Silica-Based Nanomaterials for Diabetes Mellitus Treatment
Abstract
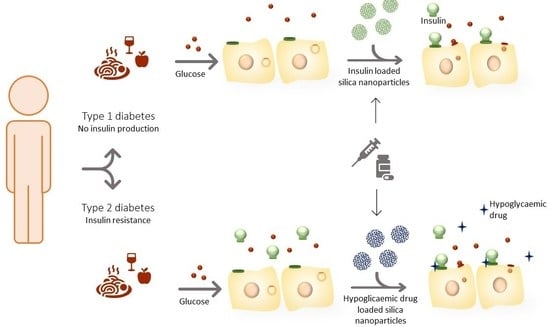
:1. Introduction
2. Diabetes Mellitus
2.1. Health Risks
2.2. Treatments
3. Silica Nanoparticles
3.1. Types and Properties
3.2. Main Preparation Methods
3.3. Cytotoxicity
4. Silica-Based Nanocarriers for Glycemia Control
4.1. Glycemia Control Mediated by Insulin
4.1.1. Oral Insulin Delivery Nanosystems
4.1.2. Non-Oral Insulin Delivery Systems
4.2. Glycemia Control Mediated by Other Drugs
4.2.1. Metformin Delivery Systems
4.2.2. Exenatide Delivery Systems
4.2.3. Other Delivery Systems
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Global Report on Diabetes; World Health Organization: Geneva, Switzerland, 2016; ISBN 978-92-4-156525-7. [Google Scholar]
- World Health Organization. Diabetes Portugal 2016 Country Profile; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Lu, Y.; Xie, J.; Zhang, E.; Zhu, H.; Du, H.; Wang, K.; Song, B.; Yang, C.; Shi, Y.; et al. Zwitterionic micelles efficiently deliver oral insulin without opening tight junctions. Nat. Nanotechnol. 2020, 15, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Kim, K.S.; Bae, Y.H. Long-term oral administration of Exendin-4 to control type 2 diabetes in a rat model. J. Control. Release 2019, 294, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Yang, T.; Fan, W.; Yang, Y.; Zhu, Q.; Guo, S.; Zhu, C.; Yuan, Y.; Zhang, T.; Gan, Y. Protein Corona Liposomes Achieve Efficient Oral Insulin Delivery by Overcoming Mucus and Epithelial Barriers. Adv. Healthc. Mater. 2019, 8, 1801123. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Han, P.; Chaudhury, R.; Khan, S.; Bickerton, S.; McHugh, M.D.; Park, H.B.; Siefert, A.L.; Rea, G.; Carballido, J.M.; et al. Metabolic and immunomodulatory control of type 1 diabetes via orally delivered bile-acid-polymer nanocarriers of insulin or rapamycin. Nat. Biomed. Eng. 2021, 5, 983–997. [Google Scholar] [CrossRef]
- Chen, X.; Ren, Y.; Feng, Y.; Xu, X.; Tan, H.; Li, J. Cp1-11 peptide/insulin complex loaded pH-responsive nanoparticles with enhanced oral bioactivity. Int. J. Pharm. 2019, 562, 23–30. [Google Scholar] [CrossRef]
- Li, Y.; Ji, W.; Peng, H.; Zhao, R.; Zhang, T.; Lu, Z.; Yang, J.; Liu, R.; Zhang, X. Charge-switchable zwitterionic polycarboxybetaine particle as an intestinal permeation enhancer for efficient oral insulin delivery. Theranostics 2021, 11, 4452–4466. [Google Scholar] [CrossRef]
- Zhou, S.; Deng, H.; Zhang, Y.; Wu, P.; He, B.; Dai, W.; Zhang, H.; Zhang, Q.; Zhao, R.; Wang, X. Thiolated Nanoparticles Overcome the Mucus Barrier and Epithelial Barrier for Oral Delivery of Insulin. Mol. Pharm. 2020, 17, 239–250. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, L.; Cao, Y.; Yu, S.; He, C.; Chen, X. A Nanocomposite Vehicle Based on Metal–Organic Framework Nanoparticle Incorporated Biodegradable Microspheres for Enhanced Oral Insulin Delivery. ACS Appl. Mater. Interfaces 2020, 12, 22581–22592. [Google Scholar] [CrossRef]
- Chen, Y.; Li, P.; Modica, J.A.; Drout, R.J.; Farha, O.K. Acid-Resistant Mesoporous Metal–Organic Framework toward Oral Insulin Delivery: Protein Encapsulation, Protection, and Release. J. Am. Chem. Soc. 2018, 140, 5678–5681. [Google Scholar] [CrossRef]
- Juère, E.; Caillard, R.; Marko, D.; Del Favero, G.; Kleitz, F. Smart Protein-Based Formulation of Dendritic Mesoporous Silica Nanoparticles: Toward Oral Delivery of Insulin. Chem. Eur. J. 2020, 26, 5195–5199. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, A.; Mousavi, S.N. Synthesis of a novel structure for the oral delivery of insulin and the study of its effect on diabetic rats. Life Sci. 2017, 186, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; He, Y.; Zhang, H.; Zhang, Y.; Gao, T.; Wang, J.-H.; Wang, S. Zwitterion-functionalized mesoporous silica nanoparticles for enhancing oral delivery of protein drugs by overcoming multiple gastrointestinal barriers. J. Colloid Interface Sci. 2021, 582, 364–375. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, W.; Zhao, R.; Zhang, X. Advances in oral peptide drug nanoparticles for diabetes mellitus treatment. Bioact. Mater. 2022, 15, 392–408. [Google Scholar] [CrossRef] [PubMed]
- Calzoni, E.; Cesaretti, A.; Polchi, A.; Di Michele, A.; Tancini, B.; Emiliani, C. Biocompatible Polymer Nanoparticles for Drug Delivery Applications in Cancer and Neurodegenerative Disorder Therapies. J. Funct. Biomater. 2019, 10, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selvarajan, V.; Obuobi, S.; Ee, P.L.R. Silica Nanoparticles—A Versatile Tool for the Treatment of Bacterial Infections. Front. Chem. 2020, 8, 602. [Google Scholar] [CrossRef]
