The Adsorption Behaviors and Mechanisms of Humic Substances by Thermally Oxidized Graphitic Carbon Nitride
Abstract
:1. Introduction
2. Materials and Methods
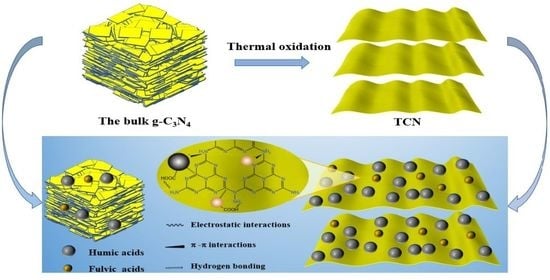
2.1. Preparation of Adsorbents and HS Materials
2.2. Characterization of Adsorbents
2.3. Adsorption Procedures
2.4. Adsorption Mechanisms
3. Results and Discussion
3.1. Characterization of Adsorbents
3.2. Comparison of HA Adsorption Using Different Adsorbents
3.3. Adsorption Kinetics
3.4. Effect of pH on Adsorption of HSs
3.5. Effect of the Common Ions on Adsorption of HSs
3.6. Adsorption Isotherms and Thermodynamic Analysis
3.7. Adsorption Mechanisms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, H.; Ai, Z.H.; Zhang, L.Z. Anoxic and oxic removal of humic acids with Fe@Fe2O3 core-shell nanowires: A comparative study. Water Res. 2014, 52, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Mohanraj, J.; Durgalakshmi, D.; Saravanan, R. Water-soluble graphitic carbon nitride for clean environmental applications. Environ. Pollut. 2021, 269, 116172. [Google Scholar] [CrossRef] [PubMed]
- Adusei-Gyamfi, J.; Ouddane, B.; Rietveld, L.; Cornard, J.-P.; Criquet, J. Natural organic matter-cations complexation and its impact on water treatment: A critical review. Water Res. 2019, 160, 130–147. [Google Scholar] [CrossRef] [PubMed]
- Goslan, E.H.; Seigle, C.; Purcell, D.; Henderson, R.; Parsons, S.A.; Jefferson, B.; Judd, S.J. Carbonaceous and nitrogenous disinfection by-product formation from algal organic matter. Chemosphere 2017, 170, 1–9. [Google Scholar] [CrossRef]
- Chen, W.M.; Li, Q.B. Elimination of uv-quenching substances from mbr- and saarb-treated mature landfill leachates in an ozonation process: A comparative study. Chemosphere 2020, 242, 125256. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.L.; Chen, W.; Liu, C. Preparation of novel magnetic chitosan nanoparticle and its application for removal of humic acid from aqueous solution. Appl. Surf. Sci. 2014, 292, 1067–1076. [Google Scholar] [CrossRef]
- Liu, Z.J.; Zhou, S.Q. Removal of humic acid from aqueous solution using polyacrylamide/chitosan semi-ipn hydrogel. Water Sci. Technol. 2018, 2017, 16–26. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Sillanpää, M. Removal of natural organic matter (NOM) and its constituents from water by adsorption—A review. Chemosphere 2017, 166, 497–510. [Google Scholar] [CrossRef]
- Giasuddin, A.B.M.; Kanel, S.R.; Choi, H. Adsorption of humic acid onto nanoscale zerovalent iron and its effect on arsenic removal. Environ. Sci. Technol. 2007, 41, 2022–2027. [Google Scholar] [CrossRef]
- Wang, R.X.; Wen, T.; Wu, X.L.; Xu, A.W. Highly efficient removal of humic acid from aqueous solutions by mg/al layered double hydroxides-Fe3O4 nanocomposites. RSC Adv. 2014, 4, 21802–21809. [Google Scholar] [CrossRef]
- Jiang, L.; Li, Y.; Shao, Y.; Zhang, Y.; Han, R.; Li, S.; Wei, W. Enhanced removal of humic acid from aqueous solution by novel stabilized nano-amorphous calcium phosphate: Behaviors and mechanisms. Appl. Surf. Sci. 2018, 427, 965–975. [Google Scholar] [CrossRef]
- Fronczak, M. Adsorption performance of graphitic carbon nitride-based materials: Current state of the art. J. Environ. Chem. Eng. 2020, 8, 104411. [Google Scholar] [CrossRef]
