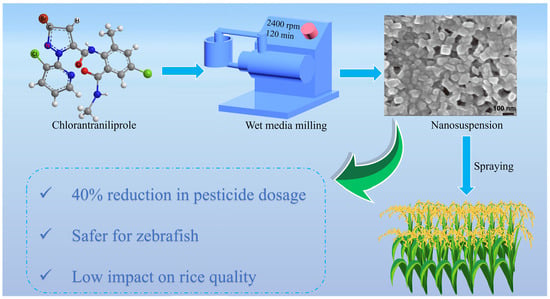
Preparation and Comprehensive Evaluation of the Efficacy and Safety of Chlorantraniliprole Nanosuspension
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of the Preparation Parameters of the Chlorantraniliprole Nanosuspension
2.2. Particle Size and Zeta Potential
2.3. Morphology
2.4. Stability
2.5. Foliar Wettability and Retention
2.6. Field Efficacy on Cnaphalocrocis Medinalis
2.7. Pesticide Residue
2.8. Impact on Rice Quality
2.9. Zebrafish Safety
3. Materials and Methods
3.1. Materials
3.2. Preparation of Chlorantraniliprole Nanosuspension
3.3. Particle Size and Zeta Potential Measurements
3.4. Morphological Characterization
3.5. Stability Test
3.6. Contact Angle Measurement
3.7. Retention Test
3.8. Field Efficacy on Cnaphalocrocis Medinalis
3.9. Residue Test
3.9.1. Sample Collection and Processing
3.9.2. Chromatographic Analysis Conditions
3.9.3. Mass Spectrometric Analysis Conditions
3.9.4. Measurement of Additive Recovery
3.10. Rice Quality Determination
3.11. Toxicity against Zebrafish
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cordova, D.; Benner, E.A.; Sacher, M.D.; Rauh, J.J.; Sopa, J.S.; Lahm, G.P.; Selby, T.P.; Stevenson, T.M.; Flexner, L.; Gutteridge, S. Anthranilic diamides: A new class of insecticides with a novel mode of action, ryanodine receptor activation. Pestic. Biochem. Physiol. 2006, 84, 196–214. [Google Scholar] [CrossRef]
- Cordova, D.; Benner, E.A.; Sacher, M.D.; Rauh, J.J.; Sopa, J.S.; Lahm, G.P.; Selby, T.P.; Stevenson, T.M.; Flexner, L.; Caspar, T. Elucidation of the Mode of Action of RynaxypyrTM, a Selective Ryanodine Receptor Activator. In Pesticide Chemistry: Crop Protection, Public Health, Environmental Safety; Wiley: Hoboken, NJ, USA, 2007; pp. 121–126. [Google Scholar]
- Sial, A.A.; Brunner, J.F.; Garczynski, S.F. Biochemical characterization of chlorantraniliprole and spinetoram resistance in laboratory-selected obliquebanded leafroller, Choristoneura rosaceana (Harris) (Lepidoptera: Tortricidae). Pestic. Biochem. Physiol. 2011, 99, 274–279. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, G.; Zhong, L.; Zhang, F.; Bai, Q.; Zheng, X.; Lu, Z. Resistance monitoring of Chilo suppressalis (Walker) (Lepidoptera: Crambidae) to chlorantraniliprole in eight field populations from east and central China. Crop. Protect. 2017, 100, 196–202. [Google Scholar] [CrossRef]
- Son, M.; Ju, J.; Lu, S.; Han, Y.; Don, Z.; Wan, Y.; Zhen, G.; Zhan, L.; Ha, R.; Jiang, L. Controlling liquid splash on superhydrophobic surfaces by a vesicle surfactant. Sci. Adv. 2017, 3, e1602188. [Google Scholar]
- Nuruzzaman, M.; Rahman, M.M.; Liu, Y.; Naidu, R. Nanoencapsulation, nano-guard for pesticides: A new window for safe application. J. Agric. Food. Chem. 2016, 64, 1447–1483. [Google Scholar] [CrossRef] [PubMed]
