Assessment of Secondary Sulfate Aqueous-Phase Formation Pathways in the Tropical Island City of Haikou: A Chemical Kinetic Perspective
Abstract
:1. Introduction
2. Methods
2.1. Sampling
2.2. Chemical Analysis
Analysis of Water-Soluble Ions, and Water-Soluble Fe and Mn
2.3. Data Analysis
2.3.1. The Concentration of nss-SO42−
2.3.2. Aerosol Water Content (AWC), Aerosol pH, and Ionic Strength
2.3.3. The Secondary SO42− Formation Rates in Aqueous-Phase Chemistry
The S(IV) Concentration
The Oxidation Rate of S(IV) by NO2
The Oxidation Rate of S(IV) by H2O2
The Oxidation Rate of S(IV) by O3
The Rate of Fe(III)- and Mn(II)-Catalyzed Oxidation of S(IV) into Secondary SO42−
Mass Transport Limitations Rate
3. Results and Discussion
3.1. Seasonal Variations in Water-Soluble Inorganic Ions, Fe and Mn in PM2.5
3.2. Seasonal Differences in H2O2, AWC, Aerosol pH, Ionic Strength, Fe(III)×Mn(II) and S(IV)
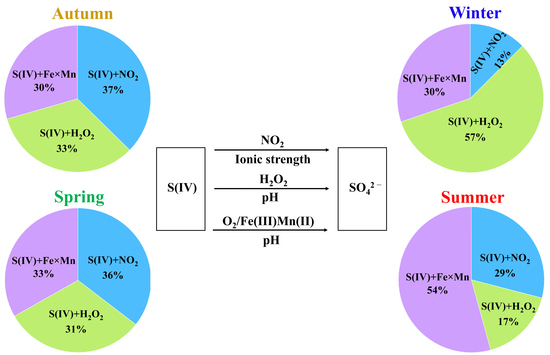
3.3. The Aqueous-Phase Formation Rates of Secondary SO42−
3.4. S(IV)+NO2 Pathway Formation Rates and their Influencing Factors
3.5. S(IV)+H2O2 and S(IV)+Fe×Mn Pathway Formation Rates and Their Influencing Factors
3.6. Comparison of Secondary SO42− Formation Rates under Different PM2.5 Levels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tian, M.; Wang, H.; Chen, Y.; Yang, F.; Zhang, X.; Zou, Q.; Zhang, R.; Ma, Y.; He, K. Characteristics of aerosol pollution during heavy haze events in Suzhou, China. Atmos. Chem. Phys. 2016, 16, 7357–7371. [Google Scholar] [CrossRef]
- He, Q.; Yan, Y.; Guo, L.; Zhang, Y.; Zhang, G.; Wang, X. Characterization and source analysis of water-soluble inorganic ionic species in PM2.5 in Taiyuan city, China. Atmos. Res. 2017, 184, 48–55. [Google Scholar] [CrossRef]
- Tian, M.; Wang, H.; Chen, Y.; Zhang, L.; Shi, G.; Liu, Y.; Yu, J.; Zhai, C.; Wang, J.; Yang, F. Highly time-resolved characterization of water-soluble inorganic ions in PM2.5 in a humid and acidic mega city in Sichuan Basin, China. Sci. Total Environ. 2017, 580, 224–234. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, Z.; Zheng, N.; Luo, L.; Xiao, H.; Xiao, H. Chemical characterization and source analysis of water-soluble inorganic ions in PM2.5 from a plateau city of Kunming at different seasons. Atmos. Res. 2020, 234, 104687. [Google Scholar] [CrossRef]
- Li, Q.; Li, X.; Yang, Z.; Cui, G.; Ding, S. Diurnal and seasonal variations in water-soluble inorganic ions and nitrate dual isotopes of PM2.5: Implications for source apportionment and formation processes of urban aerosol nitrate. Atmos. Res. 2021, 248, 105197. [Google Scholar] [CrossRef]
- Norman, A.; Anlauf, K.; Hayden, K.; Thompson, B.; Brook, J.; Li, S.; Bottenheim, J. Aerosol sulphate and its oxidation on the Pacific NW coast: S and O isotopes in PM2.5. Atmos. Environ. 2006, 40, 2676–2689. [Google Scholar] [CrossRef]
- Li, X.; Bao, H.; Gan, Y.; Zhou, A.; Liu, Y. Multiple oxygen and sulfur isotope compositions of secondary atmospheric sulfate in a mega-city in central China. Atmos. Environ. 2013, 81, 591–599. [Google Scholar] [CrossRef]
