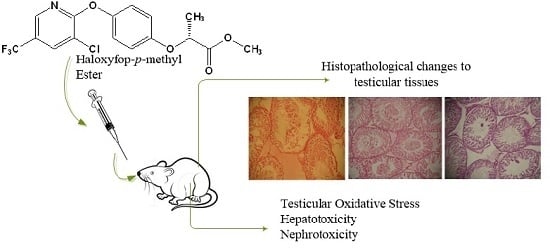
Hepatotoxicity, Nephrotoxicity and Oxidative Stress in Rat Testis Following Exposure to Haloxyfop-p-methyl Ester, an Aryloxyphenoxypropionate Herbicide
Abstract
:1. Introduction

2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Animal Selection and Care
2.3. Experimental Design
| Treatment groups | Treatments |
|---|---|
| I | Control |
| II | 6.75 mg/kg bw HPME |
| III | 13.5 mg/kg bw HPME |
| IV | 27.0 mg/kg bw HPME |
2.4. Collection of Blood and Testis
2.5. Preparation of Plasma and Sub-Cellular Fractions of Testicular Homogenates
2.6. Assay of the Biomarkers of Hepatotoxicity
2.7. Assay of the Biomarkers of Nephrotoxicity
2.8. Assay of the Testicular Protein Content
2.9. Assay of the Biomarkers of Testicular Function
2.10. Assay of the Testicular Non-Enzymatic Antioxidants
2.10.1. Assay of the Testicular Glutathione Level
2.10.2. Assay of the Testicular Ascorbic Acid Level
2.11. Assay of the Testicular Enzymic Antioxidants
2.11.1. Assay of the Testicular Glutathione-S-Transferase Activity
2.11.2. Assay of the Testicular Superoxide Dismutase Activity
2.11.3. Assay of the Testicular Catalase Activity
2.12. Assay of the Level of Lipid Peroxidation in the Testicular PMF
2.13. Testicular Histology
2.14. Statistical Analysis
3. Results
3.1. Influence of Haloxyfop-p-methyl Ester on Hepatic Function Markers in the Plasma of Rat
| Treatment groups | Bilirubin (mg/dL) | ALP (U/L) | ALT (U/L) | AST (U/L) |
|---|---|---|---|---|
| 0 (Control) | 0.29 ± 0.01 | 262.8 ± 8.6 | 30.4 ± 3.1 | 61.2 ± 5.6 |
| 6.75 mg/kg bw HPME | 0.37 ± 0.01 (28%) * | 355.4 ± 9.1 (35%) * | 36.0 ± 2.6 (18%) * | 66.4 ± 4.1 (8%) * |
| 13.50 mg/kg bw HPME | 0.47 ± 0.02 (62%) * | 384.2 ± 6.6 (46%) * | 41.1 ± 2.3 (35%) * | 73.4 ± 5.1 (20%) * |
| 27.00 mg/kg bw HPME | 0.57 ± 0.01 (97%) * | 438.8 ± 7.4 (67%) * | 45.2 ± 3.6 (49%) * | 77.0 ± 2.7 (26%) * |
3.2. Influence of Haloxyfop-p-methyl Ester on Renal Function Markers in the Plasma of Rat
| Treatment groups | Urea (mg/ dL) | Creatinine (mg/ dL) |
|---|---|---|
| 0 (Control) | 18.0 ± 2.3 | 0.42 ± 0.06 |
| 6.75 mg/kg bw HPME | 24.1 ± 1.8 (33%) * | 0.56 ± 0.03 (33%) * |
| 13.50 mg/kg bw HPME | 30.6 ± 2.4 (70%) * | 0.66 ± 0.03 (57%) * |
| 27.00 mg/kg bw HPME | 35.3 ± 3.1 (96%) * | 0.73 ± 0.07 (73%) * |
3.3. Influence of Haloxyfop-p-methyl Ester on Biomarkers of Testicular Function in Rat
3.4. Effect of Haloxyfop-p-methyl Ester on Biomarkers of Oxidative Stress in the Testis of Rat
| Treatment groups | GST (nM/min/mg protein) | SOD (units/mg protein) | CAT (μM H2O2 consumed/min/mg protein) |
|---|---|---|---|
| 0 (Control) | 22.6 ± 1.7 | 8.6 ± 0.7 | 13.9 ± 1.2 |
| 6.75 mg/kg bw HPME | 18.2 ± 1.8 (19%) * | 6.6 ± 0.5 (23%) * | 10.6 ± 0.7 (24%) * |
| 13.50 mg/kg bw HPME | 15.8 ± 2.1 (30%) * | 5.4 ± 0.6 (37%) * | 8.2 ± 0.5 (44%) * |
| 27.00 mg/kg bw HPME | 12.1 ± 1.5 (46%) * | 4.4 ± 0.4 (49%) * | 6.1 ± 0.5 (60%) * |
3.5. Effect of Haloxyfop-p-methyl Ester on Histological Characteristics of Rat Testis




4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
Abbreviations
| AOPPs | aryloxyphenoxypropionates |
| HPME | haloxyfop-p-methyl ester |
| CDNB | 1-chloro-2,4-dinitrobenzene |
| DTNB | 5,5′-dithio-bis-2-nitrobenzoic acid |
| bw | body weight |
| PMF | post mitochondrial fraction |
| BSA | bovine serum albumin |
| ddH2O | double-distilled water |
| TBILI | total bilirubin |
| ALP | alkaline phosphatase |
| ACP | acid phosphatase |
| ALT | alanine aminotransferase |
