environ. Sci. Technol. 2010, 44, 4198–4202. [Google Scholar] [CrossRef]
- Abdallah, M.A.-E.; Harrad, S.; Covaci, A. Hexabromocyclododecanes and Tetrabromobisphenol-A in Indoor Air and Dust in Birmingham, UK: Implications for Human Exposure. Environ. Sci. Technol. 2008, 42, 6855–6861. [Google Scholar] [CrossRef]
- de Wit, C.A.; Björklund, J.A.; Thuresson, K. Tri-decabrominated diphenyl ethers and hexabromocyclododecane in indoor air and dust from Stockholm microenvironments 2: Indoor sources and human exposure. Environ. Int. 2012, 39, 141–147. [Google Scholar] [CrossRef]
- Stapleton, H.M.; Misenheimer, J.; Hoffman, K.; Webster, T.F. Flame retardant associations between children’s handwipes and house dust. Chemosphere 2014, 116, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.; Allchin, C.R.; Zegers, B.N.; Haftka, J.J.H.; Boon, J.P.; Belpaire, C.; Leonards, P.E.G.; van Leeuwen, S.P.J.; de Boer, J. Distribution and Fate of HBCD and TBBPA Brominated Flame Retardants in North Sea Estuaries and Aquatic Food Webs. Environ. Sci. Technol. 2004, 38, 5497–5504. [Google Scholar] [CrossRef] [PubMed]
- Eljarrat, E.; de la Cal, A.; Raldua, D.; Duran, C.; Barcelo, D. Occurrence and Bioavailability of Polybrominated Diphenyl Ethers and Hexabromocyclododecane in Sediment and Fish from the Cinca River, a Tributary of the Ebro River (Spain). Environ. Sci. Technol. 2004, 38, 2603–2608. [Google Scholar] [CrossRef] [PubMed]
- Remberger, M.; Sternbeck, J.; Palm, A.; Kaj, L.; Strömberg, K.; Brorström-Lundén, E. The environmental occurrence of hexabromocyclododecane in Sweden. Chemosphere 2004, 54, 9–21. [Google Scholar] [CrossRef]
- de Boer, J.; Allchin, C.; Brandsma, S.H.; Kruijt, A.W.; van der Veen, I.; van Hesselingen, J.M.; Haftka, J.J.H. HBCD and TBBP-A in Sewage Sludge, Sediments and Biota, Including Interlaboratory Study; RIVO: Ymuiden, The Netherlands, 2002. [Google Scholar]
- CIS/06/01413; de Wit, C.A. Brominated Flame Retardants. Swedish Environmental Protection Agency: Stockholm, Sweden, 2000. [Google Scholar]
- de Wit, C. An overview of brominated flame retardants in the environment. Chemosphere 2002, 46, 583–624. [Google Scholar] [CrossRef]
- Law, R.J.; Allchin, C.R.; de Boer, J.; Covaci, A.; Herzke, D.; Lepom, P.; Morris, S.; Tronczynski, J.; de Wit, C. Levels and trends of brominated flame retardants in the European environment. Chemosphere 2006, 64, 187–208. [Google Scholar] [CrossRef]
- Chen, D.; La Guardia, M.J.; Luellen, D.R.; Harvey, E.; Mainor, T.M.; Hale, R.C. Do Temporal and Geographical Patterns of HBCD and PBDE Flame Retardants in U.S. Fish Reflect Evolving Industrial Usage? Environ. Sci. Technol. 2011, 45, 8254–8261. [Google Scholar] [CrossRef]
- Kupper, T.; de Alencastro, L.F.; Gatsigazi, R.; Furrer, R.; Grandjean, D.; Tarradellas, J. Concentrations and specific loads of brominated flame retardants in sewage sludge. Chemosphere 2008, 71, 1173–1180. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Q.; Wang, Y.; Xie, X. Fate of tetrabromobisphenol A and hexabromocyclododecane brominated flame retardants in soil and uptake by plants. Chemosphere 2011, 82, 204–209. [Google Scholar] [CrossRef]
- Hunziker, R.W.; Gonsior, S.; MacGregor, J.A.; Desjardins, D.; Ariano, J.; Friederich, U. Fate and effect of hexabromocyclododecane in the environment. Organohalogen Compd. 2004, 66, 2300–2305. [Google Scholar]