- Calvert, J.; Bottenheim, J.; Strausz, O. Mechanism of the homogeneous oxidation of sulfur dioxide in the troposphere. In Sulfur in the Atmosphere; Pergamon: Oxford, UK, 1978; pp. 197–226. [Google Scholar] [CrossRef]
- Ravishankara, A. Heterogeneous and multiphase chemistry in the troposphere. Science 1997, 276, 1058–1065. [Google Scholar] [CrossRef]
- Wang, T.; Liu, M.; Liu, M.; Song, Y.; Xu, Z.; Shang, F.; Huang, X.; Liao, W.; Wang, W.; Ge, M.; et al. Sulfate formation apportionment during winter haze events in North China. Environ. Sci. Technol. 2022, 56, 7771–7778. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Su, H.; Koop, T.; Mikhailov, E.; Pöschl, U. Size dependence of phase transitions in aerosol nanoparticles. Nat. Commun. 2015, 6, 5923. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Yuan, Z.; Griffith, S.M.; Yu, X.; Lau, A.; Yu, J. Sulfate formation enhanced by a cocktail of high NOx, SO2, particulate matter, and droplet pH during haze-fog events in megacities in China: An observation-based modeling investigation. Environ. Sci. Technol. 2016, 50, 7325–7334. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, R.; Gomez, M.; Yang, L.; Levy Zamora, M.; Hu, M.; Lin, Y.; Peng, J.; Guo, S.; Meng, J.; et al. Persistent sulfate formation from London Fog to Chinese haze. Proc. Natl. Acad. Sci. USA 2016, 113, 13630–13635. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Shi, G.; Zhang, Z.; Wei, Y.; Tian, X.; Feng, Y.; Russell, A.; Nenes, A. Targeting atmospheric oxidants can better reduce sulfate aerosol in China: H2O2 aqueous oxidation pathway dominates sulfate formation in haze. Environ. Sci. Technol. 2022, 56, 10608–10618. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Lu, K.; Ye, C.; Dong, H.; Li, S.; Chen, S.; Wu, Z.; Zheng, M.; Zeng, L.; Hu, M.; et al. A comprehensive observation-based multiphase chemical model analysis of sulfur dioxide oxidations in both summer and winter. Atmos. Chem. Phys. 2021, 21, 13713–13727. [Google Scholar] [CrossRef]
- Liu, P.; Ye, C.; Xue, C.; Zhang, C.; Mu, Y.; Sun, X. Formation mechanisms of atmospheric nitrate and sulfate during the winter haze pollution periods in Beijing: Gas-phase, heterogeneous and aqueous-phase chemistry. Atmos. Chem. Phys. 2020, 20, 4153–4165. [Google Scholar] [CrossRef]
- Yue, F.; Xie, Z.; Zhang, P.; Song, S.; He, P.; Liu, C.; Wang, L.; Yu, X.; Kang, H. The role of sulfate and its corresponding S(IV)+ NO2 formation pathway during the evolution of haze in Beijing. Sci. Total Environ. 2019, 687, 741–751. [Google Scholar] [CrossRef]
- Fang, X.; Bi, X.; Xu, H.; Wu, J.; Zhang, Y.; Feng, Y. Source apportionment of ambient PM10 and PM2.5 in Haikou, China. Atmos. Res. 2017, 190, 1–9. [Google Scholar] [CrossRef]
- Li, M.; Liu, H.; Geng, G.; Hong, C.; Liu, F.; Song, Y.; Tong, D.; Zheng, B.; Cui, H.; Man, H.; et al. Anthropogenic emission inventories in China: A review. Natl. Sci. Rev. 2017, 4, 834–866. [Google Scholar] [CrossRef]
- Zheng, B.; Tong, D.; Li, M.; Liu, F.; Hong, C.; Geng, G.; Li, H.; Li, X.; Peng, L.; Qi, J.; et al. Trends in China’s anthropogenic emissions since 2010 as the consequence of clean air actions. Atmos. Chem. Phys. 2018, 18, 14095–14111. [Google Scholar] [CrossRef]
