Lactational Transfer of Long-Chain Perfluorinated Carboxylic Acids in Mice: A Method to Directly Collect Milk and Evaluate Chemical Transferability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animal Handling Procedures
2.3. Milking Device
2.4. Determination of PFCAs in Plasma and Milk
2.5. M/P Concentration Ratio, Estimated Daily Intake (EDI) of Pups, EDI of Dams, and Estimated Relative Daily Intake (ERDI) between Dams and Pups
2.5.1. M/P Concentration Ratio
2.5.2. Estimated Daily Intake (EDI) of Pups
2.5.3. EDI of Dams
2.5.4. Estimated Relative Daily Intake (ERDI) between Dams and Pups
2.6. Statistical Analysis
3. Results and Discussion
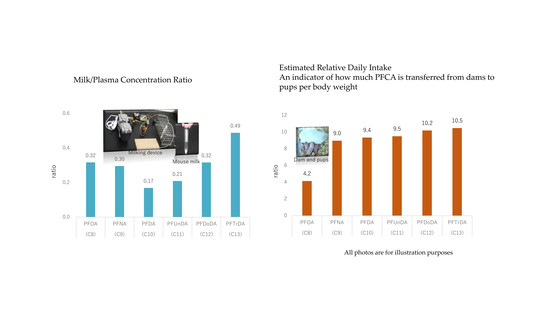
3.1. M/P Concentration Ratio of PFCAs
3.2. Estimated Lactational Transfer from Dams to Pups
3.2.1. EDI of Pups
3.2.2. EDI of Dams and ERDI between Dams and Pups
3.3. Limitations of this Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. The Optimal Duration of Exclusive Breastfeeding. 2001. Vol. WHO/NHD/01.09, WHO/FCH/CAH/01.24. Available online: https://www.who.int/nutrition/publications/infantfeeding/WHO_NHD_01.09/en/ (accessed on 24 March 2020).
- Eldelman, A.I.; Schandler, R.J. American Academy of Pediatrics policy statement: Breastfeeding and the Use of Human Milk. Pediatrics 2012, 129, e827–e841. [Google Scholar] [CrossRef] [Green Version]
- Ip, S.; Chung, M.; Raman, G.; Chew, P.; Magula, N.; DeVine, D.; Trikalinos, T.; Lau, J. Breastfeeding and maternal and infant health outcomes in developed countries. Evid. Rep./Technol. Assess. 2007, 153, 1–186. [Google Scholar]
- Chantry, C.J.; Howard, C.R.; Auinger, P. Full breastfeeding duration and associated decrease in respiratory tract infection in US children. Pediatrics 2006, 117, 425–432. [Google Scholar] [CrossRef]
- Kwan, M.L.; Buffler, P.A.; Abrams, B.; Kiley, V.A. Breastfeeding and the risk of childhood leukemia: A meta-analysis. Public Health Rep. 2004, 119, 521–535. [Google Scholar] [CrossRef] [Green Version]
- Rudant, J.; Orsi, L.; Menegaux, F.; Petit, A.; Baruchel, A.; Bertrand, Y.; Lambilliotte, A.; Robert, A.; Michel, G.; Margueritte, G.; et al. Childhood acute leukemia, early common infections, and allergy: The ESCALE Study. Am. J. Epidemiol. 2010, 172, 1015–1027. [Google Scholar] [CrossRef] [Green Version]
- Boue, G.; Cummins, E.; Guillou, S.; Antignac, J.P.; Le Bizec, B.; Membre, J.M. Public health risks and benefits associated with breast milk and infant formula consumption. Crit. Rev. Food Sci. Nutr. 2018, 58, 126–145. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M. Medications in pregnancy and lactation: Assessment of risks to fetus and infants. Organ Biol. 2011, 18, 279–286. [Google Scholar] [CrossRef]
- Larsen, L.A.; Ito, S.; Koren, G. Prediction of milk/plasma concentration ratio of drugs. Ann Pharm. 2003, 37, 1299–1306. [Google Scholar] [CrossRef]
- Glynn, A.; Berger, U.; Bignert, A.; Ullah, S.; Aune, M.; Lignell, S.; Darnerud, P.O. Perfluorinated Alkyl Acids in Blood Serum from Primiparous Women in Sweden: Serial Sampling during Pregnancy and Nursing, And Temporal Trends 1996–2010. Environ. Sci. Technol. 2012, 46, 9071–9079. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.H.; Hitomi, T.; Niisoe, T.; Takanaka, K.; Kamiyama, S.; Watanabe, T.; Moon, C.S.; Yang, H.R.; Hung, N.N.; Koizumi, A.; et al. Odd-numbered perfluorocarboxylates predominate over perfluorooctanoic acid in serum samples from Japan, Korea and Vietnam. Environ. Int. 2011, 37, 1183–1189. [Google Scholar] [CrossRef] [Green Version]