- Zhang, Y.; Sun, H.; Liu, F.; Dai, Y.; Qin, X.; Ruan, Y.; Zhao, L.; Gan, Z. Hexabromocyclododecanes in limnic and marine organisms and terrestrial plants from Tianjin, China: Diastereomer- and enantiomer-specific profiles, biomagnification, and human exposure. Chemosphere 2013, 93, 1561–1568. [Google Scholar] [CrossRef] [PubMed]
- Allchin, C.; Morris, S. Hexabromocyclododecane (HBCD) diastereoisomers and brominated diphenyl ether congener (BDE) residues in edible fish from the rivers Skerne and Tees, UK. Organohalogen Compd. 2003, 61, 41–44. [Google Scholar]
- Xia, C.; Lam, J.C.; Wu, X.; Sun, L.; Xie, Z.; Lam, P.K. Hexabromocyclododecanes (HBCDs) in marine fishes along the Chinese coastline. Chemosphere 2011, 82, 1662–1668. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, R.; Murata, S.; Ashizuka, Y.; Shintani, Y.; Hori, T.; Tsutsumi, T. Hexabromocyclododecane determination in seafood samples collected from Japanese coastal areas. Chemosphere 2010, 81, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Guerra, P.; Alaee, M.; Jiménez, B.; Pacepavicius, G.; Marvin, C.; MacInnis, G.; Eljarrat, E.; Barceló, D.; Champoux, L.; Fernie, K. Emerging and historical brominated flame retardants in peregrine falcon (Falco peregrinus) eggs from Canada and Spain. Environ. Int. 2012, 40, 179–186. [Google Scholar] [CrossRef]
- Leslie, H.A.; Leonards, P.E.; Shore, R.; Walker, L.A.; Bersuder, P.; Morris, S.; Allchin, C.R.; de Boer, J. Decabromodiphenylether and hexabromocyclododecane in wild birds from the United Kingdom, Sweden and The Netherlands: Screening and time trends. Chemosphere 2011, 82, 88–95. [Google Scholar] [CrossRef]
- Thomsen, C.; Molander, P.; Daae, H.L.; Janák, K.; Froshaug, M.; Liane, V.H.; Thorud, S.; Becher, G.; Dybing, E. Occupational Exposure to Hexabromocyclododecane at an Industrial Plant. Environ. Sci. Technol. 2007, 41, 5210–5216. [Google Scholar] [CrossRef]
- Shi, Z.; Wu, Y.-N.; Li, J.-G.; Zhao, Y.-F.; Feng, J.-F. Dietary Exposure Assessment of Chinese Adults and Nursing Infants to Tetrabromobisphenol-A and Hexabromocyclododecanes: Occurrence Measurements in Foods and Human Milk. Environ. Sci. Technol. 2009, 43, 4314–4319. [Google Scholar] [CrossRef]
- Roosens, L.; Abdallah, M.A.-E.; Harrad, S.; Neels, H.; Covaci, A. Exposure to Hexabromocyclododecanes (HBCDs) via Dust Ingestion, but Not Diet, Correlates with Concentrations in Human Serum: Preliminary Results. Environ. Health Perspect. 2009, 117, 1707–1712. [Google Scholar] [CrossRef]
- Driffield, M.; Harmer, N.; Bradley, E.; Fernandes, A.R.; Rose, M.; Mortimer, D.; Dicks, P. Determination of brominated flame retardants in food by LC–MS/MS: Diastereoisomer-specific hexabromocyclododecane and tetrabromobisphenol A. Food Addit. Contam. Part A 2008, 25, 895–903. [Google Scholar] [CrossRef]
- Eljarrat, E.; Guerra, P.; Martínez, E.; Farré, M.; Alvarez, J.G.; López-Teijón, M.; Barceló, D. Hexabromocyclododecane in Human Breast Milk: Levels and Enantiomeric Patterns. Environ. Sci. Technol. 2009, 43, 1940–1946. [Google Scholar] [CrossRef]
- Meijer, L.; Weiss, J.; van Velzen, M.; Brouwer, A.; Bergman, Å.; Sauer, P.J.J. Serum Concentrations of Neutral and Phenolic Organohalogens in Pregnant Women and Some of Their Infants in The Netherlands. Environ. Sci. Technol. 2008, 42, 3428–3433. [Google Scholar] [CrossRef]
- Weiss, J.; Wallin, E.; Axmon, A.; Jönsson, B.A.G.; Åkesson, H.; Janák, K.; Hagmar, L.; Bergman, Å. Hydroxy-PCBs, PBDEs, and HBCDDs in Serum from an Elderly Population of Swedish Fishermen’s Wives and Associations with Bone Density. Environ. Sci. Technol. 2006, 40, 6282–6289. [Google Scholar] [CrossRef]
