Pulmonary Toxicity and Inflammatory Response of Vape Cartridges Containing Medium-Chain Triglycerides Oil and Vitamin E Acetate: Implications in the Pathogenesis of EVALI
Abstract
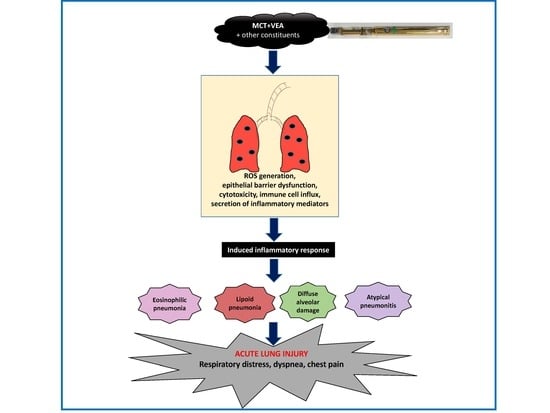
:1. Introduction
2. Material and Methods
2.1. Scientific Rigor and Reproducibility
2.2. Ethics Statement: Institutional Biosafety and Animal Protocol Approval
2.3. Aerosol Exposure Setup
2.4. Vitamin E Acetate (VEA) 50% w/v Preparation
2.5. CBD/Counterfeit Vape Cartridges
2.6. Physicochemical Characteristics of MCT and VEA
2.7. Measurement of Total Volatile Organic Compounds (VOC) Levels in Aerosols
2.8. Acellular ROS Assay
2.9. Cellular ROS Assay
2.10. Cell Culture
2.11. Aerosol Exposures and Treatments to Cells
2.12. Cytotoxicity Assay
2.13. Cytokine ELISA
2.14. Transepithelial Electrical Resistance (TEER) Measurement
2.15. In Vivo Mouse Exposures
2.16. Mouse Arterial Oxygen Saturation
2.17. Bronchoalveolar Lavage (BALF) Collection
2.18. Luminex Assay
2.19. Flow Cytometry Analysis
2.20. Western Blot Analysis
2.21. Lipidomics Analysis
2.22. Oil-Red-O Staining
2.23. Lipid-Laden-Index (LLI) Scoring
2.24. Statistical Analysis
3. Results
3.1. MCT, VEA, and CBD/Counterfeit Vape Cartridges Induce Cellular and Acellular ROS Generation and Cytotoxic Responses
3.2. MCT, Mineral Oil, VEA, and CBD/Counterfeit Vape Cartridge Aerosols Contain Volatile Organic Compounds
3.3. Exposure to MCT, VEA, and CBD/Counterfeit Vape Cartridges Elicited A Differential Inflammatory Response in Epithelial and Monocyte Cells
3.4. Reduced Epithelial Barrier Function Following Exposure to MCT, VEA, or CBD/Counterfeit Vape Cartridges
3.5. Treatment of Macrophages with MCT, VEA, and CBD/Counterfeit Vape Cartridge Liquids Caused Varying Levels of Lipid-Laden Macrophage Formation
3.6. Acute Exposure to CBD/Counterfeit Vape Aerosols did not Alter Arterial Oxygen Saturation in Mice
3.7. Exposure to CBD/Counterfeit Vape Cartridge Aerosols Induced An Inflammatory Response in Mice
3.8. Surfactant-Associated Protein A (SP-A) Reduced in VEA-Exposed Mouse Lung Homogenates
3.9. Differential Changes in Eicosanoids/Oxylipins and Short-Chain Fatty Acids in Mouse BALF Following VEA and CBD/Counterfeit Vape Cartridge Aerosols Exposure
3.10. Diradylglycerols (DG), Sterols (CE), and Glycerophosphocholines (PC) Were Significantly Altered in VEA and Cartridge Aerosol Expose Mice
3.11. E-Cig Users Exhibited Differential Changes in Eicosanoids/Oxylipins and Short-Chain Fatty Acids in Human Plasma
3.12. SARS-CoV-2 Proteins ACE2, TMPRSS2, and Furin Were Largely Unaffected by Cartridge Aerosols
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Availability Statement
Disclaimer
References
- Layden, J.E.; Ghinai, I.; Pray, I.; Kimball, A.; Layer, M.; Tenforde, M.W.; Navon, L.; Hoots, B.; Salvatore, P.P.; Elderbrook, M.; et al. Pulmonary Illness Related to E-Cigarette Use in Illinois and Wisconsin—Final Report. N. Engl. J. Med. 2020, 382, 903–916. [Google Scholar] [CrossRef]
- CDC. Outbreak of Lung Injury Associated with E-cigarette Use, or Vaping. Available online: https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html (accessed on 17 April 2019).