- Fujii, Y.; Harada, K.H.; Haraguchi, K.; Koizumi, A. Long-term trends in dietary intake of perfluoroalkyl carboxylic acids in relation to their serum concentration in two regions in Japan from 1979 to 2011. Chemosphere 2017, 176, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Okada, E.; Kashino, I.; Matsuura, H.; Sasaki, S.; Miyashita, C.; Yamamoto, J.; Ikeno, T.; Ito, Y.M.; Matsumura, T.; Tamakoshi, A.; et al. Temporal trends of perfluoroalkyl acids in plasma samples of pregnant women in Hokkaido, Japan, 2003–2011. Environ. Int 2013, 60, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Grandjean, P.; Andersen, E.W.; Budtz-Jorgensen, E.; Nielsen, F.; Molbak, K.; Weihe, P.; Heilmann, C. Serum vaccine antibody concentrations in children exposed to perfluorinated compounds. Jama 2012, 307, 391–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barouki, R.; Gluckman, P.D.; Grandjean, P.; Hanson, M.; Heindel, J.J. Developmental origins of non-communicable disease: Implications for research and public health. Environ. Health Glob. Access Sci. Source 2012, 11, 42. [Google Scholar] [CrossRef] [Green Version]
- Bruckner, J.V. Differences in sensitivity of children and adults to chemical toxicity: The NAS panel report. Regul. Toxicol. Pharmacol. Rtp. 2000, 31, 280–285. [Google Scholar] [CrossRef]
- Mogensen, U.B.; Grandjean, P.; Nielsen, F.; Weihe, P.; Budtz-Jørgensen, E. Breastfeeding as an Exposure Pathway for Perfluorinated Alkylates. Environ. Sci. Technol. 2015, 49, 10466–10473. [Google Scholar] [CrossRef]
- Kärrman, A.; Ericson, I.; Van Bavel, B.; Darnerud, P.O.; Aune, M.; Glynn, A.; Lignell, S.; Lindström, G. Exposure of perfluorinated chemicals through lactation: Levels of matched human milk and serum and a temporal trend, 1996–2004, in Sweden. Environ. Health Perspect. 2007, 115, 226–230. [Google Scholar] [CrossRef] [Green Version]
- Fujii, Y.; Yan, J.X.; Harada, K.H.; Hitomi, T.; Yang, H.; Wang, P.Y.; Koizumi, A. Levels and profiles of long-chain perfluorinated carboxylic acids in human breast milk and infant formulas in East Asia. Chemosphere 2012, 86, 315–321. [Google Scholar] [CrossRef] [Green Version]
- Fujii, Y.; Niisoe, T.; Harada, K.H.; Uemoto, S.; Ogura, Y.; Takenaka, K.; Koizumi, A. Toxicokinetics of perfluoroalkyl carboxylic acids with different carbon chain lengths in mice and humans. J. Occup. Health 2015, 57, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Oskarsson, A.; Möller, N. A method for studies on milk excretion of chemicals in mice with 2,2′,4,4′,5-pentabromodiphenyl ether (BDE-99) as a model. Toxicol. Lett. 2004, 151, 327–334. [Google Scholar] [CrossRef]
- Temple, P.L.; Kon, S.A. A simple apparatus for milking small laboratory animals. Biochem. J. 1937, 31, 2197–2198. [Google Scholar] [CrossRef] [Green Version]
- Cox, W.M., Jr.; Mueller, A.J. The Composition of Milk from Stock Rats and an Apparatus for Milking Small Laboratory Animals: Two Text Figures and one Plate (One Figure). J. Nutr. 1937, 13, 249–261. [Google Scholar] [CrossRef]
- Fujii, Y.; Harada, K.H.; Koizumi, A. Analysis of perfluoroalkyl carboxylic acids in composite dietary samples by gas chromatography/mass spectrometry with electron capture negative ionization. Environ. Sci. Technol. 2012, 46, 11235–11242. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Harada, K.H.; Koizumi, A. Occurrence of perfluorinated carboxylic acids (PFCAs) in personal care products and compounding agents. Chemosphere 2013, 93, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Doerge, D.R.; Vanlandingham, M.; Twaddle, N.C.; Delclos, K.B. Lactational transfer of bisphenol A in Sprague-Dawley rats. Toxicol Lett 2010, 199, 372–376. [Google Scholar] [CrossRef]