| AST | aspartate aminotransferase |
| γ-GT | gamma glutamyl transferase |
| LDH | lactate dehydrogenase |
| SOD | superoxide dismutase |
| CAT | catalase |
| GPx | glutathione peroxidase |
| GST | glutathione-S-transferase |
| AA | ascorbic acid |
| GSH | reduced glutathione |
| LPO | lipid peroxidation |
| MDA | malondialdehyde |
| ROS | reactive oxygen species |
| H2O2 | hydrogen peroxide |
| PUFA | polyunsaturated fatty acids |
| LM | lumen |
| OD | edema |
References
- Hamilton, D.; Ambrus, A.; Dieterle, R.; Felsot, A.; Harris, C.; Petersen, B.; Racke, K.; Wong, S.S.; Gonzalez, R.; Tanaka, K.; et al. Pesticide residues in food––Acute dietary exposure. Pest. Manag. Sci. 2004, 60, 311–339. [Google Scholar] [CrossRef] [PubMed]
- Azmi, M.A.; Naqvi, S.N.; Azmi, M.A.; Aslam, M. Effect of pesticide residues on health and different enzyme levels in the blood of farm workers from Gadap (rural area) Karachi-Pakistan. Chemosphere 2006, 64, 1739–1744. [Google Scholar] [CrossRef] [PubMed]
- Mecdad, A.A.; Ahmed, M.H.; ElHalwagy, M.E.A.; Afify, M.M.M. A study on oxidative stress biomarkers and immunomodulatory effects of pesticides in pesticide-sprayers. Egypt. J. Forens. Sci. 2011, 1, 93–98. [Google Scholar] [CrossRef]
- Nestler, H.J. Phenoxy-Phenoxypropionic Acid Derivatives and Related Compounds; Chemie der P, S., Schädling-Sbekämp Fungsmittel, W.R., Eds.; Springer-Verlag: Berlin, Germany, 1982; pp. 1–25. [Google Scholar]
- European Food Safety Authority. Reasoned opinion on the review of the existing maximum residue levels (MRLs) for haloxyfop-P. EFSA J. 2014, 12, 3861–3921. [Google Scholar]
- Banaś, A.; Johansson, I.; Stenlid, G.; Stymne, S. Investigation of the mode of action of the herbicide haloxyfop. Zesz Nauk Wyższ Szk Roln P Siedlce Ser Nauki Przyrodnicze 1993, 34, 1–19. [Google Scholar]
- Abdollahi, M.; Ranjbar, A.; Shadnia, S.; Nikfar, S.; Rezaiee, A. Pesticides and oxidative stress: A review. Med. Sci. Monit. 2004, 10, 144–147. [Google Scholar]
- Luo, X.; Sunohara, Y.; Matsumoto, H. Fluazifop-butyl causes membrane peroxidation in the herbicide-susceptible broad leaf weed bristly starbur (Acanthospermum hispidum). Pest. Biochem. Physiol. 2004, 78, 93–102. [Google Scholar] [CrossRef]
- Ore, A.; Olayinka, E.T. Fluazifop-p-butyl, an Aryloxyphenoxypropionate Herbicide Diminishes Renal and Hepatic Functions and Triggered Testicular Oxidative Stress in Orally Exposed Rats. Unpublished data. 2015. [Google Scholar]
- Jasper, R.; Locatelli, G.O.; Pilati, C.; Locatelli, C. Evaluation of biochemical, hematological and oxidative parameters in mice exposed to the herbicide glyphosate-Roundup®. Interdiscip. Toxicol. 2012, 5, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncola, J.; Cronin, M.T.D.; Mazura, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Vidyasagar, J.; Karunakar, N.; Reddy, M.S.; Rajnarayana, K.; Surender, T.; Krishna, D.R. Oxidative stress and antioxidant status in acute organophosphorous insecticide poisoning. Indian J. Pharmacol. 2005, 36, 76–79. [Google Scholar]
- Valavanidisa, A.; Vlahogiannia, T.; Dassenakisb, M.; Scoullos, M. Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants. Ecotoxicol. Environ. Saf. 2006, 64, 178–189. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Tietz, N.W. Clinical Guide to Laboratory Tests, 3rd ed.; W.B. Saunders Company: Philadelphia, PA, USA, 1995. [Google Scholar]
- Tietz, N.W.; Pruden, E.L.; Siggaard-Andersen, O. Liver Function. In Tietz Textbook of Clinical Chemistry; Burtis, A.C., Ashwood, E.R., Eds.; WB Saunders: London, UK, 1994; pp. 1354–1374. [Google Scholar]