- Gan, J.; Hussain, M.; Rathor, N.M. Behaviour of an alginate-kaolin based controlled-release formulation of the herbicide thiobencarb in simulated ecosystems. Pestic. Sci. 1994, 42, 265–272. [Google Scholar] [CrossRef]
- Mogul, M.G.; Akin, H.; Hasirci, N.; Trantolo, D.J.; Gresser, J.D.; Wise, D.L. Controlled release of biologically active agents for purposes of agricultural crop management. Resour. Conserv. Recycl. 1996, 16, 289–320. [Google Scholar] [CrossRef]
- Margni, M.D.P.O. Life cycle impact assessment of pesticides on human health and ecosystems. Agric. Ecosyst. Environ. 2002, 93, 379–392. [Google Scholar] [CrossRef]
- Gong, C.W.; Hasnain, A.; Wang, Q.L.; Liu, D.; Xu, Z.Z.; Zhan, X.X.; Liu, X.M.; Pu, J.; Sun, M.M.; Wang, X.G. Eco-friendly deacetylated chitosan base siRNA biological-nanopesticide loading cyromazine for efficiently controlling Spodoptera frugiperda. Int. J. Biol. Macromol. 2023, 241, 124575. [Google Scholar] [CrossRef]
- Song, S.J.; Wan, M.H.; Feng, W.L.; Tian, Y.; Jiang, X.F.; Luo, Y.; Shen, J. Environmentally friendly Zr-based MOF for pesticide delivery: Ultrahigh loading capacity, pH-responsive release, improved leaf affinity, and enhanced antipest activity. Langmuir 2022, 38, 10867–10874. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, J.Y.Z.; Zhou, J.; Lin, S.K.; Cheng, D.M. pH-responsive release and washout resistance of chitosan-based nano-pesticides for sustainable control of plumeria rust. Int. J. Biol. Macromol. 2022, 222, 188–197. [Google Scholar] [CrossRef]
- Rai, M.; Ingle, A. Role of nanotechnology in agriculture with special reference to management of insect pests. Appl. Microbiol. Biotechnol. 2012, 94, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Zahoor, M.; Khan, R.S.; Ikram, M.; Ul Islam, N. The impact of silver nanoparticles on the growth of plants: The agriculture applications. Heliyon 2023, 9, e16928. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xia, Q.; Gu, N. Preparation of all-trans retinoic acid nanosuspensions using a modified precipitation method. Drug Dev. Ind. Pharm. 2006, 32, 857. [Google Scholar] [CrossRef] [PubMed]
- Pandey, N.K.; Garg, V.; Bhattacharya, S.; Gulati, M.; Vaidya, Y.; Singh, S.K. Nanosuspension: Principles, perspectives and practices. Curr. Drug Del. 2016, 13, 1222–1246. [Google Scholar]
- Zhang, D.; Tan, T.; Gao, L.; Zhao, W.; Wang, P. Preparation of azithromycin nanosuspensions by high pressure homogenization and its physicochemical characteristics studies. Drug Dev. Ind. Pharm. 2007, 33, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Xiong, R.; Lu, W.; Li, J.; Wang, P.; Xu, R.; Chen, T. Preparation and characterization of intravenously injectable nimodipine nanosuspension. Int. J. Pharm. 2008, 350, 338–343. [Google Scholar] [CrossRef]
- MoSchwitzer, J.; Achleitner, G.; Pomper, H.; Müller, R.H. Development of an intravenously injectable chemically stable aqueous omeprazole formulation using nanosuspension technology. Eur. J. Pharm. Biopharm. 2004, 58, 615–619. [Google Scholar] [CrossRef]