- Wang, S.; Li, D.; Sun, C.; Yang, S.; Guan, Y.; He, H. Synthesis and characterization of g-C3N4/Ag3VO4 composites with significantly enhanced visible-light photocatalytic activity for triphenylmethane dye degradation. Appl. Catal. B: Environ. 2014, 144, 885–892. [Google Scholar] [CrossRef]
- Mkhalid, I.A.; Mohamed, R.M.; Alhaddad, M.; Basaleh, A.; Al-Hajji, L.A.; Ismail, A.A. S-scheme mesoporous Li2MnO3/g-C3N4 heterojunctions as efficient photocatalysts for the mineralization of trichloroethylene in aqueous media. J. Colloid Interface Sci. 2022, 614, 160–171. [Google Scholar] [CrossRef]
- Li, X.; Xing, J.; Zhang, C.; Han, B.; Zhang, Y.; Wen, T.; Leng, R.; Jiang, Z.; Ai, Y.; Wang, X. Adsorption of lead on sulfur-doped graphitic carbon nitride nanosheets: Experimental and theoretical calculation study. ACS Sustain. Chem. Eng. 2018, 6, 10606–10615. [Google Scholar] [CrossRef]
- Zhu, L.; You, L.J.; Wang, Y.; Shi, Z.X. The application of graphitic carbon nitride for the adsorption of Pb2+ ion from aqueous solution. Mater. Res. Express 2017, 4, 075606. [Google Scholar] [CrossRef]
- Xiao, G.; Wang, Y.Q.; Xu, S.N.; Li, P.F.; Yang, C.; Jin, Y.; Sun, Q.F.; Su, H.J. Superior adsorption performance of graphitic carbon nitride nanosheets for both cationic and anionic heavy metals from wastewater. Chin. J. Chem. Eng. 2019, 27, 305–313. [Google Scholar] [CrossRef]
- Tan, J.Z.Y.; Nursam, N.M.; Xia, F.; Sani, M.A.; Li, W.; Wang, X.D.; Caruso, R.A. High-performance coral reef-like carbon nitrides: Synthesis and application in photocatalysis and heavy metal ion adsorption. ACS Appl. Mater. Interfaces 2017, 9, 4540–4547. [Google Scholar] [CrossRef]
- Guo, S.Z.; Wu, K.L.; Gao, Y.; Liu, L.H.; Zhu, X.X.; Li, X.L.; Zhang, F. Efficient removal of Zn (II), Pb (II), and Cd (II) in waste water based on magnetic graphitic carbon nitride materials with enhanced adsorption capacity. J. Chem. Eng. Data 2018, 63, 3902–3912. [Google Scholar] [CrossRef]
- Zhu, B.; Xia, P.; Ho, W.; Yu, J. Isoelectric point and adsorption activity of porous g-C3N4. Appl. Surf. Sci. 2015, 344, 188–195. [Google Scholar] [CrossRef]
- Yan, T.; Chen, H.; Wang, X.; Jiang, F. Adsorption of perfluorooctane sulfonate (PFOS) on mesoporous carbon nitride. RSC Adv. 2013, 3, 22480–22489. [Google Scholar] [CrossRef]
- Wang, J.; Yue, D.; Cui, D.; Zhang, L.; Dong, X. Insights into adsorption of humic substances on graphitic carbon nitride. Environ. Sci. Technol. 2021, 55, 7910–7919. [Google Scholar] [CrossRef]
- Cao, S.; Low, J.; Yu, J.; Jaroniec, M. Polymeric photocatalysts based on graphitic carbon nitride. Adv. Mater. 2015, 27, 2150–2176. [Google Scholar] [CrossRef]
- Yan, H. Soft-templating synthesis of mesoporous graphitic carbon nitride with enhanced photocatalytic h2 evolution under visible light. Chem. Commun. 2012, 48, 3430–3432. [Google Scholar] [CrossRef]
- Niu, P.; Zhang, L.L.; Liu, G.; Cheng, H.M. Graphene-like carbon nitride nanosheets for improved photocatalytic activities. Adv. Funct. Mater. 2012, 22, 4763–4770. [Google Scholar] [CrossRef]
- Dong, F.; Li, Y.; Wang, Z.; Ho, W.-K. Enhanced visible light photocatalytic activity and oxidation ability of porous graphene-like g-C3N4 nanosheets via thermal exfoliation. Appl. Surf. Sci. 2015, 358, 393–403. [Google Scholar] [CrossRef]
- Xu, B.; Ahmed, M.B.; Zhou, J.L.; Altaee, A.; Xu, G.; Wu, M. Graphitic carbon nitride based nanocomposites for the photocatalysis of organic contaminants under visible irradiation: Progress, limitations and future directions. Sci. Total Environ. 2018, 633, 546–559. [Google Scholar] [CrossRef]