- Keene, W.; Pszenny, A.; Galloway, J.; Hawley, M. Sea-salt corrections and interpretation of constituent ratios in marine precipitation. J. Geophys. Res. Atmos. 1986, 91, 6647–6658. [Google Scholar] [CrossRef]
- Gao, Y.; Arimoto, R.; Duce, R.; Chen, L.; Zhou, M.; Gu, D. Atmospheric non-sea-salt sulfate, nitrate and methanesulfonate over the China Sea. J. Geophys. Res. Atmos. 1996, 101, 12601–12611. [Google Scholar] [CrossRef]
- Fountoukis, C.; Nenes, A. ISORROPIA II: A computationally efficient thermodynamic equilibrium model for K+–Ca2+–Mg2+–NH4+–Na+–SO42−–NO3−–Cl−–H2O aerosols. Atmos. Chem. Phys. 2007, 7, 4639–4659. [Google Scholar] [CrossRef]
- Gao, J.; Wei, Y.; Zhao, H.; Liang, D.; Feng, Y.; Shi, G. The role of source emissions in sulfate formation pathways based on chemical thermodynamics and kinetics model. Sci. Total Environ. 2022, 851, 158104. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Alexander, B.; Geng, L.; Chi, X.; Fan, S.; Zhan, H.; Kang, H.; Zheng, G.; Cheng, Y.; Su, H.; et al. Isotopic constraints on heterogeneous sulfate production in Beijing haze. Atmos. Chem. Phys. 2018, 18, 5515–5528. [Google Scholar] [CrossRef]
- Seinfeld, J.; Pandis, S. Atmospheric Chemistry and Physics: From Air Pollution to Climate Change, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar] [CrossRef]
- Lee, Y.; Schwartz, S. Kinetics of oxidation of aqueous sulfur (IV) by nitrogen dioxide. Precip. Scav. Dry Depos. Resuspension 1983, 1, 453–470. Available online: https://www.osti.gov/biblio/6567096 (accessed on 30 August 2022).
- Clifton, C.; Altstein, N.; Huie, R. Rate constant for the reaction of nitrogen dioxide with sulfur (IV) over the pH range 5.3–13. Environ. Sci. Technol. 1988, 22, 586–589. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Calvert, J. Chemical Transformation Modules for Eulerian Acid Deposition Models: Volume II, the Aqueous-Phase Chemistry; Atmospheric Sciences Research Laboratory, Office of Research and Development, US Environmental Protection Agency: Washington, DC, USA, 1985. [Google Scholar]
- Fu, A. Study on Peroxides Concentration and Its Influencing Factors in the Urban Atmosphere. Master’s Thesis, College of Environmental and Resource Sciences, Zhejiang University, Hangzhou, China, 2014; 56p. (In Chinese). [Google Scholar]
- Ibusuki, T.; Takeuchi, K. Sulfur dioxide oxidation by oxygen catalyzed by mixtures of manganese(II) and iron(III) in aqueous solutions at environmental reaction conditions. Atmos. Environ. 1987, 21, 1555–1560. [Google Scholar] [CrossRef]
- Zhou, M.; Qiao, L.; Zhu, S.; Li, L.; Lou, S.; Wang, H.; Wang, Q.; Tao, S.; Huang, C.; Chen, C. Chemical characteristics of fine particles and their impact on visibility impairment in Shanghai based on a 1-year period observation. J. Environ. Sci. 2016, 48, 151–160. [Google Scholar] [CrossRef]
- Qiao, B.; Chen, Y.; Tian, M.; Wang, H.; Yang, F.; Shi, G.; Zhang, L.; Peng, C.; Luo, Q.; Ding, S. Characterization of water soluble inorganic ions and their evolution processes during PM2.5 pollution episodes in a small city in southwest China. Sci. Total Environ. 2019, 650, 2605–2613. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.; Zhao, Y.; Ding, A.; Zhang, Y.; Song, T.; Zheng, J.; Ho, K.; Lee, S.; Zhong, L. Characterization of PM2.5 and the major chemical components during a 1-year campaign in rural Guangzhou, Southern China. Atmos. Res. 2016, 167, 208–215. [Google Scholar] [CrossRef]