- Chand, H.S.; Muthumalage, T.; Maziak, W.; Rahman, I. Pulmonary Toxicity and the Pathophysiology of Electronic Cigarette, or Vaping Product, Use Associated Lung Injury. Front. Pharmacol. 2019, 10, 1619. [Google Scholar] [CrossRef] [Green Version]
- Kalininskiy, A.; Bach, C.T.; Nacca, N.E.; Ginsberg, G.; Marraffa, J.; Navarette, K.A.; McGraw, M.D.; Croft, D.P. E-cigarette, or vaping, product use associated lung injury (EVALI): Case series and diagnostic approach. Lancet Respir. Med. 2019, 7, 1017–1026. [Google Scholar] [CrossRef]
- Blount, B.C.; Karwowski, M.P.; Shields, P.G.; Morel-Espinosa, M.; Valentin-Blasini, L.; Gardner, M.; Braselton, M.; Brosius, C.R.; Caron, K.T.; Chambers, D.; et al. Vitamin E Acetate in Bronchoalveolar-Lavage Fluid Associated with EVALI. N. Engl. J. Med. 2020, 382, 697–705. [Google Scholar] [CrossRef]
- Viswam, D.; Trotter, S.; Burge, P.S.; Walters, G.I. Respiratory failure caused by lipoid pneumonia from vaping e-cigarettes. BMJ Case Rep. 2018, 2018. [Google Scholar] [CrossRef]
- Sommerfeld, C.G.; Weiner, D.J.; Nowalk, A.; Larkin, A. Hypersensitivity Pneumonitis and Acute Respiratory Distress Syndrome from E-Cigarette Use. Pediatrics 2018, 141. [Google Scholar] [CrossRef]
- Muthumalage, T.; Friedman, M.R.; McGraw, M.D.; Ginsberg, G.; Friedman, A.E.; Rahman, I. Chemical Constituents Involved in E-Cigarette, or Vaping Product Use-Associated Lung Injury (EVALI). Toxics 2020, 8, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, K.P.; Lawyer, G.; Muthumalage, T.; Maremanda, K.P.; Khan, N.A.; McDonough, S.R.; Ye, D.; McIntosh, S.; Rahman, I. Systemic biomarkers in electronic cigarette users: Implications for noninvasive assessment of vaping-associated pulmonary injuries. ERJ Open Res. 2019, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Khan, N.A.; Muthumalage, T.; Lawyer, G.R.; McDonough, S.R.; Chuang, T.D.; Gong, M.; Sundar, I.K.; Rehan, V.K.; Rahman, I. Dysregulated repair and inflammatory responses by e-cigarette-derived inhaled nicotine and humectant propylene glycol in a sex-dependent manner in mouse lung. FASEB Bioadv. 2019, 1, 609–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kazachkov, M.Y.; Muhlebach, M.S.; Livasy, C.A.; Noah, T.L. Lipid-laden macrophage index and inflammation in bronchoalveolar lavage fluids in children. Eur. Respir. J. 2001, 18, 790–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.S.; Khateeb, F.; Akhtar, J.; Khan, Z.; Lal, A.; Kholodovych, V.; Hammersley, J. Organizing pneumonia related to electronic cigarette use: A case report and review of literature. Clin. Respir. J. 2018, 12, 1295–1299. [Google Scholar] [CrossRef] [PubMed]