- Fina, F.; Callear, S.K.; Carins, G.M.; Irvine, J.T.S. Structural investigation of graphitic carbon nitride via xrd and neutron diffraction. Chem. Mat. 2015, 27, 2612–2618. [Google Scholar] [CrossRef]
- Zhao, Z.W.; Sun, Y.J.; Dong, F. Graphitic carbon nitride based nanocomposites: A review. Nanoscale 2015, 7, 15–37. [Google Scholar] [CrossRef]
- Lotsch, B.V.; Doblinger, M.; Sehnert, J.; Seyfarth, L.; Senker, J.; Oeckler, O.; Schnick, W. Unmasking melon by a complementary approach employing electron diffraction, solid-state nmr spectroscopy, and theoretical calculations-structural characterization of a carbon nitride polymer. Chem. Eur. J. 2007, 13, 4969–4980. [Google Scholar] [CrossRef]
- Tong, J.H.; Li, Q.; Li, W.Y.; Wang, W.H.; Ma, W.M.; Su, B.T.; Bo, L.L. MoS2 thin sheet growing on nitrogen self-doped mesoporous graphic carbon derived from ZIF-8 with highly electrocatalytic performance on hydrogen evolution reaction. ACS Sustain. Chem. Eng. 2017, 5, 10240–10247. [Google Scholar] [CrossRef]
- Bhunia, K.; Chandra, M.; Khilari, S.; Pradhan, D. Bimetallic ptau alloy nanoparticles-integrated g-C3N4 hybrid as an efficient photocatalyst for water-to-hydrogen conversion. ACS Appl. Mater. Interfaces 2019, 11, 478–488. [Google Scholar] [CrossRef]
- Dong, F.; Ou, M.Y.; Jiang, Y.K.; Guo, S.; Wu, Z.B. Efficient and durable visible light photocatalytic performance of porous carbon nitride nanosheets for air purification. Ind. Eng. Chem. Res. 2014, 53, 2318–2330. [Google Scholar] [CrossRef]
- Wang, J.C.; Li, H.X.; Yue, D.B. Enhanced adsorption of humic/fulvic acids onto urea-derived graphitic carbon nitride. J. Hazard. Mater. 2022, 424, 127643. [Google Scholar] [CrossRef]
- Li, W.F.; Wang, Y.; Yang, X.Y.; Liu, F.Q.; Li, W.H. Graphitic carbon nitride prepared by rapid recrystallization for photoelectrochemical anticorrosion. ACS Appl. Nano Mater. 2019, 2, 7559–7565. [Google Scholar] [CrossRef]
- Wu, F.C.; Tseng, R.L.; Juang, R.S. Characteristics of elovich equation used for the analysis of adsorption kinetics in dye-chitosan systems. Chem. Eng. J. 2009, 150, 366–373. [Google Scholar] [CrossRef]
- Wang, X.; Liang, S.; Wang, Y.; Xu, B.; Bai, Y.; Shu, T.; Xing, B. Sorption of peat humic acids to multi-walled carbon nanotubes. Environ. Sci. Technol. 2011, 45, 9276–9283. [Google Scholar] [CrossRef]
- Yang, K.; Fox, J.T. Adsorption of humic acid by acid-modified granular activated carbon and powder activated carbon. J. Environ. Eng.-ASCE 2018, 144, 04018104. [Google Scholar] [CrossRef]
- Maghsoodloo, S.; Noroozi, B.; Haghi, A.K.; Sorial, G.A. Consequence of chitosan treating on the adsorption of humic acid by granular activated carbon. J. Hazard. Mater. 2011, 191, 380–387. [Google Scholar] [CrossRef]
- Lan, T.; Wu, P.; Liu, Z.; Stroet, M.; Liao, J.; Chai, Z.; Mark, A.E.; Liu, N.; Wang, D. Understanding the effect of ph on the solubility and aggregation extent of humic acid in solution by combining simulation and the experiment. Environ. Sci. Technol. 2022, 56, 917–927. [Google Scholar] [CrossRef]
- Wells, M.J.M.; Stretz, H.A. Supramolecular architectures of natural organic matter. Sci. Total Environ. 2019, 671, 1125–1133. [Google Scholar] [CrossRef]