- Antignac, J.-P.; Cariou, R.; Maume, D.; Marchand, P.; Monteau, F.; Zalko, D.; Berrebi, A.; Cravedi, J.-P.; Andre, F.; Le Bizec, B. Exposure assessment of fetus and newborn to brominated flame retardants in France: Preliminary data. Mol. Nutr. Food Res. 2008, 52, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Yamada-Okabe, T.; Sakai, H.; Kashima, Y.; Yamada-Okabe, H. Modulation at a cellular level of the thyroid hormone receptor-mediated gene expression by 1,2,5,6,9,10-hexabromocyclododecane (HBCD), 4,4′-diiodobiphenyl (DIB), and nitrofen (NIP). Toxicol. Lett. 2005, 155, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Huang, H.; Zhang, S. Accumulation and phytotoxicity of technical hexabromocyclododecane in maize. J. Environ. Sci. 2016, 42, 97–104. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, S.; Lv, J.; Wen, B.; Wang, S.; Wu, T. Experimental and Theoretical Evidence for Diastereomer—And Enantiomer-Specific Accumulation and Biotransformation of HBCD in Maize Roots. Environ. Sci. Technol. 2016, 50, 12205–12213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guo, Q.; Tan, D.; He, Z.; Wang, Y.; Liu, X. Effects of low-levels of three hexabromocyclododecane diastereomers on the metabolic profiles of pak choi leaves using high-throughput untargeted metabolomics approach. Environ. Pollut. 2018, 242, 1961–1969. [Google Scholar] [CrossRef]
- Huang, H.; Wang, D.; Wen, B.; Lv, J.; Zhang, S. Roles of maize cytochrome P450 (CYP) enzymes in stereo-selective metabolism of hexabromocyclododecanes (HBCDs) as evidenced by in vitro degradation, biological response and in silico studies. Sci. Total Environ. 2019, 656, 364–372. [Google Scholar] [CrossRef]
- Fa, S.; Pogrmic-Majkic, K.; Samardzija, D.; Hrubik, J.; Glisic, B.; Kovacevic, R.; Andric, N. HBCDD-induced sustained reduction in mitochondrial membrane potential, ATP and steroidogenesis in peripubertal rat Leydig cells. Toxicol. Appl. Pharmacol. 2015, 282, 20–29. [Google Scholar] [CrossRef]
- Shi, Y.-J.; Xu, X.-B.; Zheng, X.-Q.; Lu, Y.-L. Responses of growth inhibition and antioxidant gene expression in earthworms ( Eisenia fetida ) exposed to tetrabromobisphenol A, hexabromocyclododecane and decabromodiphenyl ether. Comp. Biochem. Physiol. Part C: Toxicol. Pharmacol. 2015, 174-175, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Wu, D.; Wang, C.; Guo, Z.; Li, B.; Zuo, Z. Hexabromocyclododecane exposure induces cardiac hypertrophy and arrhythmia by inhibiting miR-1 expression via up-regulation of the homeobox gene Nkx2.5. J. Hazard. Mater. 2016, 302, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Genskow, K.R.; Bradner, J.M.; Hossain, M.M.; Richardson, J.R.; Caudle, W.M. Selective damage to dopaminergic transporters following exposure to the brominated flame retardant, HBCDD. Neurotoxicol. Teratol. 2015, 52, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Fang, C.; Qiu, L.; Dong, S.; Zhang, X.; Yan, C. Diastereoisomer-specific effects of hexabromocyclododecanes on hepatic aryl hydrocarbon receptors and cytochrome P450s in zebrafish (Danio rerio). Chemosphere 2015, 132, 24–31. [Google Scholar] [CrossRef]
- Usenko, C.Y.; Abel, E.L.; Hopkins, A.; Martinez, G.; Tijerina, J.; Kudela, M.; Norris, N.; Joudeh, L.; Bruce, E.D. Evaluation of Common Use Brominated Flame Retardant (BFR) Toxicity Using a Zebrafish Embryo Model. Toxics 2016, 4, 21. [Google Scholar] [CrossRef]