- Baek, J.; Robert-Nicoud, G.; Herrera Hidalgo, C.; Borg, M.L.; Iqbal, M.N.; Berlin, R.; Lindgren, M.; Waara, E.; Uddén, A.; Pietiläinen, K.; et al. Engineered mesoporous silica reduces long-term blood glucose, HbA1c, and improves metabolic parameters in prediabetics. Nanomedicine 2022, 17, 9–22. [Google Scholar] [CrossRef]
- Bohara, R.A.; Thorat, N.D.; Pawar, S.H. Role of functionalization: Strategies to explore potential nano-bio applications of magnetic nanoparticles. RSC Adv. 2016, 6, 43989–44012. [Google Scholar] [CrossRef]
- Ferreira, B.J.M.L.; Martel, F.; Silva, C.; Santos, T.M.; Daniel-da-Silva, A.L. Nanostructured functionalized magnetic platforms for the sustained delivery of cisplatin: Synthesis, characterization and in vitro cytotoxicity evaluation. J. Inorg. Biochem. 2020, 213, 111258. [Google Scholar] [CrossRef]
- Nogueira, J.; Soares, S.F.; Amorim, C.O.; Amaral, J.S.; Silva, C.; Martel, F.; Trindade, T.; Daniel-da-Silva, A.L. Magnetic Driven Nanocarriers for pH-Responsive Doxorubicin Release in Cancer Therapy. Molecules 2020, 25, 333. [Google Scholar] [CrossRef]
- Fernandes, R.A.; Daniel-da-Silva, A.L.; Tavares, A.P.M.; Xavier, A.M.R.B. EDTA-Cu (II) chelating magnetic nanoparticles as a support for laccase immobilization. Chem. Eng. Sci. 2017, 158, 599–605. [Google Scholar] [CrossRef]
- Lamson, N.G.; Berger, A.; Fein, K.C.; Whitehead, K.A. Anionic nanoparticles enable the oral delivery of proteins by enhancing intestinal permeability. Nat. Biomed. Eng. 2020, 4, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Aryaeian, N.; Khorshidi Sedehi, S.; Arablou, T. Polyphenols and their effects on diabetes management: A review. Med. J. Islam. Repub. Iran 2017, 31, 886–892. [Google Scholar] [CrossRef] [Green Version]
- Shahwan, M.; Alhumaydhi, F.; Ashraf, G.M.; Hasan, P.M.Z.; Shamsi, A. Role of polyphenols in combating Type 2 Diabetes and insulin resistance. Int. J. Biol. Macromol. 2022, 206, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Rocha, S.; Lucas, M.; Ribeiro, D.; Corvo, M.L.; Fernandes, E.; Freitas, M. Nano-based drug delivery systems used as vehicles to enhance polyphenols therapeutic effect for diabetes mellitus treatment. Pharmacol. Res. 2021, 169, 105604. [Google Scholar] [CrossRef]
- Mohebian, Z.; Babazadeh, M.; Zarghami, N.; Mousazadeh, H. Anticancer efficiency of curcumin-loaded mesoporous silica nanoparticles/nanofiber composites for potential postsurgical breast cancer treatment. J. Drug Deliv. Sci. Technol. 2021, 61, 102170. [Google Scholar] [CrossRef]
- Sarkar, A.; Ghosh, S.; Chowdhury, S.; Pandey, B.; Sil, P.C. Targeted delivery of quercetin loaded mesoporous silica nanoparticles to the breast cancer cells. Biochim. Et Biophys. Acta (BBA) Gen. Subj. 2016, 1860, 2065–2075. [Google Scholar] [CrossRef]
- Chaudhary, Z.; Subramaniam, S.; Khan, G.M.; Abeer, M.M.; Qu, Z.; Janjua, T.; Kumeria, T.; Batra, J.; Popat, A. Encapsulation and Controlled Release of Resveratrol Within Functionalized Mesoporous Silica Nanoparticles for Prostate Cancer Therapy. Front. Bioeng. Biotechnol. 2019, 7, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Marinheiro, D.; Ferreira, B.; Oskoei, P.; Oliveira, H.; Daniel-da-Silva, A. Encapsulation and Enhanced Release of Resveratrol from Mesoporous Silica Nanoparticles for Melanoma Therapy. Materials 2021, 14, 1382. [Google Scholar] [CrossRef]
- Ahmadi Nasab, N.; Hassani Kumleh, H.; Beygzadeh, M.; Teimourian, S.; Kazemzad, M. Delivery of curcumin by a pH-responsive chitosan mesoporous silica nanoparticles for cancer treatment. Artif. Cells Nanomed. Biotechnol. 2018, 46, 75–81. [Google Scholar] [CrossRef]
- Yadav, Y.C.; Pattnaik, S.; Swain, K. Curcumin loaded mesoporous silica nanoparticles: Assessment of bioavailability and cardioprotective effect. Drug Dev. Ind. Pharm. 2019, 45, 1889–1895. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2013, 36, S67–S74. [CrossRef] [PubMed] [Green Version]
- Thevis, M.; Thomas, A.; Schänzer, W. Insulin. In Doping in Sports; Thieme, D., Hemmersbach, P., Eds.; Handbook of Experimental Pharmacology; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2009; Volume 195, pp. 209–226. ISBN 978-3-540-79087-7. [Google Scholar]
