Interaction of Lead and Cadmium Reduced Cadmium Toxicity in Ficus parvifolia Seedlings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
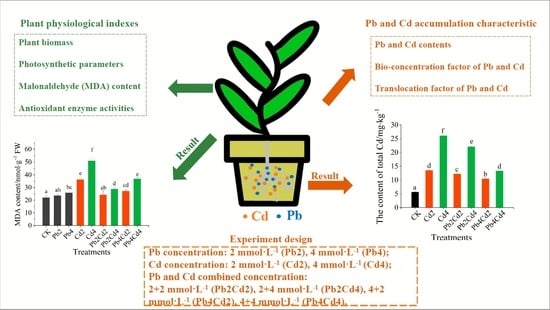
2.2. Experimental Design
2.3. Determination Methods
2.3.1. Biomass
2.3.2. Photosynthetic Characteristics and Chlorophyll Fluorescence Parameters
2.3.3. Malondialdehyde (MDA) Content and Antioxidant Enzyme Activities
2.3.4. Pb and Cd Accumulation and Translocation Characteristics in Plant
2.3.5. Physicochemical Characteristics of the Experimental Soil
2.4. Statistical Analysis
3. Results
3.1. Biomass of F. parvifolia Seedlings under Pb and Cd Stress
3.2. Photosynthetic Parameters of F. parvifolia Seedlings under Pb and Cd Stress
3.3. MDA Content and Antioxidant Enzyme Activities Change of F. parvifolia Seedlings under Pb and Cd Stress
3.4. Pb and Cd Uptake, Accumulation, and Translocation Characteristics of F. parvifolia Seedlings under Pb and Cd Stress
3.5. Pearson Correlation Analysis of Cd Content, Accumulation, and Physiological Indexes
4. Discussion
4.1. The Effects of Pb and Cd on Biomass
4.2. The Effects of Pb and Cd on Photosynthesis
4.3. The Effects of Pb and Cd on MDA Content and Antioxidant Enzymes
4.4. The Effects of Pb and Cd on Pb and Cd Accumulation and Translocation Characteristics of F. parvifolia
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, M.; Zhi, Y.; Dai, Y.Y.; Lv, J.L.; Li, Y.J.; Wu, Z.H. The detoxification mechanisms of low-accumulating and non-low-accumulating medicinal plants under Cd and Pb stress. RSC Adv. 2020, 10, 43882–43893. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Wang, B.; Shi, Z.M.; Li, L.; Li, Y.; Mao, Z.Q.; Liao, L.Y.; Zhang, H.; Wu, Y. Immobilization of heavy metals (Cd, Zn, and Pb) in different contaminated soils with swine manure biochar. Environ. Pollut. Bioavailab. 2021, 33, 55–65. [Google Scholar] [CrossRef]
- Shahid, M.A.; Balal, R.M.; Khan, N.; Zotarelli, L.; Liu, G.D.; Sarkhosh, A.; Fernández-Zapata, J.C.; Nicolás, J.J.M.; Garcia-Sanchez, F. Selenium impedes cadmium and a-rsenic toxicity in potato by modulating carbohydrate and nitrogen metabolism. Ecotoxicol. Environ. Saf. 2019, 180, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Ali, W.; Mao, K.; Zhang, H.; Junaid, M.; Xu, N.; Rasool, A.; Feng, X.; Yang, Z. Comprehensive review of the basic chemical behaviours, sources, processes, and endpoints of trace element contamination in paddy soil-rice systems in rice-growing countries. J. Hazard. Mater. 2020, 397, 122720. [Google Scholar] [CrossRef] [PubMed]
- Waheed, A.; Haxim, Y.; Islam, W.; Ahmad, M.; Ali, S.; Wen, X.; Khan, K.A.; Ghramh, H.A.; Zhang, Z.; Zhang, D. Impact of Cadmium Stress on Growth and Physio-Biochemical Attributes of Eruca sativa Mill. Plants 2022, 11, 2981. [Google Scholar] [CrossRef]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The effects of cadmium toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef]