- Reltman, S.; Frankel, S.A. Colorimetric method for the determination of serum ALT and AST. Am. J. Clin. Pathol. 1957, 28, 56–63. [Google Scholar]
- Jaffe, E.R. Oxidative Hemolysis, or What Made the Red Cell Break? N. Engl. J. Med. 1972, 286, 156–157. [Google Scholar] [PubMed]
- Gornall, A.C.; Bardwawill, C.J.; David, M.M. Determination of serum protein by means of the biuret reaction. J. Biol. Chem. 1949, 177, 751–756. [Google Scholar] [PubMed]
- Szasz, G. A kinetic photometric method for serum γ-glutamyl transpeptidase. Clin. Chem. 1969, 15, 124–136. [Google Scholar] [PubMed]
- Cabaud, P.G.; Wroblewski, F. Colorimetric measurement of lactic dehydrogenase activity of body fluids. Am. J. Clin. Pathol. 1958, 30, 234–236. [Google Scholar] [PubMed]
- Jollow, D.J.; Mitchell, J.R.; Zampaghone, N.; Gillete, J.R. Bromobenzene induced liver necrosis, protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 1974, 11, 151–169. [Google Scholar] [CrossRef] [PubMed]
- Jagota, S.K.; Dani, H.M. A new colorimetric technique for the estimation of vitamin C using Folin phenol reagent. Anal. Biochem. 1982, 127, 178–182. [Google Scholar] [CrossRef]
- Habig, W.A.; Pabst, M.J.; Jacoby, W.B. Glutathione trsansferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [PubMed]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the auto-oxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [PubMed]
- Singha, A.K. Colorimetric assay of catalase. Anal. Biochem. 1972, 47, 389–394. [Google Scholar] [CrossRef]
- Varshney, R.; Kale, R.K. Effect of calmodulin antagonist on radiation induced lipid peroxidation in microsomes. Int. J. Radiat. Biol. 1990, 58, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Baker, F.J.; Silverton, R.E. Introduction to Medical Laboratory Technology, 6th ed.; Butter Worth: London, UK, 1985. [Google Scholar]
- Maroni, M.; Fanetti, A.C.; Metruccio, F. Risk assessment and management of occupational exposure to pesticides in agriculture. Med. Lav. 2006, 97, 430–437. [Google Scholar] [PubMed]
- Damalas, C.A.; Eleftherohorinos, I.G. Pesticide Exposure, Safety Issues, and Risk Assessment Indicators. Int. J. Environ. Res. Public Health 2011, 8, 1402–1419. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, A.S.; Karunamoorthy, P.; Kondhalkar, S.J.; Ambikapathy, M.; Beerappa, R. Assessment of hematological, biochemical effects and genotoxicity among pesticide sprayers in grape garden. J. Occup. Med. Toxicol. 2015, 10, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Hauser, R.; Chen, Z.; Pothier, L.; Ryan, L.; Altshul, L. The relationship between human semen parameters and environmental exposure to polychlorinated biphenyls and p,p′-DDE. Environ Health Perspect. 2003, 111, 1505–1511. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Bhat, T.K.; Sharma, O.P. Clinical Biochemistry of Hepatotoxicity. J. Clin. Toxicol. 2011, S4, 2–19. [Google Scholar]
- Cullen, J.M. Mechanistic classification of liver injury. Toxicol. Pathol. 2005, 33, 6–8. [Google Scholar] [CrossRef] [PubMed]
- Ramaiah, S.K. A toxicologist guide to the diagnostic interpretation of hepatic biochemical parameters. Food Chem. Toxicol. 2007, 45, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Giannini, E.G.; Testa, R.; Savarino, V. Liver enzyme alteration: A guide for clinicians. CMAJ 2005, 172, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Aly, N.; El-Gendy, K. Impact of parathion exposure on some biochemical parameters in rabbit as a non-target organism. Alexandria J. Med. 2015, 51, 11–17. [Google Scholar] [CrossRef]