- Muller, R.H.M.; Peters, K. Nanosuspensions for the formulation of poorly soluble drugs. Int. J. Pharm. 1998, 160, 229–237. [Google Scholar] [CrossRef]
- Eerdenbrugh, B.V.; Mooter, G.V.D.; Augustijns, P. Top-down production of drug nanocrystals: Nanosuspension stabilization, miniaturization and transformation into solid products. Int. J. Pharm. 2008, 364, 64–75. [Google Scholar] [CrossRef]
- Chingunpitak, J.; Puttipipatkhachorn, S.; Chavalitshewinkoon-Petmitr, P.; Tozuka, Y.; Moribe, K.; Yamamoto, K. Formation, physical stability and in vitro antimalarial activity of dihydroartemisinin nanosuspensions obtained by co-grinding method. Drug Dev. Ind. Pharm. 2008, 34, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, Y.; Patel, V. Nanosuspension: An approach to enhance solubility of drugs. J. Adv. Pharm. Technol. Res. 2011, 2, 81–87. [Google Scholar] [CrossRef]
- Chin, C.-P.; Wu, H.-S.; Wang, S.S. New approach to pesticide delivery using nanosuspensions: Research and applications. Ind. Eng. Chem. Res. 2011, 50, 7637–7643. [Google Scholar] [CrossRef]
- Corrias, F.; Melis, A.; Atzei, A.; Marceddu, S.; Angioni, A. Zoxamide accumulation and retention evaluation after nanosuspension technology application in tomato plant. Pest Manag. Sci. 2021, 77, 3508–3518. [Google Scholar] [CrossRef]
- Cui, B.; Lv, Y.; Gao, F.; Wang, C.; Zeng, Z.; Wang, Y.; Sun, C.; Zhao, X.; Shen, Y.; Liu, G. Improving abamectin bioavailability via nanosuspension constructed by wet milling technique. Pest Manag. Sci. 2019, 75, 2756–2764. [Google Scholar] [CrossRef] [PubMed]
- Zhenzhong, P.; Bo, C.; Zhanghua, Z.; Lei, F.; Guoqiang, L.; Haixin, C.; Hongyu, P. Lambda-Cyhalothrin nanosuspension prepared by the melt emulsification-high pressure homogenization method. J. Nanomater. 2015, 16, 263. [Google Scholar]
- Wang, C.; Cui, B.; Guo, L.; Wang, A.; Zhao, X.; Wang, Y.; Sun, C.; Zeng, Z.; Zhi, H.; Chen, H. Fabrication and evaluation of Lambda-Cyhalothrin nanosuspension by one-Step melt emulsification technique. Nanomaterials 2019, 9, 145. [Google Scholar] [CrossRef]
- Rani, L.; Thapa, K.; Kanojia, N.; Sharma, N.; Singh, S.; Grewal, A.S.; Srivastav, A.L.; Kaushal, J. An extensive review on the consequences of chemical pesticides on human health and environment. J. Clean. Prod. 2021, 283, 124657. [Google Scholar] [CrossRef]
- Yang, B. The impact of pesticide residues on food safety. China Food Saf. Mag. 2021, 22, 152–153. [Google Scholar]
- Sumira, M.; Shilpa, P.; Shristi, K.; Abhishek, K.; Vineet, U. A perspective review on impact and molecular mechanism of environmental carcinogens on human health. Biotechnol. Genet. Eng. Rev. 2021, 37, 178–207. [Google Scholar]
- Chen, L.; Jiang, H.; Zhou, Y.; Zhou, X.; Huang, J. A Brief Analysis on the Environmental Safety of Nano-enabled Pesticides. Pestic. Sci. Adm. 2018, 39, 9. [Google Scholar]
