Additive Manufacturing of Bioactive Glass and Its Polymer Composites as Bone Tissue Engineering Scaffolds: A Review
Abstract
:1. Introduction
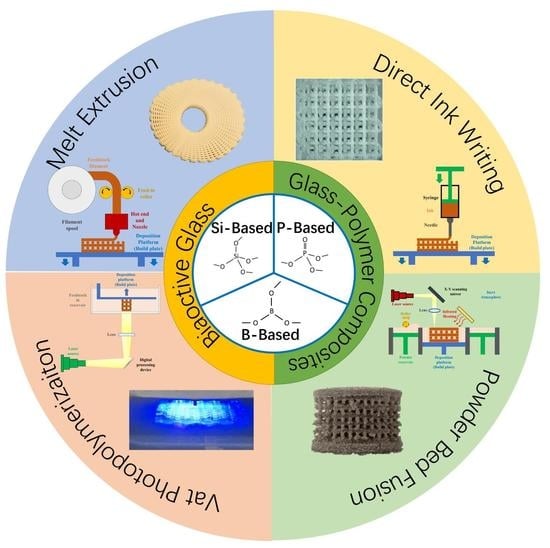
2. BG and BG/Polymer Composites Typically Applied in Bone Tissue Engineering
2.1. Bioactive Glasses (BG)
2.1.1. Silicate-Based BG
- Alkali metal cations within glass exchange with H+/H3O+ in the surrounding medium (Si-O-M + H+ → Si-OH + M+).
- Hydrolytic attacks take place at the Si-O-Si bonds within the soluble SiO2, giving rise to hydrated silica (Si-OH) at the BG–liquid interface [38].
- The hydrated silica undergoes polycondensation and repolymerization, which results in the formation of a silica-rich gel layer, as well as the depletion of metal cations from the BG.
- The calcium phosphate layer crystallizes by incorporating OH− and CO32− from the surrounding medium to form a hydroxycarbonate apatite (HCA) layer.
| Code | Composition (Oxides of Each Element) | Remark | Refs. |
|---|---|---|---|
| 45S5 | 45Si–6P–24.5Na–24.5 Ca (wt.%) | Commercialized as NovaMin® (GSK plc, London, UK) | [9] |
| 13–93 | 53Si-4P-6Na-5Mg-12K-20Ca (wt.%) | - | [51] |
| S53P4 | 53Si–4P–23Na–20Ca (wt.%) | Commercialized as Bonalive® (Bonalive Biomaterials Ltd., Turku, Finland) | [52] |
| SP-17Sr-17Ca | 44.5Si–4.5P–4Na–7.5Mg–4K–17.8Ca–17.8Sr (mol.%) | - | [53,54] |
| 58S | 60Si–4–36Ca (mol.%) | - | [39] |
| Si70-Ca30 | 70Si–30Ca | Commercialized as TheraGlass® (TheraGlass Ltd., London, UK) | [55] |
2.1.2. Phosphate-Based BG
2.1.3. Borate-Based BG

2.2. BG-Based Polymer Composites
3. Additive Manufacturing of BG and BG/Polymer Composites and Their Application to Bone Tissue Engineering
3.1. AM Technologies Applied to Fabricate BG or BG/Polymer Composite Scaffolds
3.1.1. Melt Extrusion
3.1.2. Direct Ink Writing (DIW)/Robocasting
3.1.3. Vat Photopolymerization
3.1.4. Powder Bed Fusion
3.2. Application of Additive-Manufactured BG and BG/Polymer Composites in Bone Tissue Engineering
3.2.1. Scaffold with Patient-Specific Design
3.2.2. Scaffold with the On-Demand Spatial Distribution of Biomaterials
4. Perspectives on Future Research
4.1. Toward a Higher Spatial Resolution
4.2. Binder-Free AM of Pure BG Objects

4.3. Scaffold for the Regeneration of Multiple Tissues at the Bone Defect
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AM | Additive manufacturing |
| BCP | Biphasic calcium phosphate |
| BG | Bioactive glass |
| BMP-2 | Bone morphogenic protein 2 |
| m/r/hBMSC | Mouse/rat/human bone mesenchymal stem cell |
| DIW | Direct ink writing |
| DLP | Digital light processing |
| DMD | Digital micromirror device |
| E. coli | Escherichia coli |
| EPC | Endothelial progenitor cell |
| FDM | Fused deposition modeling |
| GelMA | Gelatin methacryloyl |
| HA | Hydroxyapatite |
| hADSC | Human adipose-derived stem cell |
| HCA | Hydroxycarbonate apatite |
| hDPSC | Human dental pulp stem cell |
| HIF-1α | Hypoxia-inducible factor 1 α |
| HUVEC | Human umbilical vascular endothelial cell |
| HYSA | Hydroxy-safflower yellow A |
| MBG | Mesoporous bioactive glass |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| PACG | Poly (N-acryloyl 2-glycine) |
| PCL | Poly-ε-caprolactone |
| PDA | Polydopamine |
| PDLLA | Poly (DL-lactic acid) |
| PGA | Polyglycolic acid |
| PLA | Polylactic acid |
| PLDLA | Poly (L-co-D, L-lactic acid) |
| PLGA | Poly (lactic-co-glycolic acid) |