- Madison, M.C.; Landers, C.T.; Gu, B.H.; Chang, C.Y.; Tung, H.Y.; You, R.; Hong, M.J.; Baghaei, N.; Song, L.Z.; Porter, P.; et al. Electronic cigarettes disrupt lung lipid homeostasis and innate immunity independent of nicotine. J. Clin. Investig. 2019, 129, 4290–4304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Messina, M.D.; Levin, T.L.; Conrad, L.A.; Bidiwala, A. Vaping associated lung injury: A potentially life-threatening epidemic in US youth. Pediatr. Pulmonol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, V.; Panettieri, R.A., Jr.; Gennaro, M.L. Lipid-laden macrophages as biomarkers of vaping-associated lung injury. Lancet Respir. Med. 2020, 8, e6. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; O’Shea, D.F. Potential for release of pulmonary toxic ketene from vaping pyrolysis of vitamin E acetate. Proc. Natl. Acad. Sci. USA 2020, 117, 6349–6355. [Google Scholar] [CrossRef] [Green Version]
- Lanzarotta, A.; Falconer, T.M.; Flurer, R.; Wilson, R.A. Hydrogen Bonding between Tetrahydrocannabinol and Vitamin E Acetate in Unvaped, Aerosolized, and Condensed Aerosol e-Liquids. Anal. Chem. 2020, 92, 2374–2378. [Google Scholar] [CrossRef] [Green Version]
- Son, Y.; Mishin, V.; Laskin, J.D.; Mainelis, G.; Wackowski, O.A.; Delnevo, C.; Schwander, S.; Khlystov, A.; Samburova, V.; Meng, Q. Hydroxyl Radicals in E-Cigarette Vapor and E-Vapor Oxidative Potentials under Different Vaping Patterns. Chem. Res. Toxicol. 2019, 32, 1087–1095. [Google Scholar] [CrossRef]
- Allen, T.C.; Kurdowska, A. Interleukin 8 and acute lung injury. Arch. Pathol. Lab. Med. 2014, 138, 266–269. [Google Scholar] [CrossRef]
- Reidel, B.; Radicioni, G.; Clapp, P.W.; Ford, A.A.; Abdelwahab, S.; Rebuli, M.E.; Haridass, P.; Alexis, N.E.; Jaspers, I.; Kesimer, M. E-Cigarette Use Causes a Unique Innate Immune Response in the Lung, Involving Increased Neutrophilic Activation and Altered Mucin Secretion. Am. J. Respir. Crit. Care Med. 2018, 197, 492–501. [Google Scholar] [CrossRef]
- Cross, L.J.; Matthay, M.A. Biomarkers in acute lung injury: Insights into the pathogenesis of acute lung injury. Crit. Care Clin. 2011, 27, 355–377. [Google Scholar] [CrossRef] [Green Version]
- Muthumalage, T.; Lamb, T.; Friedman, M.R.; Rahman, I. E-cigarette flavored pods induce inflammation, epithelial barrier dysfunction, and DNA damage in lung epithelial cells and monocytes. Sci. Rep. 2019, 9, 19035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crotty Alexander, L.E.; Drummond, C.A.; Hepokoski, M.; Mathew, D.; Moshensky, A.; Willeford, A.; Das, S.; Singh, P.; Yong, Z.; Lee, J.H.; et al. Chronic inhalation of e-cigarette vapor containing nicotine disrupts airway barrier function and induces systemic inflammation and multiorgan fibrosis in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 314, R834–R847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chabot, F.; Mitchell, J.A.; Gutteridge, J.M.; Evans, T.W. Reactive oxygen species in acute lung injury. Eur. Respir. J. 1998, 11, 745–757. [Google Scholar] [PubMed]
- Triantafyllou, G.A.; Tiberio, P.J.; Zou, R.H.; Lamberty, P.E.; Lynch, M.J.; Kreit, J.W.; Gladwin, M.T.; Morris, A.; Chiarchiaro, J. Vaping-Associated Acute Lung Injury: A Case Series. Am. J. Respir. Crit. Care Med. 2019. [Google Scholar] [CrossRef]