- Satoh, T. Molecular Mechanisms for the Regulation of Insulin-Stimulated Glucose Uptake by Small Guanosine Triphosphatases in Skeletal Muscle and Adipocytes. Int. J. Mol. Sci. 2014, 15, 18677–18692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AL-Ishaq; Abotaleb; Kubatka; Kajo; Büsselberg Flavonoids and Their Anti-Diabetic Effects: Cellular Mechanisms and Effects to Improve Blood Sugar Levels. Biomolecules 2019, 9, 430. [CrossRef] [PubMed] [Green Version]
- Sapra, A.; Bhandari, P. Diabetes Mellitus. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Paulweber, B.; Valensi, P.; Lindström, J.; Lalic, N.; Greaves, C.; McKee, M.; Kissimova-Skarbek, K.; Liatis, S.; Cosson, E.; Szendroedi, J.; et al. A European Evidence-Based Guideline for the Prevention of Type 2 Diabetes. Horm. Metab. Res. 2010, 42, S3–S36. [Google Scholar] [CrossRef] [Green Version]
- The Emerging Risk Factors Collaboration Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. Lancet 2010, 375, 2215–2222. [CrossRef] [Green Version]
- Steinmetz, J.D.; Bourne, R.R.A.; Briant, P.S.; Flaxman, S.R.; Taylor, H.R.B.; Jonas, J.B.; Abdoli, A.A.; Abrha, W.A.; Abualhasan, A.; Abu-Gharbieh, E.G.; et al. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: The Right to Sight: An analysis for the Global Burden of Disease Study. Lancet Glob. Health 2021, 9, e144–e160. [Google Scholar] [CrossRef]
- Saran, R.; Li, Y.; Robinson, B.; Ayanian, J.; Balkrishnan, R.; Bragg-Gresham, J.; Chen, J.T.L.; Cope, E.; Gipson, D.; He, K.; et al. US Renal Data System 2014 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am. J. Kidney Dis. 2015, 66, A7. [Google Scholar] [CrossRef] [Green Version]
- Misra, S.; Mathieu, C. Are newer insulin analogues better for people with Type 1 diabetes? Diabet. Med. 2020, 37, 522–531. [Google Scholar] [CrossRef]
- Padhi, S.; Nayak, A.K.; Behera, A. Type II diabetes mellitus: A review on recent drug based therapeutics. Biomed. Pharmacother. 2020, 131, 110708. [Google Scholar] [CrossRef]
- Lorenzati, B.; Zucco, C.; Miglietta, S.; Lamberti, F.; Bruno, G. Oral Hypoglycemic Drugs: Pathophysiological Basis of Their Mechanism of ActionOral Hypoglycemic Drugs: Pathophysiological Basis of Their Mechanism of Action. Pharmaceuticals 2010, 3, 3005–3020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Philippe, J.; Raccah, D. Treating type 2 diabetes: How safe are current therapeutic agents? Int. J. Clin. Pract. 2009, 63, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Uppal, S.; Italiya, K.S.; Chitkara, D.; Mittal, A. Nanoparticulate-based drug delivery systems for small molecule anti-diabetic drugs: An emerging paradigm for effective therapy. Acta Biomater. 2018, 81, 20–42. [Google Scholar] [CrossRef] [PubMed]
- Souto, E.B.; Souto, S.B.; Campos, J.R.; Severino, P.; Pashirova, T.N.; Zakharova, L.Y.; Silva, A.M.; Durazzo, A.; Lucarini, M.; Izzo, A.A.; et al. Nanoparticle Delivery Systems in the Treatment of Diabetes Complications. Molecules 2019, 24, 4209. [Google Scholar] [CrossRef] [Green Version]
- Wang, A.Z.; Langer, R.; Farokhzad, O.C. Nanoparticle Delivery of Cancer Drugs. Annu. Rev. Med. 2012, 63, 185–198. [Google Scholar] [CrossRef]
- Díaz, M.R.; Vivas-Mejia, P.E. Nanoparticles as drug delivery systems in cancer medicine: Emphasis on RNAi-containing nanoliposomes. Pharmaceuticals 2013, 6, 1361–1380. [Google Scholar] [CrossRef] [Green Version]
- Bamburowicz-Klimkowska, M.; Poplawska, M.; Grudzinski, I.P. Nanocomposites as biomolecules delivery agents in nanomedicine. J. Nanobiotechnol. 2019, 17, 48. [Google Scholar] [CrossRef] [Green Version]
- Jain, R.N.; Huang, X.; Das, S.; Silva, R.; Ivanova, V.; Minko, T.; Asefa, T. Functionalized Mesoporous Silica Nanoparticles for Glucose- and pH-Stimulated Release of Insulin. Z. Anorg. Allg. Chem. 2014, 640, 616–623. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, P.; Chen, M.; Jin, T.; Wu, H.; Hei, M.; Wang, C.; Xu, Y.; Qian, X.; Zhu, W. On-demand transdermal insulin delivery system for type 1 diabetes therapy with no hypoglycemia risks. J. Colloid Interface Sci. 2022, 605, 582–591. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, S.; Hu, W.; Lin, X.; Cao, C.; Zou, S.; Tong, Z.; Jiang, G.; Kong, X. Polymer-grafted hollow mesoporous silica nanoparticles integrated with microneedle patches for glucose-responsive drug delivery. Front. Mater. Sci. 2021, 15, 98–112. [Google Scholar] [CrossRef]
- Jeelani, P.G.; Mulay, P.; Venkat, R.; Ramalingam, C. Multifaceted Application of Silica Nanoparticles. A Review. Silicon 2020, 12, 1337–1354. [Google Scholar] [CrossRef]