| PLLA | Poly (L-lactic acid) |
| PU | Polyurethane |
| S. aureus | Staphylococcus aureus |
| SLA | Stereolithography |
| SLS | Selective laser sintering |
| TIPS | Thermally induced phase separation |
| TNF-α | Tumor necrosis factor α |
| ZA | Zoledronic acid |
References
- GBD Fracture 2019 Collaborators. Global, regional, and national burden of bone fractures in 204 countries and territories, 1990–2019: A systematic analysis from the Global Burden of Disease Study 2019. Lancet Healthy Longev. 2021, 2, e580–e592. [Google Scholar] [CrossRef]
- Marieb, E.N.; Hoehn, K. Bones and Skeletal Tissues. In Human Anatomy & Physiology, 10th ed.; Pearson: Harlow, UK, 2016; pp. 193–218. [Google Scholar]
- Nauth, A.; Schemitsch, E.; Norris, B.; Nollin, Z.; Watson, J.T. Critical-Size Bone Defects: Is There a Consensus for Diagnosis and Treatment? J. Orthop. Trauma 2018, 32 (Suppl. S1), S7–S11. [Google Scholar] [CrossRef]
- Schmidt, A.H. Autologous bone graft: Is it still the gold standard? Injury 2021, 52 (Suppl. S2), S18–S22. [Google Scholar] [CrossRef]
- Rodriguez, A.E.; Nowzari, H. The long-term risks and complications of bovine-derived xenografts: A case series. J. Indian Soc. Periodontol. 2019, 23, 487–492. [Google Scholar] [CrossRef]
- Ratner, B.D. A History of Biomaterials. In Biomaterials Science, 3rd ed.; Ratner, B.D., Hoffman, A.S., Schoen, F.J., Lemons, J.E., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. xli–liii. [Google Scholar] [CrossRef]
- Hench, L.L.; Polak, J.M. Third-generation biomedical materials. Science 2002, 295, 1014–1017. [Google Scholar] [CrossRef]
- Hench, L.L. The story of Bioglass. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef]
- Hench, L.L.; Splinter, R.J.; Allen, W.C.; Greenlee, T.K. Bonding mechanisms at the interface of ceramic prosthetic materials. J. Biomed. Mater. Res. 1971, 5, 117–141. [Google Scholar] [CrossRef]
- Hench, L.L. Bioceramics: From Concept to Clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Miguez-Pacheco, V.; Hench, L.L.; Boccaccini, A.R. Bioactive glasses beyond bone and teeth: Emerging applications in contact with soft tissues. Acta Biomater. 2015, 13, 1–15. [Google Scholar] [CrossRef]
- Rocton, N.; Oudadesse, H.; Lefeuvre, B.; Peisker, H.; Rbii, K. Fine analysis of interaction mechanism of bioactive glass surface after soaking in SBF solution: AFM and ICP-OES investigations. Appl. Surf. Sci. 2020, 505, 144076. [Google Scholar] [CrossRef]
- Valimaki, V.V.; Aro, H.T. Molecular basis for action of bioactive glasses as bone graft substitute. Scand. J. Surg. 2006, 95, 95–102. [Google Scholar] [CrossRef]
- Stone-Weiss, N.; Bradtmüller, H.; Eckert, H.; Goel, A. Composition–Structure–Solubility Relationships in Borosilicate Glasses: Toward a Rational Design of Bioactive Glasses with Controlled Dissolution Behavior. ACS Appl. Mater. Interfaces 2021, 13, 31495–31513. [Google Scholar] [CrossRef]
- Yu, Y.; Mathew, R.; Edén, M. Quantitative composition–bioactivity relationships of phosphosilicate glasses: Bearings from the phosphorus content and network polymerization. J. Non-Cryst. Solids 2018, 502, 106–117. [Google Scholar] [CrossRef]
- Schumacher, M.; Habibovic, P.; van Rijt, S. Mesoporous bioactive glass composition effects on degradation and bioactivity. Bioact. Mater. 2021, 6, 1921–1931. [Google Scholar] [CrossRef]