- Butt, Y.M.; Smith, M.L.; Tazelaar, H.D.; Vaszar, L.T.; Swanson, K.L.; Cecchini, M.J.; Boland, J.M.; Bois, M.C.; Boyum, J.H.; Froemming, A.T.; et al. Pathology of Vaping-Associated Lung Injury. N. Engl. J. Med. 2019, 381, 1780–1781. [Google Scholar] [CrossRef]
- Maddock, S.D.; Cirulis, M.M.; Callahan, S.J.; Keenan, L.M.; Pirozzi, C.S.; Raman, S.M.; Aberegg, S.K. Pulmonary Lipid-Laden Macrophages and Vaping. N. Engl. J. Med. 2019, 381, 1488–1489. [Google Scholar] [CrossRef]
- Simmons, A.; Rouf, E.; Whittle, J. Not your typical pneumonia: A case of exogenous lipoid pneumonia. J. Gen. Intern. Med. 2007, 22, 1613–1616. [Google Scholar] [CrossRef] [Green Version]
- Wiedermann, F.J. Acute lung injury during G-CSF-induced neutropenia recovery: Effect of G-CSF on pro- and anti-inflammatory cytokines. Bone Marrow Transplant. 2005, 36, 731. [Google Scholar] [CrossRef] [Green Version]
- Karlin, L.; Darmon, M.; Thiery, G.; Ciroldi, M.; de Miranda, S.; Lefebvre, A.; Schlemmer, B.; Azoulay, E. Respiratory status deterioration during G-CSF-induced neutropenia recovery. Bone Marrow Transplant. 2005, 36, 245–250. [Google Scholar] [CrossRef]
- Wiedermann, F.J.; Mayr, A.J.; Kaneider, N.C.; Fuchs, D.; Mutz, N.J.; Schobersberger, W. Alveolar granulocyte colony-stimulating factor and alpha-chemokines in relation to serum levels, pulmonary neutrophilia, and severity of lung injury in ARDS. Chest 2004, 125, 212–219. [Google Scholar] [CrossRef]
- Itoh, M.; Aoshiba, K.; Herai, Y.; Nakamura, H.; Takemura, T. Lung injury associated with electronic cigarettes inhalation diagnosed by transbronchial lung biopsy. Respirol. Case Rep. 2018, 6, e00282. [Google Scholar] [CrossRef] [PubMed]
- Harris, K.; Chalhoub, M.; Maroun, R.; Abi-Fadel, F.; Zhao, F. Lipoid pneumonia: A challenging diagnosis. Heart Lung 2011, 40, 580–584. [Google Scholar] [CrossRef]
- Henry, T.S.; Kanne, J.P.; Kligerman, S.J. Imaging of Vaping-Associated Lung Disease. N. Engl. J. Med. 2019, 381, 1486–1487. [Google Scholar] [CrossRef]
- Lamkhioued, B.; Renzi, P.M.; Abi-Younes, S.; Garcia-Zepada, E.A.; Allakhverdi, Z.; Ghaffar, O.; Rothenberg, M.D.; Luster, A.D.; Hamid, Q. Increased expression of eotaxin in bronchoalveolar lavage and airways of asthmatics contributes to the chemotaxis of eosinophils to the site of inflammation. J. Immunol. 1997, 159, 4593–4601. [Google Scholar]
- Yang, M.L.; Wang, C.T.; Yang, S.J.; Leu, C.H.; Chen, S.H.; Wu, C.L.; Shiau, A.L. IL-6 ameliorates acute lung injury in influenza virus infection. Sci. Rep. 2017, 7, 43829. [Google Scholar] [CrossRef]
- Yu, M.; Zheng, X.; Witschi, H.; Pinkerton, K.E. The role of interleukin-6 in pulmonary inflammation and injury induced by exposure to environmental air pollutants. Toxicol. Sci. 2002, 68, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Ward, N.S.; Waxman, A.B.; Homer, R.J.; Mantell, L.L.; Einarsson, O.; Du, Y.; Elias, J.A. Interleukin-6-induced protection in hyperoxic acute lung injury. Am. J. Respir. Cell. Mol. Biol. 2000, 22, 535–542. [Google Scholar] [CrossRef] [PubMed]