- Tang, J.; Slowing, I.I.; Huang, Y.; Trewyn, B.G.; Hu, J.; Liu, H.; Lin, V.S.Y. Poly(lactic acid)-coated mesoporous silica nanosphere for controlled release of venlafaxine. J. Colloid Interface Sci. 2011, 360, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Cao, B.; Snyder, N.R.; Woeppel, K.M.; Eles, J.R.; Cui, X.T. ROS responsive resveratrol delivery from LDLR peptide conjugated PLA-coated mesoporous silica nanoparticles across the blood-brain barrier. J. Nanobiotechnol. 2018, 16, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anirudhan, T.S.; Nair, A.S. Temperature and ultrasound sensitive gatekeepers for the controlled release of chemotherapeutic drugs from mesoporous silica nanoparticles. J. Mater. Chem. B 2018, 6, 428–439. [Google Scholar] [CrossRef]
- Liu, J.; Luo, Z.; Zhang, J.; Luo, T.; Zhou, J.; Zhao, X.; Cai, K. Hollow mesoporous silica nanoparticles facilitated drug delivery via cascade pH stimuli in tumor microenvironment for tumor therapy. Biomaterials 2016, 83, 51–65. [Google Scholar] [CrossRef]
- Narayan, R.; Nayak, U.Y.; Raichur, A.M.; Garg, S. Mesoporous silica nanoparticles: A comprehensive review on synthesis and recent advances. Pharmaceutics 2018, 10, 118. [Google Scholar] [CrossRef] [Green Version]
- Maleki, A.; Kettiger, H.; Schoubben, A.; Rosenholm, J.M.; Ambrogi, V.; Hamidi, M. Mesoporous silica materials: From physico-chemical properties to enhanced dissolution of poorly water-soluble drugs. J. Control. Release 2017, 262, 329–347. [Google Scholar] [CrossRef]
- Kankala, R.K.; Han, Y.-H.; Xia, H.-Y.; Wang, S.-B.; Chen, A.-Z. Nanoarchitectured prototypes of mesoporous silica nanoparticles for innovative biomedical applications. J. Nanobiotechnol. 2022, 20, 126. [Google Scholar] [CrossRef]
- Hou, L.; Zheng, Y.; Wang, Y.; Hu, Y.; Shi, J.; Liu, Q.; Zhang, H.; Zhang, Z. Self-Regulated Carboxyphenylboronic Acid-Modified Mesoporous Silica Nanoparticles with “Touch Switch” Releasing Property for Insulin Delivery. ACS Appl. Mater. Interfaces 2018, 10, 21927–21938. [Google Scholar] [CrossRef]
- Xu, B.; Jiang, G.; Yu, W.; Liu, D.; Zhang, Y.; Zhou, J.; Sun, S.; Liu, Y. H2O2-Responsive mesoporous silica nanoparticles integrated with microneedle patches for the glucose-monitored transdermal delivery of insulin. J. Mater. Chem. B 2017, 5, 8200–8208. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Wu, S.-H.; Mou, C.-Y.; Lin, H.-P. Synthesis of mesoporous silica nanoparticles. Chem. Soc. Rev. 2013, 42, 3862. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.; Dumée, L.F.; Garvey, C.J.; Feng, C.; She, F.; Rookes, J.E.; Mudie, S.; Cahill, D.M.; Kong, L. A New Insight into Growth Mechanism and Kinetics of Mesoporous Silica Nanoparticles by in situ Small Angle X-ray Scattering. Langmuir 2015, 31, 8478–8487. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Ikari, K.; Imai, H. Synthesis of Silica Nanoparticles Having a Well-Ordered Mesostructure Using a Double Surfactant System. J. Am. Chem. Soc. 2004, 126, 462–463. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Zong, Y.-X.; Nie, M.-Z.; Shan, B.-Q.; Yang, T.-Q.; Hao, P.; Ma, S.-Y.; Lam, K.-F.; Zhang, K. Interfacial charge shielding directs the synthesis of dendritic mesoporous silica nanospheres by a dual-templating approach. N. J. Chem. 2019, 43, 15777–15784. [Google Scholar] [CrossRef]
- Galarneau, A.; Cambon, H.; Di Renzo, F.; Ryoo, R.; Choi, M.; Fajula, F. Microporosity and connections between pores in SBA-15 mesostructured silicas as a function of the temperature of synthesis. N. J. Chem. 2003, 27, 73–79. [Google Scholar] [CrossRef]
- Sharma, J.; Polizos, G. Hollow Silica Particles: Recent Progress and Future Perspectives. Nanomaterials 2020, 10, 1599. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Liu, T.; Lin, R. Development and Characterization of a Glimepiride-Loaded Gelatin-Coated Mesoporous Hollow Silica Nanoparticle Formulation and Evaluation of Its Hypoglycemic Effect on Type-2 Diabetes Model Rats. ASSAY Drug Dev. Technol. 2020, 18, 369–378. [Google Scholar] [CrossRef]
- Soares, S.F.; Fernandes, T.; Daniel-da-Silva, A.L.; Trindade, T. The controlled synthesis of complex hollow nanostructures and prospective applications. Proc. R. Soc. A. 2019, 475, 20180677. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Ohulchanskyy, T.Y.; Bharali, D.; Chen, Y.; Pandey, R.K.; Prasad, P.N. Organically Modified Silica Nanoparticles with Intraparticle Heavy-Atom Effect on the Encapsulated Photosensitizer for Enhanced Efficacy of Photodynamic Therapy. J. Phys. Chem. C 2009, 113, 12641–12644. [Google Scholar] [CrossRef]