- Khanam, R.; Kumar, A.; Nayak, A.K.; Shahid, M.; Tripathi, R.; Vijayakumar, S.; Bhaduri, D.; Kumar, U.; Mohanty, S.; Panneersel-vam, P.; et al. Metal(loid)s (As, Hg, Se, Pb and Cd) in paddy soil: Bioavailability and potential risk to human health. Sci. Total Environ. 2020, 699, 134330. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Redha, A.; Al-Hasan, R.; Afzal, M. Synergistic and concentration-dependent toxicity of multiple heavy metals compared with single heavy metals in Conocarpus lancifolius. Environ. Sci. Pollut. Res. 2021, 28, 23258–23272. [Google Scholar] [CrossRef]
- Alkhatib, R.; Mheidat, M.; Abdo, N.; Tadros, M.; Al-Eitan, L.; Al-Hadid, K. Effect of lead on the physiological, biochemical and ultrastructural properties of Leucaena leucocephala. Plant Biol. 2019, 21, 1132–1139. [Google Scholar] [CrossRef]
- Figlioli, F.; Sorrentino, M.C.; Memoli, V.; Arena, C.; Maisto, G.; Giordano, S.; Capozzi, F.; Spagnuolo, V. Overall plant responses to Cd and Pb metal stress in maize: Growth pattern, ultrastructure, and photosynthetic activity. Environ. Sci. Pollut. Res. 2019, 26, 1781–1790. [Google Scholar] [CrossRef] [PubMed]
- Dary, M.; Chamber-Pérez, M.A.; Palomares, A.J.; Pajuelo, E. “In situ” phytostabilisation of heavy metal polluted soils using Lupinus luteus inoculated with metal resistant plant-growth promoting rhizobacteria. J. Hazard. Mater. 2010, 177, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Lanier, C.; Bernard, F.; Dumez, S.; Leclercq-Dransart, J.; Lemière, S.; Vandenbulcke, F.; Nesslany, F.; Platel, A.; Devred, I.; Hayet, A.; et al. Combined toxic effects and DNA damage to two plant species exposed to binary metal mixtures (Cd/Pb). Ecotoxicol. Environ. Saf. 2019, 167, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Hu, S.; Fei, L.; Liu, L.; Wang, Z. Interaction between Cd and Zn on Metal Accumulation, Translocation and Mineral Nutrition in Tall Fescue (Festuca arundinacea). Int. J. Mol. Sci. 2019, 20, 3332. [Google Scholar] [CrossRef] [Green Version]
- Murtaza, B.; Naeem, F.; Shahid, M.; Abbas, G.; Shah, N.S.; Amjad, M.; Bakhat, H.F.; Imran, M.; Niazi, N.K.; Murtaza, G. A multivariate analysis of physiological and antioxidant responses and health hazards of wheat under cadmium and lead stress. Environ. Sci. Pollut. Res. 2019, 26, 362–370. [Google Scholar] [CrossRef]
- Song, P.; Xu, D.; Yue, J.; Ma, Y.; Dong, S.; Feng, J. Recent advances in soil remediation technology for heavy metal contaminated sites: A critical review. Sci. Total Environ. 2022, 838, 156417. [Google Scholar] [CrossRef]
- Sharma, J.K.; Kumar, N.; Singh, N.P.; Santal, A.R. Phytoremediation technologies and their mechanism for removal of heavy metal from contaminated soil: An approach for a sustainable environment. Front. Plant Sci. 2023, 14, 1076876. [Google Scholar] [CrossRef]
- Sabreena; Hassan, S.; Bhat, S.A.; Kumar, V.; Ganai, B.A.; Ameen, F. Phytoremediation of Heavy Metals: An Indispensable Contrivance in Green Remediation Technology. Plants 2022, 11, 1255. [Google Scholar] [CrossRef]