- De Giacomi, F.; Vassallo, R.; Yi, E.S.; Ryu, J.H. Acute Eosinophilic Pneumonia. Causes, Diagnosis, and Management. Am. J. Respir. Crit. Care Med. 2018, 197, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Fonseca Fuentes, X.; Kashyap, R.; Hays, J.T.; Chalmers, S.; Lama von Buchwald, C.; Gajic, O.; Gallo de Moraes, A. VpALI-Vaping-related Acute Lung Injury: A New Killer Around the Block. Mayo Clin. Proc. 2019, 94, 2534–2545. [Google Scholar] [CrossRef] [Green Version]
- Fryman, C.; Lou, B.; Weber, A.G.; Steinberg, H.N.; Khanijo, S.; Iakovou, A.; Makaryus, M.R. Acute Respiratory Failure Associated with Vaping. Chest 2020, 157, e63–e68. [Google Scholar] [CrossRef] [Green Version]
- Ye, P.; Rodriguez, F.H.; Kanaly, S.; Stocking, K.L.; Schurr, J.; Schwarzenberger, P.; Oliver, P.; Huang, W.; Zhang, P.; Zhang, J.; et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J. Exp. Med. 2001, 194, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Gu, Y.; Tu, Q.; Wang, K.; Gu, X.; Ren, T. Blockade of Interleukin-17 Restrains the Development of Acute Lung Injury. Scand. J. Immunol. 2016, 83, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Brix, N.; Rasmussen, F.; Poletti, V.; Bendstrup, E. Eosinophil alveolitis in two patients with idiopathic pulmonary fibrosis. Respir. Med. Case Rep. 2016, 19, 61–64. [Google Scholar] [CrossRef] [Green Version]
- Huaux, F.; Liu, T.; McGarry, B.; Ullenbruch, M.; Phan, S.H. Dual roles of IL-4 in lung injury and fibrosis. J. Immunol. 2003, 170, 2083–2092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conti, P.; DiGioacchino, M. MCP-1 and RANTES are mediators of acute and chronic inflammation. Allergy Asthma Proc. 2001, 22, 133–137. [Google Scholar] [CrossRef]
- Huaux, F.; Arras, M.; Tomasi, D.; Barbarin, V.; Delos, M.; Coutelier, J.P.; Vink, A.; Phan, S.H.; Renauld, J.C.; Lison, D. A profibrotic function of IL-12p40 in experimental pulmonary fibrosis. J. Immunol. 2002, 169, 2653–2661. [Google Scholar] [CrossRef]
- Muthumalage, T.; Rahman, I. Cannabidiol differentially regulates basal and LPS-induced inflammatory responses in macrophages, lung epithelial cells, and fibroblasts. Toxicol. Appl. Pharmacol. 2019, 382, 114713. [Google Scholar] [CrossRef]
- Ardain, A.; Porterfield, J.Z.; Kloverpris, H.N.; Leslie, A. Type 3 ILCs in Lung Disease. Front. Immunol. 2019, 10, 92. [Google Scholar] [CrossRef] [Green Version]
- Frank, J.A.; Matthay, M.A. Leukotrienes in acute lung injury: A potential therapeutic target? Am. J. Respir. Crit. Care Med. 2005, 172, 261–262. [Google Scholar] [CrossRef] [Green Version]
- Lund, S.J.; Portillo, A.; Cavagnero, K.; Baum, R.E.; Naji, L.H.; Badrani, J.H.; Mehta, A.; Croft, M.; Broide, D.H.; Doherty, T.A. Leukotriene C4 Potentiates IL-33-Induced Group 2 Innate Lymphoid Cell Activation and Lung Inflammation. J. Immunol. 2017, 199, 1096–1104. [Google Scholar] [CrossRef]