- Li, R.; Clark, A.E.; Hench, L.L. An Investigation of Bioactive Glass Powders by Sol-Gel Processing. J. Appl. Biomater. 1991, 2, 231–239. [Google Scholar] [CrossRef]
- Yan, X.; Yu, C.; Zhou, X.; Tang, J.; Zhao, D. Highly ordered mesoporous bioactive glasses with superior in vitro bone-forming bioactivities. Angew. Chem. Int. Ed. Engl. 2004, 43, 5980–5984. [Google Scholar] [CrossRef]
- Soulie, J.; Gras, P.; Marsan, O.; Laurencin, D.; Rey, C.; Combes, C. Development of a new family of monolithic calcium (pyro)phosphate glasses by soft chemistry. Acta Biomater. 2016, 41, 320–327. [Google Scholar] [CrossRef]
- Verné, E.; Vitale-Brovarone, C.; Bui, E.; Bianchi, C.L.; Boccaccini, A.R. Surface functionalization of bioactive glasses. J. Biomed. Mater. Res. Part A 2009, 90A, 981–992. [Google Scholar] [CrossRef]
- Chen, Q.-Z.; Rezwan, K.; Françon, V.; Armitage, D.; Nazhat, S.N.; Jones, F.H.; Boccaccini, A.R. Surface functionalization of Bioglass®-derived porous scaffolds. Acta Biomater. 2007, 3, 551–562. [Google Scholar] [CrossRef]
- Cazzola, M.; Vernè, E.; Cochis, A.; Sorrentino, R.; Azzimonti, B.; Prenesti, E.; Rimondini, L.; Ferraris, S. Bioactive glasses functionalized with polyphenols: In vitro interactions with healthy and cancerous osteoblast cells. J. Mater. Sci. 2017, 52, 9211–9223. [Google Scholar] [CrossRef]
- Wong, K.C. 3D-printed patient-specific applications in orthopedics. Orthop. Res. Rev. 2016, 8, 57–66. [Google Scholar] [CrossRef]
- Rahaman, M.N.; Brown, R.F.; Bal, B.S.; Day, D.E. Bioactive Glasses for Nonbearing Applications in Total Joint Replacement. Semin. Arthroplast. 2006, 17, 102–112. [Google Scholar] [CrossRef]
- Gibson, I.; Rosen, D.; Stucker, B.; Khorasani, M. Introduction and Basic Principles. In Additive Manufacturing Technologies; Gibson, I., Rosen, D., Stucker, B., Khorasani, M., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–21. [Google Scholar] [CrossRef]
- Mirkhalaf, M.; Men, Y.; Wang, R.; No, Y.; Zreiqat, H. Personalized 3D printed bone scaffolds: A review. Acta Biomater. 2023, 156, 110–124. [Google Scholar] [CrossRef]
- Hutmacher, D.W. Scaffold design and fabrication technologies for engineering tissues—State of the art and future perspectives. J. Biomater. Sci. Polym. Ed. 2001, 12, 107–124. [Google Scholar] [CrossRef]
- Cámara-Torres, M.; Fucile, P.; Sinha, R.; Mota, C.; Moroni, L. Boosting bone regeneration using augmented melt-extruded additive-manufactured scaffolds. Int. Mater. Rev. 2022, 1–31. [Google Scholar] [CrossRef]
- Shirazi, S.F.; Gharehkhani, S.; Mehrali, M.; Yarmand, H.; Metselaar, H.S.; Adib Kadri, N.; Osman, N.A. A review on powder-based additive manufacturing for tissue engineering: Selective laser sintering and inkjet 3D printing. Sci. Technol. Adv. Mater. 2015, 16, 033502. [Google Scholar] [CrossRef]
- Kumar, A.; Mandal, S.; Barui, S.; Vasireddi, R.; Gbureck, U.; Gelinsky, M.; Basu, B. Low temperature additive manufacturing of three dimensional scaffolds for bone-tissue engineering applications: Processing related challenges and property assessment. Mater. Sci. Eng. R Rep. 2016, 103, 1–39. [Google Scholar] [CrossRef]
- Egan, P.F. Integrated Design Approaches for 3D Printed Tissue Scaffolds: Review and Outlook. Materials 2019, 12, 2355. [Google Scholar] [CrossRef]
- Kim, K.; Yeatts, A.; Dean, D.; Fisher, J.P. Stereolithographic bone scaffold design parameters: Osteogenic differentiation and signal expression. Tissue Eng. Part B Rev. 2010, 16, 523–539. [Google Scholar] [CrossRef]