- Thongchai, A.; Meeinkuirt, W.; Taeprayoon, P.; Pichtel, J. Soil amendments for cadmium phytostabilization by five marigold cultivars. Environ. Sci. Pollut. Res. 2019, 26, 8737–8747. [Google Scholar] [CrossRef]
- Saleem, M.H.; Ali, S.; Rehman, M.; Rana, M.S.; Rizwan, M.; Kamran, M.; Imran, M.; Riaz, M.; Soliman, M.H.; Elkelish, A.; et al. Influence of phosphorus on copper phytoextraction via modulating cellular organelles in two jute (Corchorus capsularis L.) varieties grown in a copper mining soil of Hubei Province, China. Chemosphere 2020, 248, 126032. [Google Scholar] [CrossRef]
- Pinto-Poblete, A.; Retamal-Salgado, J.; López, M.D.; Zapata, N.; Sierra-Almeida, A.; Schoebitz, M. Combined Effect of Microplastics and Cd Alters the Enzymatic Activity of Soil and the Productivity of Strawberry Plants. Plants 2022, 11, 536. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, X.; Song, N. Polyethylene microplastics increase cadmium uptake in lettuce (Lactuca sativa L.) by altering the soil microenvironment. Sci. Total Environ. 2021, 784, 147133. [Google Scholar] [CrossRef] [PubMed]
- Ergönül, M.B.; Nassouhi, D.; Atasağun, S. Modeling of the bioaccumulative efficiency of Pistia stratiotes exposed to Pb, Cd, and Pb + Cd mixtures in nutrient-poor media. Int. J. Phytoremediation 2019, 22, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Cai, L.; Qi, S.; Wu, J.; Gu, X.S. Heavy metal remediation with Ficus microcarpa through transplantation and its environmental risks through field scale experiment. Chemosphere 2018, 193, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Bao, Y.; Qin, F.; Zhang, X.; Chen, H.; Liang, A.; Li, J.; Zhuo, Q.; Gao, Z.; Lu, P.; et al. Preliminary study on pharmacodynamics of Ficus microphylla. J. Guangdong Pharm. Univ. 2019, 35, 407–411. [Google Scholar]
- Wang, J. Occurrence and control of Ficus Gynairothrips uzeli Zimmerman in Huili region. Contemp. Hortic. 2019, 383, 161–162. [Google Scholar]
- Jiang, Y.; Tang, T.; Chen, J.; Feng, C.; Huang, X. Effects of cadmium stress on chlorophyll fluorescence parameters and physiological indexes of Ceratopteris thalictroides seedling. Jiangsu Agric. Sci. 2015, 43, 357–360. [Google Scholar]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive- substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide Dismutase: I. Occurrence in Higher Plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Lin, J.; Wang, G. Doubled CO2 could improve the drought tolerance better in sensitive cultivars than in tolerant cultivars in spring wheat. Plant Sci. 2002, 163, 627–637. [Google Scholar] [CrossRef]
- Knörzer, O.C.; Burner, J.; Boger, P. Alterations in the antioxidative system of suspension-cultured soybean cells (Glycine max) induced by oxidative stress. Physiol. Plantarum 1996, 97, 388–396. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Method. Enzymol. 1984, 105, 121–126. [Google Scholar]
- Zhou, Q.Y.; Huang, Z.M.; Chen, Y. Growth Physiology and Absorption Characteristics of Ficus Virens and Ligustrum Lucidum Under Combined Stress of Pb and Cd. J. Southwest For. Univ. (Nat. Sci.) 2019, 39, 33–40. [Google Scholar]