- Eshraghi, M.; McFall, E.; Gibeault, S.; Kothary, R. Effect of genetic background on the phenotype of the Smn2B/- mouse model of spinal muscular atrophy. Hum. Mol. Genet. 2016, 25, 4494–4506. [Google Scholar] [CrossRef] [Green Version]
- Grigor, M.R.; Thompson, M.P. Diurnal regulation of milk lipid production and milk secretion in the rat: Effect of dietary protein and energy restriction. J. Nutr. 1987, 117, 748–753. [Google Scholar] [CrossRef]
- Suzuki, K.; Sasaki, S.; Shinzawa, K.; Totani, M. Milk Intake by Breast-fed Infants before weaning. Jpn. J. Nutr. Diet. 2004, 62, 369–372. [Google Scholar] [CrossRef]
- Rosenbaum, S.E. Basic Pharmacokinetics and Pharmacodynamics: An Integrated Textbook and Computer Simulations; John Wiley & Sons, Inc.: New York, NY, USA, 2016; ISBN 9781119143185. [Google Scholar]
- Hale, T.W. Hale’s Medications and MothersʼMilk; Springer Publishing Company: New York, NY, USA, 2010. [Google Scholar]
- Astrup-Jensen, A.; Bates, C.J.; Begg, E.J.; Edwards, S.; Lazarus, C.; Matheson, I.; Mountford, P.J.; Neville, M.C.; Notarianni, L.J.; Prentiss, A.; et al. Drugs and Human Lactation, 2nd ed.; Elsevier Science: Amsterdam, The Netherlands, 1996; ISBN 9780080530550. [Google Scholar]
- Fenton, S.E.; Reiner, J.L.; Nakayama, S.F.; Delinsky, A.D.; Stanko, J.P.; Hines, E.P.; White, S.S.; Lindstrom, A.B.; Strynar, M.J.; Petropoulou, S.-S.E.; et al. Analysis of PFOA in dosed CD-1 mice. Part 2: Disposition of PFOA in tissues and fluids from pregnant and lactating mice and their pups. Reprod. Toxicol. 2009, 27, 365–372. [Google Scholar] [CrossRef] [Green Version]
- Fatemi, M.H.; Ghorbanzad’e, M. Classification of drugs according to their milk/plasma concentration ratio. Eur J Med Chem 2010, 45, 5051–5055. [Google Scholar] [CrossRef]
- Jones, P.D.; Hu, W.; De Coen, W.; Newsted, J.L.; Giesy, J.P. Binding of perfluorinated fatty acids to serum proteins. Environ. Toxicol. Chem./Setac 2003, 22, 2639–2649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Ren, X.M.; Guo, L.H. Structure-based investigation on the interaction of perfluorinated compounds with human liver fatty acid binding protein. Environ. Sci Technol 2013, 47, 11293–11301. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Alcorn, J. Xenobiotic transporter expression and function in the human mammary gland. Adv. Drug Deliv. Rev. 2003, 55, 653–665. [Google Scholar] [CrossRef]
- Nakagawa, H.; Terada, T.; Harada, K.H.; Hitomi, T.; Inoue, K.; Inui, K.-I.; Koizumi, A. Human Organic Anion Transporter hOAT4 is a Transporter of Perfluorooctanoic Acid. Basic Clin. Pharmacol. Toxicol. 2009, 105, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, H.; Hirata, T.; Terada, T.; Jutabha, P.; Miura, D.; Harada, K.H.; Inoue, K.; Anzai, N.; Endou, H.; Inui, K.-I.; et al. Roles of Organic Anion Transporters in the Renal Excretion of Perfluorooctanoic Acid. Basic Clin. Pharmacol. Toxicol. 2008, 103, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.H.; Glover, K.P.; Han, X. Characterization of cellular uptake of perfluorooctanoate via organic anion-transporting polypeptide 1A2, organic anion transporter 4, and urate transporter 1 for their potential roles in mediating human renal reabsorption of perfluorocarboxylates. Toxicol. Sci. Off. J. Soc. Toxicol. 2010, 117, 294–302. [Google Scholar] [CrossRef] [Green Version]
- Tahara, H.; Kusuhara, H.; Endou, H.; Koepsell, H.; Imaoka, T.; Fuse, E.; Sugiyama, Y. A species difference in the transport activities of H2 receptor antagonists by rat and human renal organic anion and cation transporters. J. Pharmacol. Exp. Ther. 2005, 315, 337–345. [Google Scholar] [CrossRef] [Green Version]