- Yamauchi, H.; Ishikawa, T.; Kondo, S. Surface characterization of ultramicro spherical particles of silica prepared by w/o microemulsion method. Colloids Surf. 1989, 37, 71–80. [Google Scholar] [CrossRef]
- Shirshahi, V.; Soltani, M. Solid silica nanoparticles: Applications in molecular imaging. Contrast Media Mol. Imaging 2015, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-S.; Wu, S.-H.; Tseng, C.-T.; Hung, Y.; Chang, C.; Mou, C.-Y. Synthesis of hollow silica nanospheres with a microemulsion as the template. Chem. Commun. 2009, 24, 3542–3544. [Google Scholar] [CrossRef]
- Murugadoss, S.; Lison, D.; Godderis, L.; Van Den Brule, S.; Mast, J.; Brassinne, F.; Sebaihi, N.; Hoet, P.H. Toxicology of silica nanoparticles: An update. Arch. Toxicol. 2017, 91, 2967–3010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maurer-Jones, M.A.; Lin, Y.-S.; Haynes, C.L. Functional Assessment of Metal Oxide Nanoparticle Toxicity in Immune Cells. ACS Nano 2010, 4, 3363–3373. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Teng, X.; Chen, D.; Tang, F.; He, J. The effect of the shape of mesoporous silica nanoparticles on cellular uptake and cell function. Biomaterials 2010, 31, 438–448. [Google Scholar] [CrossRef]
- Kim, I.-Y.; Joachim, E.; Choi, H.; Kim, K. Toxicity of silica nanoparticles depends on size, dose, and cell type. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1407–1416. [Google Scholar] [CrossRef]
- Code of Federal Regulations § 172.480 Silicon Dioxide. Available online: https://www.ecfr.gov/current/title-21/chapter-I/subchapter-B/part-172/subpart-E/section-172.480 (accessed on 15 December 2022).
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS); Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; et al. Re-evaluation of silicon dioxide (E 551) as a food additive. EFSA J. 2018, 16, e05088. [Google Scholar] [CrossRef] [Green Version]
- Zanoni, D.K.; Stambuk, H.E.; Madajewski, B.; Montero, P.H.; Matsuura, D.; Busam, K.J.; Ma, K.; Turker, M.Z.; Sequeira, S.; Gonen, M.; et al. Use of Ultrasmall Core-Shell Fluorescent Silica Nanoparticles for Image-Guided Sentinel Lymph Node Biopsy in Head and Neck Melanoma: A Nonrandomized Clinical Trial. JAMA Netw Open 2021, 4, e211936. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic. Bioeng. Transl. Med. 2016, 1, 10–29. [Google Scholar] [CrossRef]
- Janjua, T.I.; Cao, Y.; Yu, C.; Popat, A. Clinical translation of silica nanoparticles. Nat. Rev. Mater. 2021, 6, 1072–1074. [Google Scholar] [CrossRef] [PubMed]
- Bukara, K.; Schueller, L.; Rosier, J.; Martens, M.A.; Daems, T.; Verheyden, L.; Eelen, S.; Van Speybroeck, M.; Libanati, C.; Martens, J.A.; et al. Ordered mesoporous silica to enhance the bioavailability of poorly water-soluble drugs: Proof of concept in man. Eur. J. Pharm. Biopharm. 2016, 108, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Fonte, P.; Araújo, F.; Reis, S.; Sarmento, B. Oral Insulin Delivery: How Far are We? J. Diabetes Sci. Technol. 2013, 7, 520–531. [Google Scholar] [CrossRef] [Green Version]
- Gedawy, A.; Martinez, J.; Al-Salami, H.; Dass, C.R. Oral insulin delivery: Existing barriers and current counter-strategies. J. Pharm. Pharmacol. 2018, 70, 197–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreani, T.; de Souza, A.L.R.; Kiill, C.P.; Lorenzón, E.N.; Fangueiro, J.F.; Calpena, A.C.; Chaud, M.V.; Garcia, M.L.; Gremião, M.P.D.; Silva, A.M.; et al. Preparation and characterization of PEG-coated silica nanoparticles for oral insulin delivery. Int. J. Pharm. 2014, 473, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xiong, M.; Ni, X.; Wang, J.; Rong, H.; Su, Y.; Yu, S.; Mohammad, I.S.; Leung, S.S.Y.; Hu, H. Virus-Mimicking Mesoporous Silica Nanoparticles with an Electrically Neutral and Hydrophilic Surface to Improve the Oral Absorption of Insulin by Breaking Through Dual Barriers of the Mucus Layer and the Intestinal Epithelium. ACS Appl. Mater. Interfaces 2021, 13, 18077–18088. [Google Scholar] [CrossRef] [PubMed]
- Abeer, M.M.; Meka, A.K.; Pujara, N.; Kumeria, T.; Strounina, E.; Nunes, R.; Costa, A.; Sarmento, B.; Hasnain, S.Z.; Ross, B.P.; et al. Rationally Designed Dendritic Silica Nanoparticles for Oral Delivery of Exenatide. Pharmaceutics 2019, 11, 418. [Google Scholar] [CrossRef] [Green Version]