- Yadav, M.; Gupta, P.; Seth, C.S. Foliar application of α-lipoic acid attenuates cadmium toxicity on photosynthetic pigments and nitrogen metabolism in Solanum lycopersicum L. Acta Physiol. Plant. 2022, 44, 112. [Google Scholar] [CrossRef]
- Bao, S. Analysis of Soil Agro-Chemistry; China Agriculture Press: Beijing, China, 1999. [Google Scholar]
- Lu, R. Analytical Methods of Soil Agrochemistry; China Agriculture Science and Technique Press: Beijing, China, 1999; pp. 85–96. [Google Scholar]
- Huang, B.; Li, Z.; Huang, J.; Guo, L.; Nie, X.; Wang, Y.; Zhang, Y.; Zeng, G. Adsorption characteristics of Cu and Zn onto various size fractions of aggregates from red paddy soil. J. Hazard. Mater. 2014, 264, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Fatemi, H.; Pour, B.E.; Rizwan, M. Isolation and characterization of lead (Pb) resistant microbes and their combined use with silicon nanoparticles improved the growth, photosynthesis and antioxidant capacity of coriander (Coriandrum sativum L.) under Pb stress. Environ. Pollut. 2020, 266, 114982. [Google Scholar] [CrossRef] [PubMed]
- Nyitrai, P.; Bóka, K.; Gáspár, L.; Sárvári, É.; Lenti, K.; Kereszte, Á. Characterization of the stimulating effect of low-dose stressors in maize and bean seedlings. J. Plant Physiol. 2003, 160, 1175–1183. [Google Scholar] [CrossRef]
- Gao, M.; Liu, Y.; Dong, Y.; Song, Z. Effect of polyethylene particles on dibutyl phthalate toxicity in lettuce (Lactuca sativa L.). J. Hazard. Mater. 2020, 401, 123422. [Google Scholar] [CrossRef]
- Fu, Y.; Yang, X.; Shen, H. Root iron plaque alleviates cadmium toxicity to rice (Oryza sativa) seedlings. Ecotoxicol. Environ. Saf. 2018, 161, 534–541. [Google Scholar] [CrossRef]
- Giannakoula, A.; Therios, I.; Chatzissavvidis, C. Effect of Lead and Copper on Photosynthetic Apparatus in Citrus (Citrus aurantium L.) Plants. The Role of Antioxidants in Oxidative Damage as a Response to Heavy Metal Stress. Plants 2021, 10, 155. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Sharkey, T.D. Stomatal Conductance and Photosynthesis. Annu. Rev. Plant Physiol. 1982, 33, 317–345. [Google Scholar] [CrossRef]
- Keyster, M.; Niekerk, L.-A.; Basson, G.; Carelse, M.; Bakare, O.; Ludidi, N.; Klein, A.; Mekuto, L.; Gokul, A. Decoding Heavy Metal Stress Signalling in Plants: Towards Improved Food Security and Safety. Plants 2020, 9, 1781. [Google Scholar] [CrossRef] [PubMed]
- Arena, C.; Figlioli, F.; Sorrentino, M.C.; Izzo, L.G.; Capozzi, F.; Giordano, S.; Spagnuolo, V. Ultrastructural, protein and photosynthetic alterations induced by Pb and Cd in Cynara cardunculus L. and its potential for phytoremediation. Ecotoxicol. Environ. Saf. 2017, 145, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Gong, C.; Ju, X.; Zhu, Z.; Shen, W.; Shen, Z.; Cui, J. Hemin through the heme oxygenase 1/ferrous iron, carbon monoxide system involved in zinc tolerance in Oryza Sativa L. J. Plant Growth Regul. 2018, 37, 947–957. [Google Scholar] [CrossRef]