- Amacher, D.E. Serum Transaminase Elevations as Indicators of Hepatic Injury Following the Administration of Drugs. Regul. Toxicol. Pharmacol. 1998, 27, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Elefsiniotis, I.S.; Liatsos, G.D.; Stamelakis, D.; Moulakakis, A. Case Report: Mixed Cholestatic/Hepatocellular Liver Injury Induced by the Herbicide Quizalofop-p-ethyl. Environ. Health Perspect. 2007, 115, 1479–1481. [Google Scholar] [PubMed]
- Ferguson, M.A.; Waikar, S.S. Established and Emerging Markers of Kidney Function. Clin. Chem. 2012, 58, 4–14. [Google Scholar] [CrossRef] [PubMed]
- George, G.S.; Wakasi, M.E.; Egoro, E. Creatinine and urea levels as critical markers in end-stage renal failure. Res. Rev. J. Med. Health Sci. 2014, 3, 41–44. [Google Scholar]
- Hodgen, G.D.; Sherins, J.R. Enzymes as markers of testicular growth and development in the rat. Endocrinology 1973, 93, 985–989. [Google Scholar] [CrossRef] [PubMed]
- Sherins, R.J.; Hodgen, G.D. Testicular gamma glutamyl-transpeptidase: An index of Sertoli cell function in man. J. Reprod. Fertill. 1976, 48, 191–193. [Google Scholar] [CrossRef]
- Lushchak, V.I. Glutathione Homeostasis and Functions: Potential Targets for Medical Interventions. J. Amino Acids 2012, 2012, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Rato, L.; Alves, M.G.; Socorro, S.; Duarte, A.I.; Cavaco, J.E.; Oliveira, P.F. Metabolic regulation is important for spermatogenesis. Nat. Rev. Urol. 2012, 9, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Carreau, S.; Drosdowsky, M.A.; Foucault, P. Enzymatic properties of adult human Sertoli cells in vitro. Andrologia 1996, 28, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Jana, K.; Jana, S.; Samanta, P.K. Effects of chronic exposure to sodium arsenite on hypothalamo-pituitary-testicular activities in adult rats: Possible an estrogenic mode of action. Reprod. Biol. Endocrinol. 2006, 4, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Sing, G.B.; Srivastava, S.P.; Seth, P.K. Testicular toxicity of di-n-butyl-phthalate in adult rats: Effect on marker enzymes of spermatogenesis. Ind. J. Exp. Biol. 1990, 28, 67–70. [Google Scholar]
- Latchoumycandane, C.; Mathur, P.P. Induction of oxidative stress in the rat testis after short-term exposure to the organochlorine pesticide methoxychlor. Arch. Toxicol. 2002, 76, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Roman, S.D. Antioxidant systems and oxidative stress in the testes. Oxid. Med. Cell. Longev. 2008, 1, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Tremellen, K. Oxidative stress and male infertility—A clinical perspective. Hum. Reprod. Update 2008, 14, 243–258. [Google Scholar] [CrossRef] [PubMed]
- Mruk, D.D.; Silvestrini, B.; Mo, M.Y.; Cheng, C.Y. Antioxidant superoxide dismutase—A review: Its function, regulation in the testis, and role in male fertility. Contraception 2002, 65, 305–311. [Google Scholar] [CrossRef]
- Zini, A.; Schlegel, P.N. Catalase mRNA expression in the male rat reproductive tract. J. Androl. 1996, 17, 473–480. [Google Scholar] [PubMed]