- Sun, Y.; Liang, J.; Tank, L.; Li, H.; Zhu, Y.; Jiang, D.; Song, B.; Chen, M.; Zeng, G. Nano-pesticides: A great challenge for biodiversity? Nano Today 2019, 28, 100757. [Google Scholar] [CrossRef]
- Berger, S.; Cwiek, K. Selected aspects of adverse nutritional effects of pesticides. Ernhrung 1990, 14, 411–415. [Google Scholar]
- Rico, C.M.; Morales, M.I.; Barrios, A.C.; McCreary, R.; Hong, J.; Lee, W.-Y.; Nunez, J. Effect of cerium oxide nanoparticles on the quality of rice (Oryza sativa L.) grains. J. Agric. Food. Chem. 2013, 61, 11278–11285. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Wang, C.; Zhang, S.; Zheng, L.; Li, F.; Cao, C.; Cao, L.; Huang, Q. Fungicide-loaded mesoporous silica nanoparticles promote rice seedling growth by regulating amino acid metabolic pathways. J. Hazard. Mater. 2022, 425, 127892. [Google Scholar] [CrossRef] [PubMed]
- Cui, B.; Gao, F.; Zeng, Z.H.; Wang, C.X.; Wang, Y.; Sun, C.J.; Zhao, X.; Guo, L.; Shen, Y.; Liu, G.Q.; et al. Construction and characterization of avermectin B-2 solid nanodispersion. Sci. Rep. 2020, 10, 9096. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh, D.F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Lipinski, C.A. Drug-like properties and the causes of poor solubility and poor permeability. J. Pharmacol. Toxicol. Methods 2000, 44, 235–249. [Google Scholar] [CrossRef]
- Kawakami, K. Modification of physicochemical characteristics of active pharmaceutical ingredients and application of supersaturatable dosage forms for improving bioavailability of poorly absorbed drugs. Adv. Drug Deliv. Rev. 2012, 64, 480–495. [Google Scholar] [CrossRef]
- Kipp, J.E. The role of solid nanoparticle technology in the parenteral delivery of poorly water-soluble drugs. Int. J. Pharm. 2004, 284, 109–122. [Google Scholar] [CrossRef]
- Gittings, M.R.; Saville, D.A. The determination of hydrodynamic size and zeta potential from electrophoretic mobility and light scattering measurements. Colloids Surf. A Physicochem. Eng. Asp. 1998, 141, 111–117. [Google Scholar] [CrossRef]
- Tapak, N.S.; Nawawi, M.A.; Mohamed, A.H.; Tjih, E.T.T.; Mohd, Y.; Ab Rashid, A.H.B.; Abdullah, J.; Yusof, N.A.; Ahmad, N.M. Chemical synthesis of metal oxide nanoparticles via ionic liquid as capping agent: Principle, preparation and applications. Malays. J. Anal. Sci. 2022, 26, 1394–1420. [Google Scholar]
- Feng, J.; Lu, F.; Li, M.; Li, W.; Wang, X. Stability of suspension solution and development of suspension concentrate products. Agric. Res. Appl. 2009, 3, 12–19. [Google Scholar]
- Chunxin, W.; Bo, C.; Xiang, Z.; Yan, W.; Zhanghua, Z.; Changjiao, S.; Dongsheng, Y.; Guoqiang, L.; Haixin, C. Optimization and characterization of lambda-cyhalothrin solid nanodispersion by self-dispersing method. Pest Manag. Sci. 2018, 75, 380–389. [Google Scholar]