- Huang, X.; Kuang, J.; Li, W.; Guan, J.; Cheng, X. Effects of Pb2+ and Cd2+ Stresses on Leaf Ultrastructure of Ficus parvifolia. J. Southwest For. Univ. (Nat. Sci.) 2017, 37, 41–47. [Google Scholar]
- Piao, L.; Wang, Y.; Liu, X.; Sun, G.; Zhang, S.; Yan, J.; Chen, Y.; Meng, Y.; Li, M.; Gu, W. Exogenous Hemin alleviated cadmium stress in maize (Zea mays L.) by enhancing leaf photosynthesis, AsA-GSH cycle and polyamine metabolism. Front. Plant Sci. 2022, 13, 993675. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, X.; Li, K.; Wu, M.; Zhang, R.; Zhang, L.; Chen, G. Photosynthetic responses of Oryza sativa L. seedlings to cadmium stress: Physiological, biochemical and ultrastructural analyses. Biometals 2014, 27, 389–401. [Google Scholar] [CrossRef]
- Li, L.; Gu, W.; Li, J.; Li, C.; Xie, T.; Qu, D.; Meng, Y.; Li, C.; Wei, S. Exogenously applied spermidine alleviates photosynthetic inhibition under drought stress in maize (Zea mays L.) seedlings associated with changes in endogenous polyamines and phytohormones. Plant Physiol. Biochem. 2018, 129, 35–55. [Google Scholar] [CrossRef]
- Haider, F.U.; Cai, L.; Coulter, J.A.; Cheema, S.A.; Wu, J.; Zhang, R.; Ma, W.; Farooq, M. Cadmium toxicity in plants: Impacts and remediation strategies. Ecotoxicol. Environ. Saf. 2021, 211, 111887. [Google Scholar] [CrossRef]
- Chandrasekhar, C.; Ray, J.G. Lead accumulation, growth responses and biochemical changes of three plant species exposed to soil amended with different concentrations of lead nitrate. Ecotoxicol. Environ. Saf. 2019, 171, 26–36. [Google Scholar] [CrossRef]
- Khanna, K.; Jamwal, V.L.; Kohli, S.K.; Gandhi, S.G.; Ohri, P.; Bhardwaj, R.; Abd-Allah, E.F.; Hashem, A.; Ahmad, P. Plant growth promoting rhizo-bacteria induced Cd tolerance in Lycopersicon esculentum through altered antioxidative defense expression. Chemosphere 2019, 217, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhang, H.; Wang, Y.; He, G.; Wang, J.; Guo, D.; Li, T.; Sun, G.; Zhang, H. The role of antioxidant mechanism in photosynthesis under heavy metals Cd or Zn exposure in tobacco leaves. J. Plant Interact. 2021, 16, 354–366. [Google Scholar] [CrossRef]
- Sun, M.; Li, S.; Gong, Q.; Xiao, Y.; Peng, F. Leucine Contributes to Copper Stress Tolerance in Peach (Prunus persica) Seedlings by Enhancing Photosynthesis and the Antioxidant Defense System. Antioxidants 2022, 11, 2455. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Seth, C.S. Salicylic acid alleviates chromium (VI) toxicity by restricting its uptake, improving photosynthesis and augmenting antioxidant defense in Solanum lycopersicum L. Physiol. Mol. Biol. Plants 2021, 27, 2651–2664. [Google Scholar] [CrossRef]
- Kumar, D.; Seth, C.S. Photosynthesis, lipid peroxidation, and antioxidative responses of Helianthus annuus L. against chromium (VI) accumulation. Int. J. Phytoremediat. 2022, 24, 590–599. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, C.; Wu, Z.; Liu, X.; Cai, M.; Jia, W.; Zhao, X. Selenium reduces cadmium accumulation in seed by increasing cadmium retention in root of oilseed rape (Brassica napus L.). Environ. Exp. Bot. 2019, 158, 161–170. [Google Scholar] [CrossRef]