- Sheehan, D.; Meade, D.; Foley, V.M.; Dowd, C.A. Structure, function and evolution of glutathione transferases: Implications for classification of non-mammalian members of an ancient enzyme superfamily. Biochem. J. 2001, 360, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Aravinda, S.; Gopalakrishnan, B.; Dey, C.S.; Totey, S.M.; Pawshe, C.H.; Salunke, D.; Kaur, K.; Shaha, C. A Testicular Protein Important for Fertility Has Glutathione-S-Transferase Activity and Is Localized Extracellularly in the Seminiferous Tubules. J. Biol. Chem. 1995, 270, 15675–15685. [Google Scholar] [CrossRef] [PubMed]
- Abarikwu, S.O.; Adesiyan, A.C.; Oyeloja, T.O.; Oyeyemi, M.O.; Farombi, E.O. Changes in sperm characteristics and induction of oxidative stress in the testis and epididymis of experimental rats by a herbicide, atrazine. Arch. Environ. Contam. Toxicol. 2010, 58, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Peltola, V.; Huhtaniemi, I.; Ahotupa, M. Antioxidant enzyme activity in the maturing rat testis. J. Androl. 1992, 13, 450–455. [Google Scholar] [PubMed]
- Furland, N.E.; Zanetti, S.R.; Oresti, G.M.; Maldonado, E.N.; Aveldano, M.I. Ceramides and Sphingomyelins with High Proportions of Very Long-Chain Polyunsaturated Fatty Acids in Mammalian Germ Cells. J. Biol. Chem. 2007, 282, 18141–18150. [Google Scholar] [CrossRef] [PubMed]
- Mylonas, C.; Kouretas, D. Lipid peroxidation and tissue damage. In Vivo 1999, 13, 295–309. [Google Scholar] [PubMed]
- Joshi, S.C.; Tibrewal, P.; Sharma, A.; Sharma, P. Evaluation of toxic effect of 2,4-D (2,4-dichlorophenoxyacetic acid) on fertility and biochemical parameters of male reproductive system of albino rats. Int. J. Pharm. Pharm. Sci. 2012, 4, 338–342. [Google Scholar]
- Swapna, I.; SathyaSaiKumar, K.V.; Murthy, R.K.; Dutta-Gupta, A.; Senthilkumaran, B. Changes in cerebral membrane lipid composition and fluidity during thioacetamide-induced hepatic encephalopathy. J. Neurochem. 2006, 98, 1899–1907. [Google Scholar] [CrossRef] [PubMed]
- Bell, M.; Sikka, S.C.; Rajasekaran, M.; Hellstrom, W. Time course of hydrogen peroxide induced changes in the lipid peroxidation of human sperm membranes. Adv. Contracept. Deliv. Syst. 1992, 8, 144–150. [Google Scholar] [PubMed]
- Lenzi, A.; Gandini, L.; Picardo, M.; Tramer, F.; Sandri, G.; Panfili, E. Lipoperoxidation damage of spermatozoa polyunsaturated fatty acids (PUFA): Scavenger mechanisms and possible scavenger therapies. Front. Biosci. 2000, 1, E1–E15. [Google Scholar] [CrossRef]
- Lerda, D.; Rizzi, R. Study of reproductive function in persons occupationally exposed to 2,4-dichlorophenoxyacetic acid (2,4-D). Mutat. Res. 1991, 262, 47–50. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olayinka, E.T.; Ore, A. Hepatotoxicity, Nephrotoxicity and Oxidative Stress in Rat Testis Following Exposure to Haloxyfop-p-methyl Ester, an Aryloxyphenoxypropionate Herbicide. Toxics 2015, 3, 373-389. https://doi.org/10.3390/toxics3040373
Olayinka ET, Ore A. Hepatotoxicity, Nephrotoxicity and Oxidative Stress in Rat Testis Following Exposure to Haloxyfop-p-methyl Ester, an Aryloxyphenoxypropionate Herbicide. Toxics. 2015; 3(4):373-389. https://doi.org/10.3390/toxics3040373
Chicago/Turabian StyleOlayinka, Ebenezer Tunde, and Ayokanmi Ore. 2015. "Hepatotoxicity, Nephrotoxicity and Oxidative Stress in Rat Testis Following Exposure to Haloxyfop-p-methyl Ester, an Aryloxyphenoxypropionate Herbicide" Toxics 3, no. 4: 373-389. https://doi.org/10.3390/toxics3040373
APA StyleOlayinka, E. T., & Ore, A. (2015). Hepatotoxicity, Nephrotoxicity and Oxidative Stress in Rat Testis Following Exposure to Haloxyfop-p-methyl Ester, an Aryloxyphenoxypropionate Herbicide. Toxics, 3(4), 373-389. https://doi.org/10.3390/toxics3040373