- Stephenson, A.H.; Sprague, R.S.; Dahms, T.E.; Lonigro, A.J. Increased leukotriene C4 in ethchlorvynol-induced acute lung injury in dogs. J. Appl. Physiol. 1987, 62, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Mishra, N.C.; Rir-Sima-Ah, J.; Langley, R.J.; Singh, S.P.; Pena-Philippides, J.C.; Koga, T.; Razani-Boroujerdi, S.; Hutt, J.; Campen, M.; Kim, K.C.; et al. Nicotine primarily suppresses lung Th2 but not goblet cell and muscle cell responses to allergens. J. Immunol. 2008, 180, 7655–7663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clapp, P.W.; Jaspers, I. Electronic Cigarettes: Their Constituents and Potential Links to Asthma. Curr. Allergy Asthma Rep. 2017, 17, 79. [Google Scholar] [CrossRef] [PubMed]
- Seet, R.C.; Lee, C.Y.; Loke, W.M.; Huang, S.H.; Huang, H.; Looi, W.F.; Chew, E.S.; Quek, A.M.; Lim, E.C.; Halliwell, B. Biomarkers of oxidative damage in cigarette smokers: Which biomarkers might reflect acute versus chronic oxidative stress? Free Radic. Biol. Med. 2011, 50, 1787–1793. [Google Scholar] [CrossRef]
- Bittleman, D.B.; Casale, T.B. 5-Hydroxyeicosatetraenoic acid (HETE)-induced neutrophil transcellular migration is dependent upon enantiomeric structure. Am. J. Respir. Cell Mol. Biol. 1995, 12, 260–267. [Google Scholar] [CrossRef]
- Zarbock, A.; Distasi, M.R.; Smith, E.; Sanders, J.M.; Kronke, G.; Harry, B.L.; von Vietinghoff, S.; Buscher, K.; Nadler, J.L.; Ley, K. Improved survival and reduced vascular permeability by eliminating or blocking 12/15-lipoxygenase in mouse models of acute lung injury (ALI). J. Immunol. 2009, 183, 4715–4722. [Google Scholar] [CrossRef] [Green Version]
- Cruickshank-Quinn, C.; Powell, R.; Jacobson, S.; Kechris, K.; Bowler, R.P.; Petrache, I.; Reisdorph, N. Metabolomic similarities between bronchoalveolar lavage fluid and plasma in humans and mice. Sci. Rep. 2017, 7, 5108. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Pohlmann, S. A Multibasic Cleavage Site in the Spike Protein of SARS-CoV-2 Is Essential for Infection of Human Lung Cells. Mol. Cell 2020, 78, 779–784.e5. [Google Scholar] [CrossRef]
- Russo, P.; Bonassi, S.; Giacconi, R.; Malavolta, M.; Tomino, C.; Maggi, F. COVID-19 and smoking: Is nicotine the hidden link? Eur. Respir. J. 2020, 55. [Google Scholar] [CrossRef]
- Wang, Q.; Sundar, I.K.; Li, D.; Lucas, J.H.; Muthumalage, T.M.; cDonough, S.R.; Rahman, I. E-cigarette-induced pulmonary inflammation and dysregulated repair are mediated by nAChR α7 receptor: Role of nAChR α7 in SARS-CoV-2 Covid-19 ACE2 receptor regulation. Respir. Res. 2020, 21, 154. [Google Scholar]
- Shukla, P.; Dwivedi, P.; Gupta, P.K.; Mishra, P.R. Optimization of novel tocopheryl acetate nanoemulsions for parenteral delivery of curcumin for therapeutic intervention of sepsis. Expert Opin. Drug Deliv. 2014, 11, 1697–1712. [Google Scholar] [CrossRef]