- Lanza, M.G.D.B.; Reis, A.R.D. Roles of selenium in mineral plant nutrition: ROS scavenging responses against abiotic stresses. Plant Physiol. Biochem. 2021, 164, 27–43. [Google Scholar] [CrossRef]
- Wu, W.; Nan, Z.; Wang, S.; Zhao, Z.; Zhou, T. Uptake Effect of Cd and Pb by Rape under Single Cd/Pb and Cd-Pb Combined Stress. Environ. Sci. 2012, 33, 3253–3260. [Google Scholar]
- Chen, Z.; Tang, Y.-T.; Yao, A.-J.; Cao, J.; Wu, Z.-H.; Peng, Z.-R.; Wang, S.-Z.; Xiao, S.; Baker, A.J.M.; Qiu, R.-L. Mitigation of Cd accumulation in paddy rice (Oryza sativa L.) by Fe fertilization. Environ. Pollut. 2017, 231, 549–559. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, J.; Huang, Q.; Tang, S.; Wang, J.; Hu, P.; Shao, G. Can liming reduce Cadmium (Cd) accumulation in rice (Oryza sativa) in slightly acidic soils? A contradictory dynamic equilibrium between Cd uptake capacity of roots and Cd immobilization in soils. Chemosphere 2018, 193, 547–556. [Google Scholar] [CrossRef]
- Welch, R.M.; Hart, J.J.; Norvell, W.A.; Sullivan, L.A.; Kochian, L.V. Effects of nutrient solution zinc activity on net uptake, translocation, and root export of cadmium and zinc by separated sections of intact durum wheat (Triticum turgidum L. var durum) seedling roots. Plant Soil 1999, 208, 243–250. [Google Scholar] [CrossRef]
- Liu, Z.; Bai, Y.; Yang, T.M. Effect of Cd on the Uptake and Translocation of Pb, Cu, Zn, and Ni in Potato and Wheat Grown in Sierozem. Soil Sediment Contam. 2019, 28, 650–669. [Google Scholar]
- Du, J.; Zeng, J.; Ming, X.; He, Q.; Tao, Q.; Jiang, M.; Gao, S.; Li, X.; Lei, T.; Pan, Y.; et al. The presence of zinc reduced cadmium uptake and translocation in Cosmos bipinnatu sseedlings under cadmium/zinc combined stress. Plant Physiol. Biochem. 2020, 151, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Lu, L. Advances in Genes-Encoding Transporters for Cadmium Uptake, Translocation, and Accumulation in Plants. Toxics 2022, 10, 411. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Chen, D.; Xue, R.; Long, J.; Lin, X.; Lin, Y.; Jia, L.; Zeng, R.; Song, Y. Effects of boron, silicon and their interactions on cadmium accumulation and toxicity in rice plants. J. Hazard. Mater. 2019, 367, 447–455. [Google Scholar] [CrossRef]
- Zeng, J.; Li, X.; Wang, X.; Zhang, K.; Wang, Y.; Kang, H.; Chen, G.; Lan, T.; Zhang, Z.; Yuan, S.; et al. Cadmium and lead mixtures are less toxic to the Chinese medicinal plant Ligusticum chuanxiong Hort. than either metal alone. Ecotoxic. Environ. Saf. 2020, 193, 110342. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, L.; Huang, X.; Zhou, Y.; Quan, Q.; Li, Y.; Zhu, X. Response of photosynthesis to different concentrations of heavy metals in Davidia involucrata. PLoS ONE 2020, 15, e0228563. [Google Scholar] [CrossRef] [Green Version]