- Carlsson, N.; Gustafsson, H.; Thörn, C.; Olsson, L.; Holmberg, K.; Åkerman, B. Enzymes immobilized in mesoporous silica: A physical–chemical perspective. Adv. Colloid Interface Sci. 2014, 205, 339–360. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, L.; Yu, H.; Ur-Rahman, K. Advances in phenylboronic acid-based closed-loop smart drug delivery system for diabetic therapy. J. Control. Release 2019, 305, 50–64. [Google Scholar] [CrossRef]
- Ma, R.; Shi, L. Phenylboronic acid-based glucose-responsive polymeric nanoparticles: Synthesis and applications in drug delivery. Polym. Chem. 2014, 5, 1503–1518. [Google Scholar] [CrossRef]
- Sun, X.; James, T.D. Glucose Sensing in Supramolecular Chemistry. Chem. Rev. 2015, 115, 8001–8037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brooks, W.L.A.; Deng, C.C.; Sumerlin, B.S. Structure–Reactivity Relationships in Boronic Acid–Diol Complexation. ACS Omega 2018, 3, 17863–17870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Z.; Li, Q.; Mei, L. pH-Sensitive nanoscale materials as robust drug delivery systems for cancer therapy. Chin. Chem. Lett. 2020, 31, 1345–1356. [Google Scholar] [CrossRef]
- Qin, T.; Yan, L.; Wang, X.; Lin, S.; Zeng, Q. Glucose-Responsive Polyelectrolyte Complexes Based on Dendritic Mesoporous Silica for Oral Insulin Delivery. AAPS PharmSciTech 2021, 22, 226. [Google Scholar] [CrossRef]
- Oroval, M.; Díez, P.; Aznar, E.; Coll, C.; Marcos, M.D.; Sancenón, F.; Villalonga, R.; Martínez-Máñez, R. Self-Regulated Glucose-Sensitive Neoglycoenzyme-Capped Mesoporous Silica Nanoparticles for Insulin Delivery. Chem. Eur. J. 2017, 23, 1353–1360. [Google Scholar] [CrossRef]
- Zou, Z.; He, D.; Cai, L.; He, X.; Wang, K.; Yang, X.; Li, L.; Li, S.; Su, X. Alizarin Complexone Functionalized Mesoporous Silica Nanoparticles: A Smart System Integrating Glucose-Responsive Double-Drugs Release and Real-Time Monitoring Capabilities. ACS Appl. Mater. Interfaces 2016, 8, 8358–8366. [Google Scholar] [CrossRef]
- Weinstain, R.; Segal, E.; Satchi-Fainaro, R.; Shabat, D. Real-time monitoring of drug release. Chem. Commun. 2010, 46, 553–555. [Google Scholar] [CrossRef]
- Ock, K.; Jeon, W.I.; Ganbold, E.O.; Kim, M.; Park, J.; Seo, J.H.; Cho, K.; Joo, S.-W.; Lee, S.Y. Real-Time Monitoring of Glutathione-Triggered Thiopurine Anticancer Drug Release in Live Cells Investigated by Surface-Enhanced Raman Scattering. Anal. Chem. 2012, 84, 2172–2178. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S.; An, F.-F.; Liu, J.; Jin, S.; Zhang, J.-C.; Wang, P.C.; Zhang, X.; Lee, C.-S.; Liang, X.-J. Self-carried curcumin nanoparticles for in vitro and in vivo cancer therapy with real-time monitoring of drug release. Nanoscale 2015, 7, 13503–13510. [Google Scholar] [CrossRef] [Green Version]
- Jana, A.; Devi, K.S.P.; Maiti, T.K.; Singh, N.D.P. Perylene-3-ylmethanol: Fluorescent Organic Nanoparticles as a Single-Component Photoresponsive Nanocarrier with Real-Time Monitoring of Anticancer Drug Release. J. Am. Chem. Soc. 2012, 134, 7656–7659. [Google Scholar] [CrossRef]
- Yang, Z.; Song, J.; Tang, W.; Fan, W.; Dai, Y.; Shen, Z.; Lin, L.; Cheng, S.; Liu, Y.; Niu, G.; et al. Stimuli-Responsive Nanotheranostics for Real-Time Monitoring Drug Release by Photoacoustic Imaging. Theranostics 2019, 9, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wu, T.; Kong, J.-L. In Situ Monitoring of Intracellular Controlled Drug Release from Mesoporous Silica Nanoparticles Coated with pH-Responsive Charge-Reversal Polymer. ACS Appl. Mater. Interfaces 2014, 6, 17446–17453. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Shah, B.P.; Zhang, Y.; Yang, L.; Lee, K.-B. Real-Time Monitoring of ATP-Responsive Drug Release Using Mesoporous-Silica-Coated Multicolor Upconversion Nanoparticles. ACS Nano 2015, 9, 5234–5245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Lu, W.; Guo, Y.; Zhu, Y.; Song, Y. Chitosan-Gated Fluorescent Mesoporous Silica Nanocarriers for the Real-Time Monitoring of Drug Release. Langmuir 2020, 36, 6749–6756. [Google Scholar] [CrossRef]
- Patiño-Herrera, R.; Louvier-Hernández, J.F.; Escamilla-Silva, E.M.; Chaumel, J.; Escobedo, A.G.P.; Pérez, E. Prolonged release of metformin by SiO2 nanoparticles pellets for type II diabetes control. Eur. J. Pharm. Sci. 2019, 131, 1–8. [Google Scholar] [CrossRef]
- Chen, C.; Zheng, H.; Xu, J.; Shi, X.; Li, F.; Wang, X. Sustained-release study on Exenatide loaded into mesoporous silica nanoparticles: In vitro characterization and in vivo evaluation. DARU J. Pharm. Sci. 2017, 25, 20. [Google Scholar] [CrossRef]