| Factor | Value | Reference |
|---|---|---|
| (A) PFCA concentrations in milk (C8–C13) | Shown in Table 2 (μmol/L) | the present study |
| (B) Body weight of FVB mice at PND 8 | 4.7 g | Eshraghi et al., 2016 [27] |
| (C) Body weight of FVB mice at PND 13 | 6.8 g | Eshraghi et al., 2016 [27] |
| (D) Body weight gain between PND 8 and PND 13 | 2.1 g | (C)–(B) |
| (E) Average daily body weight gain | 0.42 g/day | (D)/5 |
| (F) Estimated milk consumption weight per day | 0.84 g/day | (E) × 2 (Grigor and Thompson, 1987 [28], Goerge et al., 2010 [26]) |
| (G) Estimated milk consumption volume per day | 8.5 × 10−4 L/day | (F) × 1.017 (Breastmilk specific weight, g/L) × 10−3 (Suzuki et al., 2004 [29]) |
| (H) Body weight of pups at PND 10 | 5.5g | (B) + (E) × 2 |
| (I) EDI of pups | Shown in Table 3 (μmol/kg/day) | (A) × (G)/(H) …Equation (2) |
| (J) PFCA concentrations in plasma (C8–C13) | Shown in Table 2 (μmol/L) | the present study |
| (K) Total PFCA clearances (C8–C13) | C8; 0.012, C9; 0.005, C10; 0.003, C11; 0.003, C12; 0.005, C13; 0.007 (L/kg/day) | Fujii et al., 2015 [20] |
| (L) EDI of dams | Shown in Table 3 (μmol/kg/day) | (J) × (K) …Equation (3) |
| (M) ERDI between dams and pups | Shown in Table 3 (ratio) | (I)/(L) …Equation (4) |
| PFOA | PFNA | PFDA | PFUnDA | PFDoDA | PFTrDA | ||
|---|---|---|---|---|---|---|---|
| (C8) | (C9) | (C10) | (C11) | (C12) | (C13) | ||
| Milk | µmol/L | 4.38 (1.15) * | 3.30 (2.15) * | 0.57 (0.20) * | 0.46 (0.19) * | 0.29 (0.10) * | 0.23 (0.07) * |
| Plasma | µmol/L | 13.78 (2.21) | 11.00 (5.46) | 3.43 (0.75) | 2.42 (1.63) | 1.10 (0.53) | 0.63 (0.39) |
| Milk/Plasma a | ratio | 0.32 (0.07) | 0.30 (0.10) | 0.17 (0.07) | 0.21 (0.07) | 0.32 (0.15) | 0.49 (0.27) |
| PFOA | PFNA | PFDA | PFUnDA | PFDoDA | PFTrDA | ||
|---|---|---|---|---|---|---|---|
| (C8) | (C9) | (C10) | (C11) | (C12) | (C13) | ||
| EDI of pups a | µmol/kg/day | 0.68 (0.18) | 0.51 (0.33) | 0.09 (0.03) | 0.07 (0.03) | 0.05 (0.02) | 0.04 (0.01) |
| EDI of dams b | µmol/kg/day | 0.163 (0.025) | 0.056 (0.027) | 0.010 (0.002) | 0.008 (0.005) | 0.005 (0.002) | 0.005 (0.003) |
| ERDI between dams and pups c | ratio | 4.16 (0.87) | 8.98 (3.15) | 9.35 (3.84) | 9.51 (2.96) | 10.20 (4.95) | 10.49 (5.87) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujii, Y.; Harada, K.H.; Kobayashi, H.; Haraguchi, K.; Koizumi, A. Lactational Transfer of Long-Chain Perfluorinated Carboxylic Acids in Mice: A Method to Directly Collect Milk and Evaluate Chemical Transferability. Toxics 2020, 8, 23. https://doi.org/10.3390/toxics8020023
Fujii Y, Harada KH, Kobayashi H, Haraguchi K, Koizumi A. Lactational Transfer of Long-Chain Perfluorinated Carboxylic Acids in Mice: A Method to Directly Collect Milk and Evaluate Chemical Transferability. Toxics. 2020; 8(2):23. https://doi.org/10.3390/toxics8020023
Chicago/Turabian StyleFujii, Yukiko, Kouji H. Harada, Hatasu Kobayashi, Koichi Haraguchi, and Akio Koizumi. 2020. "Lactational Transfer of Long-Chain Perfluorinated Carboxylic Acids in Mice: A Method to Directly Collect Milk and Evaluate Chemical Transferability" Toxics 8, no. 2: 23. https://doi.org/10.3390/toxics8020023
APA StyleFujii, Y., Harada, K. H., Kobayashi, H., Haraguchi, K., & Koizumi, A. (2020). Lactational Transfer of Long-Chain Perfluorinated Carboxylic Acids in Mice: A Method to Directly Collect Milk and Evaluate Chemical Transferability. Toxics, 8(2), 23. https://doi.org/10.3390/toxics8020023