- Amjad, M.; Iqbal, M.; Abbas, G.; Farooq, A.B.U.; Naeem, M.A.; Imran, M.; Murtaza, B.; Nadeem, M.; Jacobsen, S.-E. Assessment of cadmium and lead tolerance potential of quinoa (Chenopodium quinoa Willd) and its implications for phytoremediation andhuman health. Environ. Geochem. Health 2022, 44, 1487–1500. [Google Scholar] [CrossRef]
- Li, H.; Li, Z.; Huang, L.; Mao, X.; Dong, Y.; Fu, S.; Su, R.; Chang, Y.; Zhang, C. Environmental Factors Influence the Effects of Biochar on the Bioavailability of Cd and Pb in Soil Under Flooding Condition. Water Air Soil Pollut. 2023, 234, 100. [Google Scholar] [CrossRef]
- Liu, H.; Qian, T.; Zhang, M. The sorption of selenite from aqueous solutions by pyrite. Toxicol. Environ. Chem. 2015, 97, 282–290. [Google Scholar] [CrossRef]
- Huo, L.; Xie, W.; Qian, T.; Guan, X.; Zhao, D. Reductive immobilization of pertechnetate in soil and groundwater using synthetic pyrite nanoparticles. Chemosphere 2017, 174, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Qian, T.; Huo, L.; Li, Y.; Zhao, D. Immobilization of hexavalent chromium in soil and groundwater using synthetic pyrite particles. Environ. Pollut. 2019, 255, 112992. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Qu, M.; Chen, J.; Guang, X.; Zhang, J.; Liu, M.; Kang, J.; Zhao, Y.; Huang, B. Heavy metal accumulation in the surrounding areas affected by mining in China: Spatial distribution patterns, risk assessment, and influencing factors. Sci. Total Environ. 2022, 825, 154004. [Google Scholar] [CrossRef] [PubMed]
- Pusz, A.; Wiśniewska, M.; Rogalski, D. Assessment of the Accumulation Ability of Festuca rubra L. and Alyssum saxatile L. Tested on Soils Contaminated with Zn, Cd, Ni, Pb, Cr, and Cu. Resources 2021, 10, 46. [Google Scholar] [CrossRef]





| Soil Characteristic | Value |
|---|---|
| pH | 6.90 ± 0.01 |
| Organic matter | 156.50 ± 0.56 g·kg−1 |
| Organic carbon | 90.80 ± 0.32 g·kg−1 |
| Total nitrogen | 1.25 ± 0.02 g·kg−1 |
| available phosphorus | 10.59 ± 0.10 g·kg−1 |
| Total potassium | 8.24 ± 0.17 g·kg−1 |
| Pb content | 23.17 ± 1.04 mg·kg−1 |
| Cd content | 0.45 ± 0.02 mg·kg−1 |
| Treatments | Pb Concentration (mmol·L−1) | Cd Concentration (mmol·L−1) |
|---|---|---|
| CK | 0 | 0 |
| Pb2 | 2 | 0 |
| Pb4 | 4 | 0 |
| Cd2 | 0 | 2 |
| Cd4 | 0 | 4 |
| Pb2Cd2 | 2 | 2 |
| Pb2Cd4 | 2 | 4 |
| Pb4Cd2 | 4 | 2 |
| Pb4Cd4 | 4 | 4 |
| Treatments | Root Biomass (g) | Stem Biomass (g) | Leaf Biomass (g) | Total Biomass (g) |
|---|---|---|---|---|
| CK | 5.08 ± 0.04bc | 2.96 ± 0.49c | 1.10 ± 0.45de | 9.14 ± 0.82d |
| Pb2 | 6.08 ± 0.33c | 2.73 ± 0.66bc | 0.95 ± 0.20cd | 9.77 ± 0.93d |
| Pb4 | 5.00 ± 1.02bc | 2.74 ± 0.65bc | 1.33 ± 0.35e | 9.07 ± 1.65d |
| Cd2 | 3.08 ± 0.37a | 1.89 ± 0.55ab | 0.28 ± 0.12a | 5.25 ± 0.85ab |
| Cd4 | 2.81 ± 0.56a | 2.02 ± 0.57ab | 0.27 ± 0.09a | 5.07 ± 0.93a |
| Pb2Cd2 | 5.11 ± 0.59bc | 2.27 ± 0.48abc | 0.54 ± 0.16ab | 7.92 ± 1.18bc |
| Pb2Cd4 | 5.23 ± 1.42bc | 3.03 ± 0.57c | 0.71 ± 0.18bc | 8.97 ± 1.70d |
| Pb4Cd2 | 4.30 ± 1.60ab | 2.38 ± 0.34abc | 0.81 ± 0.20bc | 7.48 ± 2.03bcd |