- Huang, P.-K.; Lin, S.-X.; Tsai, M.-J.; Leong, M.; Lin, S.-R.; Kankala, R.; Lee, C.-H.; Weng, C.-F. Encapsulation of 16-Hydroxycleroda-3,13-Dine-16,15-Olide in Mesoporous Silica Nanoparticles as a Natural Dipeptidyl Peptidase-4 Inhibitor Potentiated Hypoglycemia in Diabetic Mice. Nanomaterials 2017, 7, 112. [Google Scholar] [CrossRef] [Green Version]
- Geng, S.; Qin, L.; He, Y.; Li, X.; Yang, M.; Li, L.; Liu, D.; Li, Y.; Niu, D.; Yang, G. Effective and safe delivery of GLP-1AR and FGF-21 plasmids using amino-functionalized dual-mesoporous silica nanoparticles in vitro and in vivo. Biomaterials 2021, 271, 120763. [Google Scholar] [CrossRef]
- Corcoran, C.; Jacobs, T.F. Metformin. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Wang, Y.-W.; He, S.-J.; Feng, X.; Cheng, J.; Luo, Y.-T.; Tian, L.; Huang, Q. Metformin: A review of its potential indications. Drug Des. Dev. Ther. 2017, 11, 2421–2429. [Google Scholar] [CrossRef] [Green Version]
- Pernicova, I.; Korbonits, M. Metformin—mode of action and clinical implications for diabetes and cancer. Nat. Rev. Endocrinol. 2014, 10, 143–156. [Google Scholar] [CrossRef]
- Bray, G.M. Exenatide. Am. J. Health-Syst. Pharm. 2006, 63, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Malone-Povolny, M.J.; Merricks, E.P.; Wimsey, L.E.; Nichols, T.C.; Schoenfisch, M.H. Long-Term Accurate Continuous Glucose Biosensors via Extended Nitric Oxide Release. ACS Sens. 2019, 4, 3257–3264. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Yu, C.; Zhang, Y.; Xu, S. Efficient core shell structured dual response ratiometric fluorescence probe for determination of H2O2 and glucose via etching of silver nanoprisms. Anal. Chim. Acta 2019, 1048, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Yu, C.; Quan, S.; Xu, S. A novel dual response ratiometric fluorescent probe for the determination of H2O2 and glucose via etching of silver nanoparticles. Analyst 2019, 144, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Razavi, M.; Primavera, R.; Kevadiya, B.D.; Wang, J.; Ullah, M.; Buchwald, P.; Thakor, A.S. Retraction of “Controlled Nutrient Delivery to Pancreatic Islets Using Polydopamine-Coated Mesoporous Silica Nanoparticles”. Nano Lett. 2022, 22, 3174. [Google Scholar] [CrossRef]
- Malone-Povolny, M.J.; Bradshaw, T.M.; Merricks, E.P.; Long, C.T.; Nichols, T.C.; Schoenfisch, M.H. Combination of Nitric Oxide Release and Surface Texture for Mitigating the Foreign Body Response. ACS Biomater. Sci. Eng. 2021, 7, 2444–2452. [Google Scholar] [CrossRef]
- Ren, X.; Han, Y.; Wang, J.; Jiang, Y.; Yi, Z.; Xu, H.; Ke, Q. An aligned porous electrospun fibrous membrane with controlled drug delivery—An efficient strategy to accelerate diabetic wound healing with improved angiogenesis. Acta Biomater. 2018, 70, 140–153. [Google Scholar] [CrossRef]
- López-Goerne, T.; Ramírez, P.; Alvarez, D.; Rodríguez-Reinoso, F.; Silvestre-Albero, A.M.; Gómez, E.; Rodríguez-Castellon, E. Physicochemical properties and in vivo evaluation of Pt/TiO2 –SiO2 nanopowders. Nanomedicine 2018, 13, 2171–2185. [Google Scholar] [CrossRef]
- Gan, J.; Liu, C.; Li, H.; Wang, S.; Wang, Z.; Kang, Z.; Huang, Z.; Zhang, J.; Wang, C.; Lv, D.; et al. Accelerated wound healing in diabetes by reprogramming the macrophages with particle-induced clustering of the mannose receptors. Biomaterials 2019, 219, 119340. [Google Scholar] [CrossRef]
- Mao, C.-F.; Zhang, X.-R.; Johnson, A.; He, J.-L.; Kong, Z.-L. Modulation of Diabetes Mellitus-Induced Male Rat Reproductive Dysfunction with Micro-Nanoencapsulated Echinacea purpurea Ethanol Extract. BioMed Res. Int. 2018, 2018, 1–17. [Google Scholar] [CrossRef]
- Sudirman, S.; Hsu, Y.-H.; Johnson, A.; Tsou, D.; Kong, Z.-L. Amelioration effects of nanoencapsulated triterpenoids from petri dish-cultured Antrodia cinnamomea on reproductive function of diabetic male rats. Int. J. Nanomed. 2018, 13, 5059–5073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Gong, X.; Fang, L.; Fan, Q.; Cai, L.; Qiu, X.; Zhang, B.; Chang, J.; Lu, Y. Potential of CeCl3 @mSiO2 nanoparticles in alleviating diabetic cataract development and progression. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Feng, J.; Xiao, Y.; Bao, C. Grafting resveratrol onto mesoporous silica nanoparticles towards efficient sustainable immunoregulation and insulin resistance alleviation for diabetic periodontitis therapy. J. Mater. Chem. B 2022, 10, 4840–4855. [Google Scholar] [CrossRef] [PubMed]








| Administration Routes | Nanoparticle Type | Stimuli Response | Chemical Modifications | In Vivo Tested | Main Conclusions | Ref. |