- Miller, I.; Serchi, T.; Cambier, S.; Diepenbroek, C.; Renaut, J.; Van der Berg, J.; Kwadijk, C.; Gutleb, A.; Rijntjes, E.; Murk, A. Hexabromocyclododecane (HBCD) induced changes in the liver proteome of eu- and hypothyroid female rats. Toxicol. Lett. 2016, 245, 40–51. [Google Scholar] [CrossRef]
- Bernhard, A.; Berntssen, M.H.; Lundebye, A.-K.; Alvheim, A.R.; Myrmel, L.S.; Fjære, E.; Torstensen, B.E.; Kristiansen, K.; Madsen, L.; Brattelid, T.; et al. Marine fatty acids aggravate hepatotoxicity of α-HBCD in juvenile female BALB/c mice. Food Chem. Toxicol. 2016, 97, 411–423. [Google Scholar] [CrossRef]
- Miller, I.; Diepenbroek, C.; Rijntjes, E.; Renaut, J.; Teerds, K.J.; Kwadijk, C.; Cambier, S.; Murk, A.J.; Gutleb, A.C.; Serchi, T. Gender specific differences in the liver proteome of rats exposed to short term and low-concentration hexabromocyclododecane (HBCD). Toxicol. Res. 2016, 5, 1273–1283. [Google Scholar] [CrossRef]
- Shi, Y.; Xu, X.; Chen, J.; Liang, R.; Zheng, X.; Shi, Y.; Wang, Y. Antioxidant gene expression and metabolic responses of earthworms (Eisenia fetida) after exposure to various concentrations of hexabromocyclododecane. Environ. Pollut. 2018, 232, 245–251. [Google Scholar] [CrossRef]
- Reffatto, V.; Rasinger, J.D.; Carroll, T.S.; Ganay, T.; Lundebye, A.-K.; Sekler, I.; Hershfinkel, M.; Hogstrand, C. Parallel in vivo and in vitro transcriptomics analysis reveals calcium and zinc signalling in the brain as sensitive targets of HBCD neurotoxicity. Arch. Toxicol. 2018, 92, 1189–1203. [Google Scholar] [CrossRef]
- Rasinger, J.D.; Carroll, T.S.; Maranghi, F.; Tassinari, R.; Moracci, G.; Altieri, I.; Mantovani, A.; Lundebye, A.-K.; Hogstrand, C. Low dose exposure to HBCD, CB-153 or TCDD induces histopathological and hormonal effects and changes in brain protein and gene expression in juvenile female BALB/c mice. Reprod. Toxicol. 2018, 80, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Lu, G.; Yan, Z.; Liu, J.; Nkoom, M.; Yang, H. Responses of antioxidant and biotransformation enzymes in Carassius carassius exposed to hexabromocyclododecane. Environ. Toxicol. Pharmacol. 2018, 62, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Farmahin, R.; Gannon, A.M.; Gagné, R.; Rowan-Carroll, A.; Kuo, B.; Williams, A.; Curran, I.; Yauk, C.L. Hepatic transcriptional dose-response analysis of male and female Fischer rats exposed to hexabromocyclododecane. Food Chem. Toxicol. 2019, 133, 110262. [Google Scholar] [CrossRef]
- Chen, H.; Guo, S.; Li, H.; Zhou, D.; Cao, X.; Wang, C.; Liu, Y.; Xiang, M.; Li, L.; Yu, Y. Multi-generational effects and variations of stress response by hexabromocyclododecane (HBCD) exposure in the nematode Caenorhabditis elegans. J. Environ. Manag. 2019, 245, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zhang, L.; Liu, X.; Yu, Y.; Liu, S.; Chen, M.; Huang, C.; Hu, G. The enrichment and purification of hexabromocyclododecanes and its effects on thyroid in zebrafish. Ecotoxicol. Environ. Saf. 2019, 185, 109690. [Google Scholar] [CrossRef]
- Xie, X.; Yu, C.; Ren, Q.; Wen, Q.; Zhao, C.; Tang, Y.; Du, Y. Exposure to HBCD promotes adipogenesis both in vitro and in vivo by interfering with Wnt6 expression. Sci. Total. Environ. 2020, 705, 135917. [Google Scholar] [CrossRef]