- Liu, H.H.; Surawanvijit, S.; Rallo, R.; Orkoulas, G.; Cohen, Y. Analysis of nanoparticle agglomeration in aqueous suspensions via constant-number Monte Carlo simulation. Environ. Sci. Technol. 2011, 45, 9284–9292. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and zeta potential–what they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Müller, K.H.; Motskin, M.; Philpott, A.J.; Routh, A.F.; Shanahan, C.M.; Duer, M.J.; Skepper, J.N. The effect of particle agglomeration on the formation of a surface-connected compartment induced by hydroxyapatite nanoparticles in human monocyte-derived macrophages. Biomaterials 2014, 35, 1074–1088. [Google Scholar] [CrossRef] [PubMed]
- Farrell, E.; Brousseau, J.-L. Guide for DLS sample preparation. Brookhaven. Instrum. 2014, 1, 1–3. [Google Scholar]
- Ma, H.; Xia, S.; Li, N.; Wang, T.; Zheng, W.; Yu, T.; Shu, Q.; Han, Y. Emulsifying stability and viscosity reduction for heavy crude oil in surfactant-polymer composite system. J. Mol. Liq. 2022, 362, 119713. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, Y.; Wang, S.; Guo, Z.; Zhang, C.; Zhu, X.; Zhang, G. Hyperbranched ionic surfactants with polyether skeleton: Synthesis, properties and used as stabilizer for emulsion polymerization. J. Mol. Liq. 2022, 355, 118937. [Google Scholar] [CrossRef]
- Bao, Z.; Wu, Y.; Liu, R.; Zhang, S.; Chen, Y.; Wu, T.; Gao, Y.; Zhang, C.; Du, F. Molecular selection and environmental evaluation of eco-friendly surfactants to efficiently reduce pesticide pollution. J. Clean. Prod. 2023, 416, 137954. [Google Scholar] [CrossRef]
- He, J.; Li, J.; Gao, Y.; He, X.; Hao, G. Nano-based smart formulations: A potential solution to the hazardous effects of pesticide on the environment. J. Hazard. Mater. 2023, 456, 131599. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Zeb, A.; Ali, Z.; Shah, S.M. Impact of five insecticides on chickpea (Cicer arietinum L.) nodulation, yield and nitrogen fixing rhizospheric bacteria. Soil Environ. 2009, 28, 56–59. [Google Scholar]
- Reddy, N.S.; Dashsontakke, S. Effect of spraying selected pesticides on the contents of specified minerals in cabbage. Plant Foods Hum. Nutr. (Former. Qual. Plant.) 1997, 51, 357–363. [Google Scholar] [CrossRef]
- Ferree, D.C. Influence of Pesticides on Photosynthesis of Crop Plants. In Proceedings of the Photosynthesis and Plant Development, Diepenbeek, Belgium, 23–29 July 1978. [Google Scholar]
- Hu, J. Effects of Chemical Pesticide on Physiology and Biochemistry of Rice Plant and Rice Quality and Analysis of Residues; Yangzhou University: Yangzhou, China, 2008. [Google Scholar]
- Luo, S.; Wang, Z.; Feng, X.; Xu, J.; Ding, H.; Wu, J.; Ge, C.; Ma, F. Study on tracer dynamics of effects of pesticides on export rate of photosynthate of rice leaves. Sci. Agric. Sin. 2002, 35, 5. [Google Scholar]
- Yuan, S.; Wu, J.; Xu, J.; Li, G. Influences of herbicides on physiology and biochemistry of rice. Acta Phytophylacica Sin. 2001, 28, 5. [Google Scholar]