- Xue, Y.; Williams, T.L.; Li, T.; Umbehr, J.; Fang, L.; Wang, W.; Baybutt, R.C. Type II pneumocytes modulate surfactant production in response to cigarette smoke constituents: Restoration by vitamins A and E. Toxicol. In Vitro 2005, 19, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Beattie, J.R.; Schock, B.C. Identifying the spatial distribution of vitamin E, pulmonary surfactant and membrane lipids in cells and tissue by confocal Raman microscopy. Methods Mol. Biol. 2009, 579, 513–535. [Google Scholar] [CrossRef] [PubMed]










| Analyte | Air Mean | ± SEM | VEA Mean | ± SEM | p-Value | Significance | Cartridge Mean | ± SEM | p-Value | Significance |
|---|---|---|---|---|---|---|---|---|---|---|
| (vs. Air) | (vs. Air) | |||||||||
| DG(16:0_18:2_0:0) | 06.49 | ±0.20 | 05.05 | ±0.36 | <0.001 | *** | 04.38 | ±0.04 | <0.001 | *** |
| DG(16:0_16:0_0:0) | 00.19 | ±0.01 | 00.25 | ±0.04 | 0.5741 | NS | 00.40 | ±0.04 | 0.0031 | ** |
| CE(20:4) | 02.77 | ±0.10 | 02.52 | ±0.09 | 0.0002 | *** | 02.87 | ±0.24 | 0.2751 | NS |
| PC(16:0/16:0) | 29.86 | ±2.18 | 31.48 | ±2.41 | <0.001 | *** | 40.82 | ±2.64 | <0.001 | *** |
| PC(16:0_16:1) | 16.77 | ±1.42 | 17.59 | ±0.85 | 0.0295 | * | 23.46 | ±1.17 | <0.001 | *** |
| PC(16:0_18:2) | 04.31 | ±0.28 | 05.02 | ±0.48 | 0.0644 | NS | 06.74 | ±0.75 | <0.001 | *** |
| PC(16:0_18:1) | 04.63 | ±0.44 | 04.88 | ±0.17 | 0.6911 | NS | 06.64 | ±0.41 | <0.001 | *** |
| PC(14:0_16:0) | 04.22 | ±0.37 | 04.59 | ±0.34 | 0.4375 | NS | 05.79 | ±0.58 | <0.001 | *** |
| PC(16:0_22:6) | 01.86 | ±0.18 | 02.33 | ±0.32 | 0.2937 | NS | 03.18 | ±0.51 | <0.001 | *** |
| PG(16:0_18:1) | 02.70 | ±0.27 | 02.93 | ±0.11 | 0.7163 | NS | 03.85 | ±0.21 | 0.0014 | ** |
| PG(18:2_16:0) | 02.36 | ±0.16 | 02.84 | ±0.22 | 0.2688 | NS | 03.93 | ±0.37 | <0.001 | *** |
| PG(16:0/16:0) | 01.73 | ±0.14 | 01.85 | ±0.07 | 0.9170 | NS | 02.58 | ±0.12 | 0.0237 | * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muthumalage, T.; Lucas, J.H.; Wang, Q.; Lamb, T.; McGraw, M.D.; Rahman, I. Pulmonary Toxicity and Inflammatory Response of Vape Cartridges Containing Medium-Chain Triglycerides Oil and Vitamin E Acetate: Implications in the Pathogenesis of EVALI. Toxics 2020, 8, 46. https://doi.org/10.3390/toxics8030046
Muthumalage T, Lucas JH, Wang Q, Lamb T, McGraw MD, Rahman I. Pulmonary Toxicity and Inflammatory Response of Vape Cartridges Containing Medium-Chain Triglycerides Oil and Vitamin E Acetate: Implications in the Pathogenesis of EVALI. Toxics. 2020; 8(3):46. https://doi.org/10.3390/toxics8030046
Chicago/Turabian StyleMuthumalage, Thivanka, Joseph H. Lucas, Qixin Wang, Thomas Lamb, Matthew D. McGraw, and Irfan Rahman. 2020. "Pulmonary Toxicity and Inflammatory Response of Vape Cartridges Containing Medium-Chain Triglycerides Oil and Vitamin E Acetate: Implications in the Pathogenesis of EVALI" Toxics 8, no. 3: 46. https://doi.org/10.3390/toxics8030046
APA StyleMuthumalage, T., Lucas, J. H., Wang, Q., Lamb, T., McGraw, M. D., & Rahman, I. (2020). Pulmonary Toxicity and Inflammatory Response of Vape Cartridges Containing Medium-Chain Triglycerides Oil and Vitamin E Acetate: Implications in the Pathogenesis of EVALI. Toxics, 8(3), 46. https://doi.org/10.3390/toxics8030046