- Huang, Y.F.; Chen, H.L.; Han, G.H. Research on the adsorption of humic acid on pyrite surface. In Proceedings of the Light Metals Symposium at the 147th Annual TMS Meeting and Exhibition, Phoenix, AZ, USA, 11–15 March 2018; pp. 197–202. [Google Scholar]
- Lozano, P.; Berge, N.D. Single-walled carbon nanotube behavior in representative mature leachate. Waste Manage. 2012, 32, 1699–1711. [Google Scholar] [CrossRef] [PubMed]
- Han, H.W.; Rafiq, M.K.; Zhou, T.Y.; Xu, R.; Masek, O.; Li, X.K. A critical review of clay-based composites with enhanced adsorption performance for metal and organic pollutants. J. Hazard. Mater. 2019, 369, 780–796. [Google Scholar] [CrossRef]
- Lee, S.; Hur, J. Heterogeneous adsorption behavior of landfill leachate on granular activated carbon revealed by fluorescence excitation emission matrix (EEM)-parallel factor analysis PARAFAC). Chemosphere 2016, 149, 41–48. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Da'ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef]
- Sun, W.L.; Xia, J.; Li, S.; Sun, F. Effect of natural organic matter (NOM) on Cu (II) adsorption by multi-walled carbon nanotubes: Relationship with nom properties. Chem. Eng. J. 2012, 200, 627–636. [Google Scholar] [CrossRef]
- Wang, M.S.; Liao, L.B.; Zhang, X.L.; Li, Z.H. Adsorption of low concentration humic acid from water by palygorskite. Appl. Clay Sci. 2012, 67–68, 164–168. [Google Scholar] [CrossRef]
- Wang, J.; Yue, D.; Li, M.; Wang, H.; Wang, J.; Wang, C.; Wang, H. Application of carbon nitride nanosheets for adsorption of various humic substances from aqueous solutions. Chem. Eng. J. 2023, 454, 140296. [Google Scholar] [CrossRef]
- Wang, J.; Wang, C.; Shi, A.; Shi, Y.; Yue, D.; Zhang, L.; Wang, J.; Wang, H.; Wang, C.; Cui, D. An innovative approach for landfill leachate treatment based on selective adsorption of humic acids with carbon nitride. Chem. Eng. J. 2023, 461, 142090. [Google Scholar] [CrossRef]
- Karanfil, T.; Kitis, M.; Kilduff, J.E.; Wigton, A. Role of granular activated carbon surface chemistry on the adsorption of organic compounds. 2. Natural organic matter. Environ. Sci. Technol. 1999, 33, 3225–3233. [Google Scholar] [CrossRef]
- Ateia, M.; Apul, O.G.; Shimizu, Y.; Muflihah, A.; Yoshimura, C.; Karanfil, T. Elucidating adsorptive fractions of natural organic matter on carbon nanotubes. Environ. Sci. Technol. 2017, 51, 7101–7110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.R.; Wang, F.; Wang, Y.X. Electrospun cellulose acetate/chitosan fibers for humic acid removal: Construction guided by intermolecular interaction study. ACS Appl. Polym. Mater. 2021, 3, 5022–5029. [Google Scholar] [CrossRef]
- Yang, K.; Xing, B.S. Adsorption of fulvic acid by carbon nanotubes from water. Environ. Pollut. 2009, 157, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zhan, Y. Adsorption of humic acid from aqueous solution onto unmodified and surfactant-modified chitosan/zeolite composites. Chem. Eng. J. 2012, 200–202, 202–213. [Google Scholar] [CrossRef]
- Ning, Y.; Luo, Z.Q.; Li, Y.L.; Yang, Z.; Liu, D.Q.; Zhang, Y.Y. Alkaline leaching characteristics of uranium from lincang coal: Correlation with the dissolution of coal humic substances. Fuel 2021, 305, 121507. [Google Scholar] [CrossRef]
- Lee, B.-M.; Seo, Y.-S.; Hur, J. Investigation of adsorptive fractionation of humic acid on graphene oxide using fluorescence eem-parafac. Water Res. 2015, 73, 242–251. [Google Scholar] [CrossRef]