- Huang, X.; Bian, Q.; Ng, W.; Louie, P.; Yu, J. Characterization of PM2.5 major components and source investigation in suburban Hong Kong: A one year monitoring study. Aerosol Air Qual. Res. 2014, 14, 237–250. [Google Scholar] [CrossRef]
- Zheng, G.; Duan, F.; Su, H.; Ma, Y.; Cheng, Y.; Zheng, B.; Zhang, Q.; Huang, T.; Poschl, U.; Cheng, Y.; et al. Exploring the severe winter haze in Beijing: The impact of synoptic weather, regional transport and heterogeneous reactions. Atmos. Chem. Phys. 2015, 15, 2969–2983. [Google Scholar] [CrossRef]
- Lin, Y.; Hsu, S.; Chou, C.; Zhang, R.; Wu, Y.; Kao, S.; Luo, L.; Huang, C.; Lin, S.; Huang, Y.T. Wintertime haze deterioration in Beijing by industrial pollution deduced from trace metal fingerprints and enhanced health risk by heavy metals. Environ. Pollut. 2016, 208, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, S.; Luo, L.; Yang, S.; Lu, B.; Wang, C.; Hsu, S.; Kao, S. Seasonal variations, source apportionment and dry deposition of chemical species of total suspended particulate in Pengjia Yu Island, East China Sea. Mar. Pollut. Bull. 2023, 187, 114608. [Google Scholar] [CrossRef]
- Lei, Y.; Li, D.; Lu, D.; Zhang, T.; Sun, J.; Wang, X.; Xu, H.; Shen, Z. Insights into the roles of aerosol soluble iron in secondary aerosol formation. Atmos. Environ. 2023, 294, 119507. [Google Scholar] [CrossRef]
- Karthikeyan, S.; Joshi, U.; Balasubramanian, R. Microwave assisted sample preparation for determining water-soluble fraction of trace elements in urban airborne particulate matter: Evaluation of bioavailability. Anal. Chim. Acta 2006, 576, 23–30. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, W.; Lin, Q.; Yuan, Q.; Liu, L.; Zhang, J.; Zhang, Y.; Shao, L.; Niu, H.; Yang, S.; et al. Iron solubility in fine particles associated with secondary acidic aerosols in east China. Environ. Pollut. 2020, 264, 114769. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, M.; Wang, T.; Song, Y.; Zhou, L.; Cao, J.; Hu, J.; Tang, G.; Chen, Z.; Li, Z.; et al. Sulfate formation is dominated by manganese-catalyzed oxidation of SO2 on aerosol surfaces during haze events. Nat. Commun. 2021, 12, 1993. [Google Scholar] [CrossRef]
- Hsu, S.; Wong, G.; Gong, G.; Shiah, F.; Huang, Y.; Kao, S.; Tsai, F.; Lung, S.; Lin, F.; Lin, I.; et al. Sources, solubility, and dry deposition of aerosol trace elements over the East China Sea. Mar. Chem. 2010, 120, 116–127. [Google Scholar] [CrossRef]
- Luo, L.; Pan, Y.; Zhu, R.; Zhang, Z.; Zheng, N.; Liu, Y.; Liu, Y.; Liu, C.; Xiao, H.; Xiao, H. Assessment of the seasonal cycle of nitrate in PM2.5 using chemical compositions and stable nitrogen and oxygen isotopes at Nanchang, China. Atmos. Environ. 2020, 225, 117371. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Y.; Wang, Y.; Yu, J.; Shao, J.; Liu, P.; Zhu, W.; Cheng, Z.; Li, Z.; Yan, N.; et al. Organosulfates in atmospheric aerosols in Shanghai, China: Seasonal and interannual variability, origin, and formation mechanisms. Atmos. Chem. Phys. 2021, 21, 2959–2980. [Google Scholar] [CrossRef]