- Bertucci, J.; Bandaralage, S.M.I.; Hecker, M. Assessing the cytotoxic effect of hexabromocyclododecane (HBCD) on liver tissue cultures from fathead minnow (Pimephales promelas). Aquat. Toxicol. 2020, 225, 105523. [Google Scholar] [CrossRef]
- Park, K.; Kwak, I.-S. Apoptotic p53 Gene Expression in the Regulation of Persistent Organic Pollutant (POP)-Induced Oxidative Stress in the Intertidal Crab Macrophthalmusjaponicus. Antioxidants 2022, 11, 771. [Google Scholar] [CrossRef]
- Hakk, H. Comparative Metabolism Studies of Hexabromocyclododecane (HBCD) Diastereomers in Male Rats Following a Single Oral Dose. Environ. Sci. Technol. 2016, 50, 89–96. [Google Scholar] [CrossRef]
- Bradshaw, C.; Näslund, J.; Hansen, J.; Kozlowsky-Suzuki, B.; Sundström, B.; Gustafsson, K. Hexabromocyclododecane affects benthic-pelagic coupling in an experimental ecosystem. Environ. Pollut. 2015, 206, 306–314. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, P.; Wang, X.; Wang, Y.; Zhou, Z.; Zhu, W. NMR- and LC–MS/MS-based urine metabolomic investigation of the subacute effects of hexabromocyclododecane in mice. Environ. Sci. Pollut. Res. 2016, 23, 8500–8507. [Google Scholar] [CrossRef] [PubMed]
- Ratel, J.; Planche, C.; Mercier, F.; Blinet, P.; Kondjoyan, N.; Marchand, P.; Fournier, A.; Travel, A.; Jondreville, C.; Engel, E. Liver volatolomics to reveal poultry exposure to γ-hexabromocyclododecane (HBCD). Chemosphere 2017, 189, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Szabo, D.T.; Pathmasiri, W.; Sumner, S.; Birnbaum, L.S. Serum Metabolomic Profiles in Neonatal Mice following Oral Brominated Flame Retardant Exposures to Hexabromocyclododecane (HBCD) Alpha, Gamma, and Commercial Mixture. Environ. Health Perspect. 2017, 125, 651–659. [Google Scholar] [CrossRef]
- Dong, H.; Lu, G.; Yan, Z.; Liu, J.; Yang, H.; Nkoom, M. Bioconcentration and effects of hexabromocyclododecane exposure in crucian carp (Carassius auratus). Ecotoxicology 2018, 27, 313–324. [Google Scholar] [CrossRef]
- Wang, B.; Wang, H.; Xiao, D.; Han, D. In vitro effects of brominated flame retardants, selected metals and their mixtures on ethoxyresorufin-O-deethylase activity in Mossambica tilapia liver. Ecotoxicol. Environ. Saf. 2018, 161, 350–355. [Google Scholar] [CrossRef]
- Huang, L.; Wang, W.; Zanaroli, G.; Xu, P.; Tang, H. Hexabromocyclododecanes Are Dehalogenated by CYP168A1 from Pseudomonas aeruginosa Strain HS9. Appl. Environ. Microbiol. 2021, 87, e00826-21. [Google Scholar] [CrossRef] [PubMed]
- Maia, M.L.; Sousa, S.; Pestana, D.; Faria, A.; Teixeira, D.; Delerue-Matos, C.; Domingues, V.F.; Calhau, C. Impact of brominated flame retardants on lipid metabolism: An in vitro approach. Environ. Pollut. 2022, 294, 118639. [Google Scholar] [CrossRef] [PubMed]
- Koike, E.; Yanagisawa, R.; Takano, H. Brominated flame retardants, hexabromocyclododecane and tetrabromobisphenol A, affect proinflammatory protein expression in human bronchial epithelial cells via disruption of intracellular signaling. Toxicol. In Vitro 2016, 32, 212–219. [Google Scholar] [CrossRef]
- Dungen, M.W.V.D.; Rijk, J.C.; Kampman, E.; Steegenga, W.T.; Murk, A.J. Steroid hormone related effects of marine persistent organic pollutants in human H295R adrenocortical carcinoma cells. Toxicol. In Vitro 2015, 29, 769–778. [Google Scholar] [CrossRef]
- An, J.; Guo, P.; Shang, Y.; Zhong, Y.; Zhang, X.; Yu, Y.; Yu, Z. The “adaptive responses” of low concentrations of HBCD in L02 cells and the underlying molecular mechanisms. Chemosphere 2016, 145, 68–76. [Google Scholar] [CrossRef]