- Giménez-Moolhuyzen, M.; van der Blom, J.; Lorenzo-Mínguez, P.; Cabello, T.; Crisol-Martínez, E. Photosynthesis inhibiting effects of pesticides on sweet pepper leaves. Insects 2020, 11, 69. [Google Scholar] [CrossRef]
- Khidir, S.M. Effect of some soil treated pesticides on growth characteristics of faba bean and wheat plants. Int. Journ. Emerg. Techn. Comput. Appl. Sci. 2013, 5, 7–20. [Google Scholar]
- Bashir, K.; Seki, M.; Nishizawa, N.K. The transport of essential micronutrients in rice. Mol. Breed. 2019, 39, 168. [Google Scholar] [CrossRef]
- Scheepmaker, J.W.A.; Kassteele, J.V.D. Effects of chemical control agents and microbial biocontrol agents on numbers of non-target microbial soil organisms: A meta-analysis. Biocontrol. Sci. Technol. 2011, 21, 1225–1242. [Google Scholar] [CrossRef]
- Ding, J.; Liu, J. Acute toxicity test of 200 g/L chloride worm benzamide suspension agent against zebrafish. J. Agric. Catastrophology 2022, 12, 43–45. [Google Scholar]
- Huang, W. Toxcity Study of Two Kinds of Diamide Insecticides to Zebrafish Embryo; Hainan University: Haikou, China, 2017. [Google Scholar]
- Du, J.; Fu, Y. Diamide insecticides targeting insect ryanodine receptors: Mechanism and application prospect. Biochem. Biophys. Res. Commun. 2023, 670, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Cui, B.; Feng, L.; Wang, C.; Yang, D.; Cui, H. Stability and biological activity evaluation of chlorantraniliprole solid nanodispersions prepared by high pressure homogenization. PLoS ONE 2016, 11, e0160877. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Qi, S.; Yang, D. Study on the point of run-off and the maximum retention of spray liquid on crop leaves. Chin. J. Pestic. Sci. 2000, 2, 66–71. [Google Scholar]






| Size (nm) | PDI | ||
|---|---|---|---|
| Surfactant/pesticide ratio | 30% | 167.1 ± 1.2 | 0.174 ± 0.013 |
| 50% | 161.9 ± 0.2 | 0.186 ± 0.010 | |
| 70% | 160.3 ± 0.8 | 0.153 ± 0.007 | |
| Milling time (h) | 0.5 | 288.4 ± 2.9 | 0.253 ± 0.009 |
| 1 | 244.2 ± 3.1 | 0.174 ± 0.024 | |
| 2 | 161.9 ± 0.2 | 0.186 ± 0.010 | |
| 3 | 181.0 ± 1.4 | 0.176 ± 0.015 | |
| Formulation | Size (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|
| Nanosuspension | 161.9 ± 0.2 b | 0.186 ± 0.010 b | −43.1 ± 0.7 b |
| SC | 678.7 ± 27.3 a | 0.446 ± 0.021 a | −29.4 ± 0.3 a |
| Time (Day) | 4 °C | 25 °C | 54 °C | |||
|---|---|---|---|---|---|---|
| Size (nm) | PDI | Size (nm) | PDI | Size (nm) | PDI | |
| 0 | 161.9 ± 0.2 e | 0.186 ± 0.010 a | 161.9 ± 0.2 bc | 0.186 ± 0.010 a | 161.9 ± 0.2 e | 0.186 ± 0.010 a |
| 2 | 166.1 ± 0.6 d | 0.143 ± 0.006 e | 159.0 ± 1.3 c | 0.141 ± 0.008 b | 156.7 ± 0.5 f | 0.163 ± 0.004 ab |
| 4 | 166.4 ± 0.6 d | 0.137 ± 0.005 e | 162.9 ± 1.5 bc | 0.134 ± 0.008 b | 173.9 ± 1.3 c | 0.167 ± 0.002 ab |
| 6 | 176.1 ± 0.4 c | 0.159 ± 0.005 cd | 164.6 ± 2.8 ab | 0.147 ± 0.003 b | 169.4 ± 0.3 d | 0.149 ± 0.007 bc |
| 8 | 175.8 ± 0.2 c | 0.166 ± 0.011 bc | 169.3 ± 3.2 a | 0.146 ± 0.019 b | 169.8 ± 0.7 d | 0.133 ± 0.012 cd |