- Bougiatioti, A.; Nikolaou, P.; Stavroulas, I.; Kouvarakis, G.; Weber, R.; Nenes, A.; Kanakidou, M.; Mihalopoulos, N. Particle water and pH in the eastern Mediterranean: Source variability and implications for nutrient availability. Atmos. Chem. Phys. 2016, 16, 4579–4591. [Google Scholar] [CrossRef]
- Murphy, J.; Gregoire, P.; Tevlin, A.; Wentworth, G.; Ellis, R.; Markovic, M.; VandenBoer, T. Observational constraints on particle acidity using measurements and modelling of particles and gases. Faraday Discuss. 2017, 200, 379–395. [Google Scholar] [CrossRef]
- Xu, L.; Guo, H.; Boyd, C.; Klein, M.; Bougiatioti, A.; Cerully, K.; Hite, J.; Isaacman-VanWertz, G.; Kreisberg, N.; Knote, C.; et al. Effects of anthropogenic emissions on aerosol formation from isoprene and monoterpenes in the southeastern United States. Proc. Natl. Acad. Sci. USA 2015, 112, 37–42. [Google Scholar] [CrossRef]
- Liu, M.; Song, Y.; Zhou, T.; Xu, Z.; Yan, C.; Zheng, M.; Wu, Z.; Hu, M.; Wu, Y.; Zhu, T. Fine particle pH during severe haze episodes in northern China. Geophys. Res. Lett. 2017, 44, 5213–5221. [Google Scholar] [CrossRef]
- Li, P.; Li, X.; Yang, C.; Wang, X.; Chen, J.; Collett, J., Jr. Fog water chemistry in Shanghai. Atmos. Environ. 2011, 45, 4034–4041. [Google Scholar] [CrossRef]
- Shao, J.; Chen, Q.; Wang, Y.; Lu, X.; He, P.; Sun, Y.; Shah, V.; Martin, R.; Philip, S.; Song, S.; et al. Heterogeneous sulfate aerosol formation mechanisms during wintertime Chinese haze events: Air quality model assessment using observations of sulfate oxygen isotopes in Beijing. Atmos. Chem. Phys. 2019, 19, 6107–6123. [Google Scholar] [CrossRef]
- Liu, T.; Clegg, S.; Abbatt, J. Fast oxidation of sulfur dioxide by hydrogen peroxide in deliquesced aerosol particles. Proc. Natl. Acad. Sci. USA 2020, 117, 1354–1359. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Chu, B.; Ge, Y.; Zhang, S.; Ma, Q.; He, H.; Li, S. Enhancement of aqueous sulfate formation by the coexistence of NO2/NH3 under high ionic strengths in aerosol water. Environ. Pollut. 2019, 252, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Nash, T. The effect of nitrogen dioxide and of some transition metals on the oxidation of dilute bisulphite solutions. Atmos. Environ. (1967) 1979, 13, 1149–1154. [Google Scholar] [CrossRef]
- Liu, T.; Abbatt, J. Oxidation of sulfur dioxide by nitrogen dioxide accelerated at the interface of deliquesced aerosol particles. Nat. Chem. 2021, 13, 1173–1177. [Google Scholar] [CrossRef] [PubMed]
- Guieu, C.; Duce, R.; Arimoto, R. Dissolved input of manganese to the ocean: Aerosol source. J. Geophys. Res. Atmos. 1994, 99, 18789–18800. [Google Scholar] [CrossRef]
- Mackie, D.; Boyd, P.; Hunter, K.; McTainsh, G. Simulating the cloud processing of iron in Australian dust: pH and dust concentration. Geophys. Res. Lett. 2005, 32, L06809. [Google Scholar] [CrossRef]