| Pb4Cd4 | 3.64 ± 1.01ab | 1.79 ± 0.33a | 0.64 ± 0.31ab | 6.07 ± 1.13abc |
| Pb | ** | ns | * | ** |
| Cd | * | * | *** | *** |
| Pb × Cd | ns | ns | ns | ns |
| Treatments | Pn (μmol·m−2·s−1) | Gs (mol·m−2·s−1) | Ci (μmol·mol−1) | Tr (mol·m−2·s−1) |
|---|---|---|---|---|
| CK | 6.27 ± 1.02c | 0.126 ± 0.013de | 296.88 ± 6.27bc | 2.72 ± 0.21d |
| Pb2 | 7.89 ± 0.11d | 0.137 ± 0.002e | 281.38 ± 0.69b | 2.70 ± 0.05d |
| Pb4 | 8.40 ± 0.92d | 0.197 ± 0.020f | 305.48 ± 2.62bc | 3.55 ± 0.28e |
| Cd2 | 4.78 ± 1.43b | 0.109 ± 0.023cd | 311.37 ± 11.81c | 1.80 ± 0.42bc |
| Cd4 | 2.99 ± 1.07a | 0.052 ± 0.022a | 282.84 ± 16.92b | 0.90 ± 0.33a |
| Pb2Cd2 | 4.21 ± 1.01ab | 0.088 ± 0.010bc | 301.86 ± 20.49bc | 2.03 ± 0.19c |
| Pb2Cd4 | 4.09 ± 0.21ab | 0.072 ± 0.007ab | 289.81 ± 5.54bc | 1.65 ± 0.14bc |
| Pb4Cd2 | 5.28 ± 1.40bc | 0.068 ± 0.033ab | 244.30 ± 24.89a | 1.59 ± 0.60bc |
| Pb4Cd4 | 4.66 ± 0.83b | 0.060 ± 0.012ab | 251.45 ± 29.92a | 1.48 ± 0.25b |
| Pb | ** | ns | *** | * |
| Cd | *** | *** | * | *** |
| Pb × Cd | ns | *** | *** | *** |
| Treatments | Fv/Fm | Y(II) | qp | NPQ | ETR |
|---|---|---|---|---|---|
| CK | 0.7290 ± 0.0164d | 0.6363 ± 0.0168d | 0.9218 ± 0.0062e | 0.3163 ± 0.0177a | 16.85 ± 0.40f |
| Pb2 | 0.7034 ± 0.0144bc | 0.5954 ± 0.0184c | 0.8983 ± 0.0075d | 0.2989 ± 0.0269a | 15.25 ± 0.46e |
| Pb4 | 0.7220 ± 0.0338cd | 0.6090 ± 0.0239cd | 0.8990 ± 0.0298d | 0.3085 ± 0.0550a | 16.26 ± 1.09f |
| Cd2 | 0.6883 ± 0.0209ab | 0.5558 ± 0.0124b | 0.8584 ± 0.0281b | 0.2997 ± 0.0328a | 13.15 ± 0.54b |
| Cd4 | 0.6685 ± 0.0303a | 0.5036 ± 0.0532a | 0.8262 ± 0.0443a | 0.5036 ± 0.1163b | 12.18 ± 1.12a |
| Pb2Cd2 | 0.7253 ± 0.0241cd | 0.5891 ± 0.0097c | 0.8633 ± 0.0471bc | 0.2772 ± 0.0279a | 14.08 ± 0.50cd |
| Pb2Cd4 | 0.6998 ± 0.0333bc | 0.5861 ± 0.0345c | 0.8553 ± 0.0313b | 0.3074 ± 0.0495a | 13.96 ± 0.19cd |
| Pb4Cd2 | 0.7323 ± 0.0176d | 0.6019 ± 0.0404c | 0.8915 ± 0.0198cd | 0.2728 ± 0.0310a | 14.48 ± 0.71d |
| Pb4Cd4 | 0.7003 ± 0.0269bc | 0.5173 ± 0.0602a | 0.8636 ± 0.0366bc | 0.2889 ± 0.0154a | 13.48 ± 0.85bc |
| Pb | * | * | ns | ns | * |
| Cd | *** | *** | * | *** | *** |
| Pb × Cd | ** | *** | ns | * | *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Cheng, X.; Feng, C.; Huang, X. Interaction of Lead and Cadmium Reduced Cadmium Toxicity in Ficus parvifolia Seedlings. Toxics 2023, 11, 271. https://doi.org/10.3390/toxics11030271
Li Y, Cheng X, Feng C, Huang X. Interaction of Lead and Cadmium Reduced Cadmium Toxicity in Ficus parvifolia Seedlings. Toxics. 2023; 11(3):271. https://doi.org/10.3390/toxics11030271
Chicago/Turabian StyleLi, Yan, Xiaomao Cheng, Chengcheng Feng, and Xiaoxia Huang. 2023. "Interaction of Lead and Cadmium Reduced Cadmium Toxicity in Ficus parvifolia Seedlings" Toxics 11, no. 3: 271. https://doi.org/10.3390/toxics11030271
APA StyleLi, Y., Cheng, X., Feng, C., & Huang, X. (2023). Interaction of Lead and Cadmium Reduced Cadmium Toxicity in Ficus parvifolia Seedlings. Toxics, 11(3), 271. https://doi.org/10.3390/toxics11030271