- Wang, X.; Xu, S.; Zhou, S.; Xu, W.; Leary, M.; Choong, P.; Qian, M.; Brandt, M.; Xie, Y.M. Topological design and additive manufacturing of porous metals for bone scaffolds and orthopaedic implants: A review. Biomaterials 2016, 83, 127–141. [Google Scholar] [CrossRef]
- Du, X.; Fu, S.; Zhu, Y. 3D printing of ceramic-based scaffolds for bone tissue engineering: An overview. J. Mater. Chem. B 2018, 6, 4397–4412. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Koski, C.; Vu, A.A. Additive manufacturing of natural biopolymers and composites for bone tissue engineering. Mater. Horiz. 2020, 7, 2011–2027. [Google Scholar] [CrossRef]
- Mohammadi Zerankeshi, M.; Bakhshi, R.; Alizadeh, R. Polymer/metal composite 3D porous bone tissue engineering scaffolds fabricated by additive manufacturing techniques: A review. Bioprinting 2022, 25, e00191. [Google Scholar] [CrossRef]
- Barbeck, M.; Serra, T.; Booms, P.; Stojanovic, S.; Najman, S.; Engel, E.; Sader, R.; Kirkpatrick, C.J.; Navarro, M.; Ghanaati, S. Analysis of the in vitro degradation and the in vivo tissue response to bi-layered 3D-printed scaffolds combining PLA and biphasic PLA/bioglass components—Guidance of the inflammatory response as basis for osteochondral regeneration. Bioact. Mater. 2017, 2, 208–223. [Google Scholar] [CrossRef] [PubMed]
- Brauer, D.S.; Möncke, D. Introduction to the Structure of Silicate, Phosphate and Borate Glasses. In Bioactive Glasses: Fundamentals, Technology and Applications; Boccaccini, A.R., Brauer, D.S., Hupa, L., Eds.; The Royal Society of Chemistry: London, UK, 2016. [Google Scholar] [CrossRef]
- Sepulveda, P.; Jones, J.R.; Hench, L.L. Characterization of melt-derived 45S5 and sol-gel-derived 58S bioactive glasses. J. Biomed. Mater. Res. 2001, 58, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Chai, Y.; Ma, Y.; Duan, B.; Yuan, Y.; Liu, C. Rapid initiation of guided bone regeneration driven by spatiotemporal delivery of IL-8 and BMP-2 from hierarchical MBG-based scaffold. Biomaterials 2019, 196, 122–137. [Google Scholar] [CrossRef]
- Schumacher, M.; Reither, L.; Thomas, J.; Kampschulte, M.; Gbureck, U.; Lode, A.; Gelinsky, M. Calcium phosphate bone cement/mesoporous bioactive glass composites for controlled growth factor delivery. Biomater. Sci. 2017, 5, 578–588. [Google Scholar] [CrossRef]
- Kim, T.-H.; Singh, R.K.; Kang, M.S.; Kim, J.-H.; Kim, H.-W. Inhibition of osteoclastogenesis through siRNA delivery with tunable mesoporous bioactive nanocarriers. Acta Biomater. 2016, 29, 352–364. [Google Scholar] [CrossRef]
- Kaur, G.; Pickrell, G.; Sriranganathan, N.; Kumar, V.; Homa, D. Review and the state of the art: Sol–gel and melt quenched bioactive glasses for tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 104, 1248–1275. [Google Scholar] [CrossRef]
- Wu, C.; Chang, J. Mesoporous bioactive glasses: Structure characteristics, drug/growth factor delivery and bone regeneration application. Interface Focus 2012, 2, 292–306. [Google Scholar] [CrossRef]
- Vallet-Regi, M. Ordered mesoporous materials in the context of drug delivery systems and bone tissue engineering. Chemistry 2006, 12, 5934–5943. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L. Bioceramics. J. Am. Ceram. Soc. 2005, 81, 1705–1728. [Google Scholar] [CrossRef]