- Wang, S.; Wang, L.; Fan, X.; Wang, N.; Ma, S.; Zhang, R. Formation pathway of secondary inorganic aerosol and its influencing factors in Northern China: Comparison between urban and rural sites. Sci. Total Environ. 2022, 840, 156404. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Liu, P.; Ma, Z.; Xue, C.; Zhang, C.; Zhang, Y.; Liu, J.; Liu, C.; Sun, X.; Mu, Y. High H2O2 concentrations observed during haze periods during the winter in Beijing: Importance of H2O2 oxidation in sulfate formation. Environ. Sci. Technol. Lett. 2018, 5, 757–763. [Google Scholar] [CrossRef]
- Xue, J.; Yu, X.; Yuan, Z.; Griffith, S.; Lau, A.; Seinfeld, J.; Yu, J. Efficient control of atmospheric sulfate production based on three formation regimes. Nat. Geosci. 2019, 12, 977–982. [Google Scholar] [CrossRef]
- Yang, J.; Wang, S.; Zhang, R.; Yin, S. Elevated particle acidity enhanced the sulfate formation during the COVID-19 pandemic in Zhengzhou, China. Environ. Pollut. 2022, 296, 118716. [Google Scholar] [CrossRef]
- Denbigh, K. The Principles of Chemical Equilibrium: With Applications in Chemistry and Chemical Engineering, 4th ed.; Cambridge University Press: Cambridge, UK, 1981. [Google Scholar] [CrossRef]
- Herrmann, H. Kinetics of aqueous phase reactions relevant for atmospheric chemistry. Chem. Rev. 2003, 103, 4691–4716. [Google Scholar] [CrossRef]
- Maaß, F.; Elias, H.; Wannowius, K. Kinetics of the oxidation of hydrogen sulfite by hydrogen peroxide in aqueous solution: Ionic strength effects and temperature dependence. Atmos. Environ. 1999, 33, 4413–4419. [Google Scholar] [CrossRef]
- Lagrange, J.; Pallares, C.; Lagrange, P. Electrolyte effects on aqueous atmospheric oxidation of sulphur dioxide by ozone. J. Geophys. Res. Atmos. 1994, 99, 14595–14600. [Google Scholar] [CrossRef]
- Martin, L.; Hill, M. The iron catalyzed oxidation of sulfur: Reconciliation of the literature rates. Atmos. Environ. 1967, 21, 1487–1490. [Google Scholar] [CrossRef]
- Martin, L.; Hill, M.; Tai, A.; Good, T. The iron catalyzed oxidation of sulfur(IV) in aqueous solution: Differing effects of organics at high and low pH. J. Geophys. Res. Atmos. 1991, 96, 3085–3097. [Google Scholar] [CrossRef]
- Siefert, R.; Johansen, A.M.; Hoffmann, M.; Pehkonen, S. Measurements of trace metal (Fe, Cu, Mn, Cr) oxidation states in fog and stratus clouds. J. Air Waste Manag. Assoc. 1998, 48, 128–143. [Google Scholar] [CrossRef]
- Graedel, T.; Weschler, C. Chemistry within aqueous atmospheric aerosols and raindrops. Rev. Geophys. 1981, 19, 505–539. [Google Scholar] [CrossRef]
- Martin, L.; Hill, M. The effect of ionic strength on the manganese catalyzed oxidation of sulfur(IV). Atmos. Environ. 1987, 21, 2267–2270. [Google Scholar] [CrossRef]
- Jacob, D. Heterogeneous chemistry and tropospheric ozone. Atmos. Environ. 2000, 34, 2131–2159. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, Y.; Zeng, L.; Shao, K.; Qi, B. Study of atmospheric peroxides in Guangzhou city. China Environ. Sci. 2001, 21, 221–225. (In Chinese) [Google Scholar]
- Peng, Y.; Chen, K.; Lai, C.; Lu, P.; Kao, J.H. Concentrations of H2O2 and HNO3 and O3–VOC–NOx sensitivity in ambient air in southern Taiwan. Atmos. Environ. 2006, 40, 6741–6751. [Google Scholar] [CrossRef]