- Almughamsi, H.; Whalen, M.M. Hexabromocyclododecane and tetrabromobisphenol A alter secretion of interferon gamma (IFN-γ) from human immune cells. Arch. Toxicol. 2016, 90, 1695–1707. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Chen, C.; Shang, Y.; Zhong, Y.; Ren, G.; Yu, Z.; An, J. In vitro study on the biotransformation and cytotoxicity of three hexabromocyclododecane diastereoisomers in liver cells. Chemosphere 2016, 161, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Erratico, C.; Zheng, X.; Eede, N.V.D.; Tomy, G.; Covaci, A. Stereoselective Metabolism of α-, β-, and γ-Hexabromocyclododecanes (HBCDs) by Human Liver Microsomes and CYP3A4. Environ. Sci. Technol. 2016, 50, 8263–8273. [Google Scholar] [CrossRef] [PubMed]
- Krivoshiev, B.V.; Dardenne, F.; Covaci, A.; Blust, R.; Husson, S.J. Assessing in-vitro estrogenic effects of currently-used flame retardants. Toxicol. In Vitro 2016, 33, 153–162. [Google Scholar] [CrossRef]
- Kim, S.-H.; Nam, K.-H.; Hwang, K.-A.; Choi, K.-C. Influence of hexabromocyclododecane and 4-nonylphenol on the regulation of cell growth, apoptosis and migration in prostatic cancer cells. Toxicol. In Vitro 2016, 32, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Anisuzzaman, S.; Whalen, M.M. Tetrabromobisphenol A and hexabromocyclododecane alter secretion of IL-1β from human immune cells. J. Immunotoxicol. 2016, 13, 403–416. [Google Scholar] [CrossRef]
- Canbaz, D.; Lebre, M.C.; Logiantara, A.; van Ree, R.; van Rijt, L.S. Indoor pollutant hexabromocyclododecane enhances house dust mite-induced activation of human monocyte-derived dendritic cells. J. Immunotoxicol. 2016, 13, 810–816. [Google Scholar] [CrossRef]
- Li, R.J.; Gao, H.; Na, G.S.; Lu, Z.H.; Yao, Y.; Yang, F. Hexabromocyclododecane-induced Genotoxicity in Cultured Human Breast Cells through DNA Damage. Biomed. Environ. Sci. 2017, 30, 296–300. [Google Scholar]
- Yasmin, S.; Whalen, M. Flame retardants, hexabromocyclododecane (HCBD) and tetrabromobisphenol a (TBBPA), alter secretion of tumor necrosis factor alpha (TNFα) from human immune cells. Arch. Toxicol. 2018, 92, 1483–1494. [Google Scholar] [CrossRef]
- Jin, Y.; Shang, Y.; Zhang, D.; An, J.; Pan, D. Hexabromocyclododecanes promoted autophagy through the PI3K/Akt/mTOR pathway in L02 cells. J. Environ. Manag. 2019, 244, 77–82. [Google Scholar] [CrossRef]
- Shi, X.; Zha, J.; Wen, B.; Zhang, S. Diastereoisomer-specific neurotoxicity of hexabromocyclododecane in human SH-SY5Y neuroblastoma cells. Sci. Total Environ. 2019, 686, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Sousa, S.; Maia, M.L.; Pestana, D.; Teixeira, D.; Ângelo-Dias, M.; Martins, C.; Borrego, L.M.; Delerue-Matos, C.; Calhau, C.; Domingues, V.F.; et al. Brominated flame retardants effect in MCF-7 cells: Impact on vitamin D pathway. J. Steroid Biochem. Mol. Biol. 2022, 219, 106079. [Google Scholar] [CrossRef] [PubMed]
- Ongono, J.S.; Dow, C.; Gambaretti, J.; Severi, G.; Boutron-Ruault, M.-C.; Bonnet, F.; Fagherazzi, G.; Mancini, F.R. Dietary exposure to brominated flame retardants and risk of type 2 diabetes in the French E3N cohort. Environ. Int. 2019, 123, 54–60. [Google Scholar] [CrossRef]
- Huang, M.; Li, J.; Xiao, Z.; Shi, Z. Tetrabromobisphenol A and hexabromocyclododecane isomers in breast milk from the general population in Beijing, China: Contamination levels, temporal trends, nursing infant’s daily intake, and risk assessment. Chemosphere 2020, 244, 125524. [Google Scholar] [CrossRef] [PubMed]