| 10 | 177.5 ± 1.0 bc | 0.148 ± 0.009 de | 167.9 ± 2.6 a | 0.151 ± 0.012 b | 178.3 ± 2.5 b | 0.165 ± 0.010 ab |
| 12 | 178.8 ± 1.4 b | 0.174 ± 0.007 ab | 166.9 ± 2.6 ab | 0.141 ± 0.008 b | 176.4 ± 0.7 b | 0.117 ± 0.023 d |
| 14 | 182.5 ± 0.4 a | 0.168 ± 0.007 bc | 166.1 ± 0.8 ab | 0.134 ± 0.017 b | 182.3 ± 0.3 a | 0.168 ± 0.012 ab |
| Sample | Treatment | Residue Amount (mg/kg) |
|---|---|---|
| Leaves | SC 30 g a.i./hm2 | 0.074 ± 0.0021 |
| nanosuspension 30 g a.i./hm2 | 0.063 ± 0.0006 | |
| Grains | SC 30 g a.i./hm2 | 0.042 ± 0.0006 |
| nanosuspension 30 g a.i./hm2 | Not detected |
| Nutrient | Control Group | Nanosuspension | SC |
|---|---|---|---|
| Energy | 1550 kJ/100 g | 1511 kJ/100 g | 1498 kJ/100 g |
| Protein | 7.2 g/100 g | 7.2 g/100 g | 7.2 g/100 g |
| Fat | 1.4 g/100 g | 0.9 g/100 g | 0.9 g/100 g |
| Carbohydrate | 80.0 g/100 g | 78.6 g/100 g | 77.8 g/100 g |
| Dietary fiber | 2.0 g/100 g | 2.4 g/100 g | 2.5 g/100 g |
| Calcium | 89.4 mg/kg | 88.2 mg/kg | 81.7 mg/kg |
| Magnesium | 587 mg/kg | 556 mg/kg | 413 mg/kg |
| Iron | 7.82 mg/kg | 6.21 mg/kg | 5.41 mg/kg |
| Zinc | 21.4 mg/kg | 23.1 mg/kg | 19.6 mg/kg |
| Potassium | 1630 mg/kg | 1480 mg/kg | 1170 mg/kg |
| Time (min) | Flow Rate (mL/min) | Water Phase | Acetonitrile |
|---|---|---|---|
| 0 | 0.6 | 60 | 40 |
| 0.5 | 0.6 | 60 | 40 |
| 5 | 0.6 | 5 | 95 |
| 5.5 | 0.6 | 5 | 95 |
| 6 | 0.6 | 60 | 40 |
| 7 | 0.6 | 60 | 40 |
| Treatment | Number | 10 μg/L | 20 μg/L | 30 μg/L | RSD (%) |
|---|---|---|---|---|---|
| Chlorantraniliprole (leaf) | 1 | 79.6% | 89.7% | 109.2% | 0.9 |
| 2 | 78.4% | 87.6% | 107.4% | ||
| 3 | 82.3% | 85.4% | 112.3% | ||
| average | 80.10% | 87.57% | 109.63% | ||
| Chlorantraniliprole (grain) | 1 | 80.2% | 85.6% | 105.9% | 4.2 |
| 2 | 78.9% | 84.3% | 107.3% | ||
| 3 | 84.1% | 80.1% | 99.6% | ||
| average | 81.07% | 83.33% | 104.20% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, X.; Guo, L.; Du, Q.; Wang, T.; Zeng, Z.; Wang, Y.; Cui, H.; Gao, F.; Cui, B. Preparation and Comprehensive Evaluation of the Efficacy and Safety of Chlorantraniliprole Nanosuspension. Toxics 2024, 12, 78. https://doi.org/10.3390/toxics12010078
Ding X, Guo L, Du Q, Wang T, Zeng Z, Wang Y, Cui H, Gao F, Cui B. Preparation and Comprehensive Evaluation of the Efficacy and Safety of Chlorantraniliprole Nanosuspension. Toxics. 2024; 12(1):78. https://doi.org/10.3390/toxics12010078
Chicago/Turabian StyleDing, Xiquan, Liang Guo, Qian Du, Tingyu Wang, Zhanghua Zeng, Yan Wang, Haixin Cui, Fei Gao, and Bo Cui. 2024. "Preparation and Comprehensive Evaluation of the Efficacy and Safety of Chlorantraniliprole Nanosuspension" Toxics 12, no. 1: 78. https://doi.org/10.3390/toxics12010078
APA StyleDing, X., Guo, L., Du, Q., Wang, T., Zeng, Z., Wang, Y., Cui, H., Gao, F., & Cui, B. (2024). Preparation and Comprehensive Evaluation of the Efficacy and Safety of Chlorantraniliprole Nanosuspension. Toxics, 12(1), 78. https://doi.org/10.3390/toxics12010078