- Hua, W.; Chen, Z.; Jie, C.; Kondo, Y.; Hofzumahaus, A.; Takegawa, N.; Chang, C.; Lu, K.; Miyazaki, Y.; Kita, K.; et al. Atmospheric hydrogen peroxide and organic hydroperoxides during PRIDE-PRD’06, China: Their concentration, formation mechanism and contribution to secondary aerosols. Atmos. Chem. Phys. 2008, 8, 6755–6773. [Google Scholar] [CrossRef]
- Guo, J.; Tilgner, A.; Yeung, C.; Wang, Z.; Louie, P.; Luk, C.; Xu, Z.; Yuan, C.; Gao, Y.; Poon, S.; et al. Atmospheric peroxides in a polluted subtropical environment: Seasonal variation, sources and sinks, and importance of heterogeneous processes. Environ. Sci. Technol. 2014, 48, 1443–1450. [Google Scholar] [CrossRef] [PubMed]






| Regions | Sampling Time | PM2.5 Concentrations | Main Pathway | Mean Rate | Range | Relative Contributions | References | |
|---|---|---|---|---|---|---|---|---|
| (μg m−3) | (μg m−3 h−1) | (μg m−3 h−1) | (%) | |||||
| Haikou | Winter | December 2021–February 2022 | 6.2–52 | S(IV)+H2O2 | 7.2 × 10−3 | 3.0 × 10−4–9.5 × 10−2 | 57 | this study |
| Beijing | December 2017 | 18 ± 10–52 ± 10 | S(IV)+Fe×Mn | − | 0.9–1.0 a | − | [15] | |
| Beijing | January 2013 | >75(haze) | S(IV)+NO2 | − | 1–7a | − | [11] | |
| Beijing | January 2016 | >75(haze) | S(IV)+H2O2 | − | ~2.7 × 10−1 a | − | [59] | |
| Beijing | December 2017 | >75(haze) | S(IV)+Fe×Mn | − | 1.0–1.8 a,b | − | [15] | |
| Guangzhou | 2005 | haze-fog | S(IV)+NO2 | − | 1.0–6.4 | − | [60] | |
| Zhengzhou | January2020–February 2020 | >75(haze) | S(IV)+Fe×Mn | − | 2.0 × 10−2–1.2 × 10−1 | − | [61] | |
| Zhengzhou | January 2018 | >75(haze) | S(IV)+Fe×Mn | − | 10−1–100 a | − | [58] | |
| Xinxiang | January 2018 | >75(haze) | S(IV)+H2O2 | 10−1 a | − | [58] | ||
| Haikou | Summer | June 2022–August 2022 | 3.9–15 | S(IV)+Fe×Mn | 3.7 × 10−3 | 2.1 × 10−5–3.8 × 10−2 | 54 | this study |
| Wangdu | June 2014 | 20 ± 10–55 ± 12 | S(IV)+Fe×Mn | − | 1.2–2.3 a,b | − | [15] | |
| Wangdu | June 2014 | >75(haze) | S(IV)+Fe×Mn | − | 1.9–3.6 a,b | − | [15] | |
| Tianjin | June 2018–August 2018, June 2019–August 2019 | >75(haze) | S(IV)+Fe×Mn | − | − | 55 | [14] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Luo, L.; Xu, Z.; Liu, S.; Li, Y.; Ni, Y.; Kao, S.-J. Assessment of Secondary Sulfate Aqueous-Phase Formation Pathways in the Tropical Island City of Haikou: A Chemical Kinetic Perspective. Toxics 2024, 12, 105. https://doi.org/10.3390/toxics12020105
Wang C, Luo L, Xu Z, Liu S, Li Y, Ni Y, Kao S-J. Assessment of Secondary Sulfate Aqueous-Phase Formation Pathways in the Tropical Island City of Haikou: A Chemical Kinetic Perspective. Toxics. 2024; 12(2):105. https://doi.org/10.3390/toxics12020105
Chicago/Turabian StyleWang, Chen, Li Luo, Zifu Xu, Shuhan Liu, Yuxiao Li, Yuanzhe Ni, and Shuh-Ji Kao. 2024. "Assessment of Secondary Sulfate Aqueous-Phase Formation Pathways in the Tropical Island City of Haikou: A Chemical Kinetic Perspective" Toxics 12, no. 2: 105. https://doi.org/10.3390/toxics12020105
APA StyleWang, C., Luo, L., Xu, Z., Liu, S., Li, Y., Ni, Y., & Kao, S. -J. (2024). Assessment of Secondary Sulfate Aqueous-Phase Formation Pathways in the Tropical Island City of Haikou: A Chemical Kinetic Perspective. Toxics, 12(2), 105. https://doi.org/10.3390/toxics12020105