- Zainab, B.; Ayaz, Z.; Rashid, U.; Al Farraj, D.A.; Alkufeidy, R.M.; AlQahtany, F.S.; Aljowaie, R.M.; Abbasi, A.M. Role of Persistent Organic Pollutants in Breast Cancer Progression and Identification of Estrogen Receptor Alpha Inhibitors Using In-Silico Mining and Drug-Drug Interaction Network Approaches. Biology 2021, 10, 681. [Google Scholar] [CrossRef] [PubMed]


| Study | Study Organism | Exposure | Effects | Reference |
|---|---|---|---|---|
| In vitro | Maize roots and shoots | HBCD—0, 3.12 × 10−9, 7.8 × 10−9, 1.6 × 10−8, 3.12 × 10−8 and 7.8 × 10−8 mol/L | Roots present the highest HBCD levels. Inhibition of germination rate, root biomass, root elongation, shoot biomass, and shoot elongation. | [69] |
| Maize roots | α-HBCD—3.9 × 10−11 mol/L, β-HBCD—2.7 × 10−11 mol/L and γ-HBCD—3.12 × 10−12 mol/L | Presence of the three enantiomers: α-, β-, and γ-HBCD in the maize roots. | [70] | |
| Pak Choi leaves | Seeds were put into glass pots with Hewitt’s nutrient solution and HBCD | Alteration on several metabolic pathways. | [71] | |
| In silico and in vitro | Maize | Microsome prepared with 10 × 10−3 mol/L of technical HBCD | Decreased and alteration in the protein content of maize CYPs. | [72] |
| Study | Study Organism | Exposure | Effects | Reference |
|---|---|---|---|---|
| In vitro | normal human bronchial epithelial cell line, BEAS-2B | Exposure to 1.6 × 10−8–1.6 × 10−5 mol/L of HBCD | Increased cell proliferation at low concentrations and decreased at higher concentrations. Increased ICAM-1 expression, IL-6 and IL-8 production. Increased EGF production. EGFR-specific tyrosine kinase inhibitor (AG1478), blocked the HBCD-induced IL-6 production. | [101] |
| H295R human adrenocortical carcinoma cells | Exposure to 1 × 10−6 mol/L of HBCD to cytotoxicity tests. | No alteration in the hormone levels. | [102] | |
| The human hepatic cell line L02 | Exposure to 10−13, 10−11, and 5 × 10−5 mol/L of HBCD | Suppressed cell survival. PI3K/Akt pathway, AMPK signaling, and p38 MAPK pathway regulation. | [103] | |
| PBMCs and monocyte-depleted PBMCS/NK cells | Exposure to HBCD (5 × 10−8 to 5 × 10−6 mol/L) for 24 h, 48 h, and 6 days. | Increased IFN-γ secretion from NK, PBMC, and MD-PBMC cells. Decreased the secretion of some enzyme inhibitors. | [104] | |
| Human hepatoma HepG2 cells and L02 cells | Exposure to 0, 10−7, 10−6, and 10−5 mol/L of HBCD. | No inhibitory effects. Intracellular redox state and DNA damage alterations. Increased ROS levels. | [105] | |
| pooled human liver microsomes | Exposure to 1 × 10−8 to 1 × 10−4 mol/L of HBCD. | Increased hydroxylated metabolites formation. rCYP2B6 and rCYP3A4 formed HBCD metabolites. | [106] | |
| The human breast adenocarcinoma cell line, MCF-7 | Exposure to 1 × 10−4 mol/L of HBCD. | Increased estrogenic activity. Modulation of the cell viability. | [107] | |
| LNCaP prostate cancer cells | Exposure to 10−8–10−5 mol/L of HBCD. | Increased LNCaP cell proliferation and migration. Increased cyclin D1 expression. | [108] | |
| NK cells/PMBC and monocyte-depleted (MD) PBMC | Exposure to HBCD (5 × 10−8 to 5 × 10−6 mol/L) for 24 h, 48 h, and 6 days. | Increased IL-1β secretion from NK, MD-PBMC, and PBMC cells. Some enzyme inhibitors could decrease the secretion of IL-1β caused by HBCD. | [109] | |