- Liu, J.; Cao, J.; Chen, H.; Zhou, D. Adsorptive removal of humic acid from aqueous solution by micro- and mesoporous covalent triazine-based framework. Colloid. Surface. A 2015, 481, 276–282. [Google Scholar] [CrossRef]
- Derakhshani, E.; Naghizadeh, A. Optimization of humic acid removal by adsorption onto bentonite and montmorillonite nanoparticles. J. Mol. Liq. 2018, 259, 76–81. [Google Scholar] [CrossRef]
- Ngah, W.S.W.; Fatinathan, S.; Yosop, N.A. Isotherm and kinetic studies on the adsorption of humic acid onto chitosan-H2SO4 beads. Desalination 2011, 272, 293–300. [Google Scholar] [CrossRef]
- Doulia, D.; Leodopoulos, C.; Gimouhopoulos, K.; Rigas, F. Adsorption of humic acid on acid-activated Greek bentonite. J. Colloid Interface Sci. 2009, 340, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.Y.; Song, S.; Zhang, R.; Wen, T.; Wang, X.X.; Yu, S.J.; Song, W.C.; Hayat, T.; Alsaedi, A.; Wang, X.K. Construction of Layered Double Hydroxides/Hollow Carbon Microsphere Composites and Its Applications for Mutual Removal of Pb(II) and Humic Acid from Aqueous Solutions. ACS Sustain. Chem. Eng. 2017, 5, 11268–11279. [Google Scholar] [CrossRef]
- Tao, Q.; Xu, Z.Y.; Wang, J.H.; Liu, F.L.; Wan, H.Q.; Zheng, S.R. Adsorption of humic acid to aminopropyl functionalized SBA-15. Microporous Mesoporous Mater. 2010, 131, 177–185. [Google Scholar] [CrossRef]
- Wang, Q.Z.; Chen, X.G.; Liu, N.; Wang, S.X.; Liu, C.S.; Meng, X.H.; Liu, C.G. Protonation constants of chitosan with different molecular weight and degree of deacetylation. Carbohydr. Polym. 2006, 65, 194–201. [Google Scholar] [CrossRef]
- Wang, S.B.; Terdkiatburana, T.; Tade, M.O. Single and co-adsorption of heavy metals and humic acid on fly ash. Sep. Purif. Technol. 2008, 58, 353–358. [Google Scholar] [CrossRef]
- Jayalath, S.; Larsen, S.C.; Grassian, V.H. Surface adsorption of Nordic aquatic fulvic acid on amine-functionalized and non-functionalized mesoporous silica nanoparticles. Environ. Sci.-Nano 2018, 5, 2162–2171. [Google Scholar] [CrossRef]
- Zhang, J.; Gong, J.L.; Zenga, G.M.; Ou, X.M.; Jiang, Y.; Chang, Y.N.; Guo, M.; Zhang, C.; Liu, H.Y. Simultaneous removal of humic acid/fulvic acid and lead from landfill leachate using magnetic graphene oxide. Appl. Surf. Sci. 2016, 370, 335–350. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Wang, J.; Yue, D.; Wang, J.; Tang, C.; Zhang, L. The Adsorption Behaviors and Mechanisms of Humic Substances by Thermally Oxidized Graphitic Carbon Nitride. Toxics 2023, 11, 369. https://doi.org/10.3390/toxics11040369
Li H, Wang J, Yue D, Wang J, Tang C, Zhang L. The Adsorption Behaviors and Mechanisms of Humic Substances by Thermally Oxidized Graphitic Carbon Nitride. Toxics. 2023; 11(4):369. https://doi.org/10.3390/toxics11040369
Chicago/Turabian StyleLi, Hongxin, Jianlong Wang, Dongbei Yue, Jianchao Wang, Chu Tang, and Lingyue Zhang. 2023. "The Adsorption Behaviors and Mechanisms of Humic Substances by Thermally Oxidized Graphitic Carbon Nitride" Toxics 11, no. 4: 369. https://doi.org/10.3390/toxics11040369
APA StyleLi, H., Wang, J., Yue, D., Wang, J., Tang, C., & Zhang, L. (2023). The Adsorption Behaviors and Mechanisms of Humic Substances by Thermally Oxidized Graphitic Carbon Nitride. Toxics, 11(4), 369. https://doi.org/10.3390/toxics11040369