|---|---|---|---|---|---|---|
| Oral | Non-porous silica | None | Non-modified, COO− and NH3+ | Yes | Enhanced intestinal permeation of insulin by negatively charged and small-size NPs | [24] |
| MSNs | pH and glucose | Functionalized with boric acid and coated with polyacrylic acid | No | pH-dependent and glucose-triggered release of insulin | [52] | |
| Dendritic MSN | pH | Thiol groups | No | Succinylated β-lactoglobulin tablets with dendritic MSNs with good pH-dependent release (80% at pH 7.4) | [13] | |
| Dendritic MSN | pH and glucose | Alginate-g-3-aminophenylboronic acid or chitosan-g-3-fluoro-4-carboxyphenylboronic acid coating | Yes | Self-regulates insulin release, demonstrating a significant hypoglycaemic effect on diabetic rats | [99] | |
| MSNs | pH | Polyamide amine coating | Yes | Chitosan-gelatine scaffolds containing NPs enhanced permeability of insulin and reduced blood glucose levels in rats | [14] | |
| MSNs | pH | Deoxycholic acid and coated with sulfobeataine 12 | Yes | Increased absorption of loaded insulin and hypoglycaemic effect in diabetic rats | [15] | |
| MSNs | None | Several virus-mimicking functional groups | Yes | Enhanced penetration through the mucus layer and epithelium and effective hypoglycaemic effect | [91] | |
| Intravenous | MSNs | Glucose | Carboxyphenylboronic modified and sodium alginate coated | Yes | Effective blood glucose levels control in response to glucose concentration | [63] |
| Intravenous and transdermal | MSNs | Glucose | Phenylboronic acid zinc oxide NPs | Yes | No hypoglycaemic risk and effective blood glucose level control | [53] |
| Transdermal | MSNs | Glucose | 4-(imidazoyl carbamate)phenylboronic acid pinacol ester, α-cyclodextrin and glucose oxidase | Yes | Blood glucose levels control without hypoglycaemic effect | [64] |
| Not proposed | MSNs | Glucose | 1-propyl-1-H-benzimidazole and cyclodextrin-modified glucose oxidase coating | No | Self-regulated delivery system with enlarged pores with great insulin release control | [100] |
| MSNs | Glucose | Alizarin complexone and gluconated insulin | No | Self-regulated release of insulin and hypoglycaemic drug rosiglitazone maleate and insulin, together with real-time release monitoring | [101] |
| Drug | Administration Routes | Nanoparticle Type | Stimuli Response | Chemical Modifications | In Vivo Tested | Main Conclusions | Ref. |
|---|---|---|---|---|---|---|---|
 Metformin | Transdermal | HMSNs | Glucose | poly(3-acrylamidophenylboronic acid) | Yes | Effective glucose-responsive release to control blood glucose levels | [54] |
| Not proposed | MSNs | pH | Chitosan coating | No | pH-dependent controlled release of metformin | [110] | |
| Exenatide (peptide) | Intravenous | MSNs | None | None | Yes | Peptide half-life time extended up to 14.5 h, increased bioavailability and prolonged hypoglycaemic effect | [111] |
| Not proposed | Dendritic MSNs | pH | Phosphonate groups and chitosan coating | No | Bioavailability of exenatide increased 1.7 times, and controlled release dependent on the pH | [92] | |
 Glimepiride | Oral | HMSNs | None | Gelatine coating | Yes | Improved bioavailability of glimepiride and hypoglycaemic effect | [72] |
 16-hydroxycleroda-3,13-dine-16,15-olide | Oral | MSNs | None | NH2 | Yes | Improved bioavailability and blood glucose levels control | [112] |
| Liraglutide and fibroblast growth factor 21 (peptides) | Intravenous | MSNs | None | NH2 | Yes | Blood glucose level control owing to the synergistic effect of both drugs | [113] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marinheiro, D.; Martel, F.; Ferreira, B.J.M.L.; Daniel-da-Silva, A.L. Silica-Based Nanomaterials for Diabetes Mellitus Treatment. Bioengineering 2023, 10, 40. https://doi.org/10.3390/bioengineering10010040
Marinheiro D, Martel F, Ferreira BJML, Daniel-da-Silva AL. Silica-Based Nanomaterials for Diabetes Mellitus Treatment. Bioengineering. 2023; 10(1):40. https://doi.org/10.3390/bioengineering10010040
Chicago/Turabian StyleMarinheiro, Diogo, Fátima Martel, Bárbara J. M. L. Ferreira, and Ana L. Daniel-da-Silva. 2023. "Silica-Based Nanomaterials for Diabetes Mellitus Treatment" Bioengineering 10, no. 1: 40. https://doi.org/10.3390/bioengineering10010040
APA StyleMarinheiro, D., Martel, F., Ferreira, B. J. M. L., & Daniel-da-Silva, A. L. (2023). Silica-Based Nanomaterials for Diabetes Mellitus Treatment. Bioengineering, 10(1), 40. https://doi.org/10.3390/bioengineering10010040