| Peripheral blood mononuclear cells (PBMC) | Exposure to 0.1 × 10−7, 1 × 10−6, 1 × 10−5, and 2 × 10−5 mol/L of HBCD | Enhanced CD86 expression. Increased IL-8 production. | [110] | |
| human breast cells HBL-100 | Exposure to 0, 7.8 × 10−6, 1.6 × 10−5, and 7.8 × 10−5 mol/L of HBCD. | Low concentrations increased proliferation rate, contrarily to higher concentrations. Increased ROS production and the DNA tail. BRCA1 was promoted with HBCD increase, which exhibited a prognostic of breast cancer. | [111] | |
| PBMCs, and monocyte-depleted PBMCS/NK cells | Exposure to HBCD (5 × 10−8 to 5 × 10−6 mol/L) for 24 h, 48 h, and 6 days. | Decreased TNFα secretion from NK cells. Increased TNFα secretion by MD-PBMC cells and PBMC cells. | [112] | |
| L02 cell line | Exposure to HBCD (5 × 10−8 to 5 × 10−6 mol/L) for 24 h, 48 h, and 6 days. | Increased cellular apoptosis. Increased LC3-I (initial step of autophagy) and LC3-II proteins. Increased PI3K/Akt/mTOR pathway. Increased autophagy. | [113] | |
| 3T3-L1 preadipocytes and Human/Preadipocytes from visceral adipose (HPA-V) | Exposure to 2 × 10−5 and 4 × 10−5 mol/L of HBCD. | Increased lipid droplets formation. Induced adipogenesis during the first 2 days. | [89] | |
| SH-SY5Y human neuroblastoma cell line | Exposure to 1 × 10−10, 1 × 10−9, and 1 × 10−8 mol/L of HBCD. | Decreased cell viability. Increased cellular apoptosis and necrosis. Increased ROS level. | [114] | |
| The murine cell line 3T3-L1/The human cell line HepG2 | Incubation of 24 h with HBCD for short-term exposure and long-term exposure incubation, at least for 4 weeks, was used 2.5 × 10−7 mol/L of HBCD. | Decreased cell proliferation. Reduced MMP-9 expression and cell migration. Long-term exposure induced an uncommon response to calcitriol | [100] | |
| MCF-7 cells/HepG2 cells | Incubated for 24 h with HBCD (1 × 10−8 to 5 × 10−7 mol/L). Long-term exposure, for at least 4 weeks, used 2.5 × 10−7 mol/L HBCD. | Decreased cell proliferation in a dose-dependent manner. Reduced the expression of MMP-9 and cell migration. Long-term exposure induced an uncommon response to calcitriol. | [115] | |
| Epidemiological | French E3N cohort | 71,415 women, of which 3667 were (type 2 diabetes) T2D cases | Dietary exposure to HBCD was linearly associated with a T2D risk increase. Non-processed white meat and processed meat were the main groups that contributed to HBCD dietary exposure. | [116] |
| Breast milk | 111 individual samples were obtained. | HBCD was detected in almost all samples and the concentrations were between the limit of detection that was set as zero and 36.3 n/g lw | [117] | |
| In silico | Breast cancer | Involved in breast cancer prevailing. Showed strong interactions with the Erα protein. | [118] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marques, M.L.; Cairrao, E. Occurrence and Health Effects of Hexabromocyclododecane: An Updated Review. Toxics 2023, 11, 409. https://doi.org/10.3390/toxics11050409
Marques ML, Cairrao E. Occurrence and Health Effects of Hexabromocyclododecane: An Updated Review. Toxics. 2023; 11(5):409. https://doi.org/10.3390/toxics11050409
Chicago/Turabian StyleMarques, Maria Lopes, and Elisa Cairrao. 2023. "Occurrence and Health Effects of Hexabromocyclododecane: An Updated Review" Toxics 11, no. 5: 409. https://doi.org/10.3390/toxics11050409
APA StyleMarques, M. L., & Cairrao, E. (2023). Occurrence and Health Effects of Hexabromocyclododecane: An Updated Review. Toxics, 11(5), 409. https://doi.org/10.3390/toxics11050409