- Montazerian, M.; Zanotto, E.D. Bioactive Glass-ceramics: Processing, Properties and Applications. In Bioactive Glasses: Fundamentals, Technology and Applications; Boccaccini, A.R., Brauer, D.S., Hupa, L., Eds.; The Royal Society of Chemistry: London, UK, 2016. [Google Scholar] [CrossRef]
- Huang, W.; Day, D.E.; Kittiratanapiboon, K.; Rahaman, M.N. Kinetics and mechanisms of the conversion of silicate (45S5), borate, and borosilicate glasses to hydroxyapatite in dilute phosphate solutions. J. Mater. Sci. Mater. Med. 2006, 17, 583–596. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y.; Sayes, C.M. A toxicological profile of silica nanoparticles. Toxicol. Res. 2022, 11, 565–582. [Google Scholar] [CrossRef] [PubMed]
- Kusaka, T.; Nakayama, M.; Nakamura, K.; Ishimiya, M.; Furusawa, E.; Ogasawara, K. Effect of silica particle size on macrophage inflammatory responses. PLoS ONE 2014, 9, e92634. [Google Scholar] [CrossRef]
- Kolan, K.C.; Leu, M.C.; Hilmas, G.E.; Brown, R.F.; Velez, M. Fabrication of 13-93 bioactive glass scaffolds for bone tissue engineering using indirect selective laser sintering. Biofabrication 2011, 3, 025004. [Google Scholar] [CrossRef]
- Peltola, M.; Aitasalo, K.; Suonpaa, J.; Varpula, M.; Yli-Urpo, A. Bioactive glass S53P4 in frontal sinus obliteration: A long-term clinical experience. Head Neck 2006, 28, 834–841. [Google Scholar] [CrossRef]
- Baino, F.; Hamzehlou, S.; Kargozar, S. Bioactive Glasses: Where Are We and Where Are We Going? J. Funct. Biomater. 2018, 9, 25. [Google Scholar] [CrossRef]
- Sriranganathan, D.; Kanwal, N.; Hing, K.A.; Hill, R.G. Strontium substituted bioactive glasses for tissue engineered scaffolds: The importance of octacalcium phosphate. J. Mater. Sci. Mater. Med. 2016, 27, 39. [Google Scholar] [CrossRef]
- Macon, A.L.; Kim, T.B.; Valliant, E.M.; Goetschius, K.; Brow, R.K.; Day, D.E.; Hoppe, A.; Boccaccini, A.R.; Kim, I.Y.; Ohtsuki, C.; et al. A unified in vitro evaluation for apatite-forming ability of bioactive glasses and their variants. J. Mater. Sci. Mater. Med. 2015, 26, 115. [Google Scholar] [CrossRef]
- Brauer, D.S. Phosphate Glasses. In Bio-Glasses; John Wiley & Sons: Hoboken, NJ, USA, 2012; pp. 45–64. [Google Scholar] [CrossRef]
- Abou Neel, E.A.; Salih, V.; Knowles, J.C. 1.18 Phosphate-Based Glasses. In Comprehensive Biomaterials II; Ducheyne, P., Ed.; Elsevier: Oxford, UK, 2017; pp. 392–405. [Google Scholar] [CrossRef]
- Ahmed, I.; Lewis, M.; Olsen, I.; Knowles, J.C. Phosphate glasses for tissue engineering: Part 1. Processing and characterisation of a ternary-based P2O5–CaO–Na2O glass system. Biomaterials 2004, 25, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Tan, T.; Wang, D. Dissolution mechanism and release kinetics of phosphate controlled release glasses in aqueous medium. J. Control. Release. 2004, 96, 29–36. [Google Scholar] [CrossRef]
- De Rooij, J.F.; Heughebaert, J.C.; Nancollas, G.H. A ph study of calcium phosphate seeded precipitation. J. Colloid Interface Sci. 1984, 100, 350–358. [Google Scholar] [CrossRef]
- Kasuga, T. Bioactive calcium pyrophosphate glasses and glass-ceramics. Acta Biomater. 2005, 1, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Kasuga, T.; Hosoi, Y.; Nogami, M.; Niinomi, M. Apatite Formation on Calcium Phosphate Invert Glasses in Simulated Body Fluid. J. Am. Ceram. Soc. 2004, 84, 450–452. [Google Scholar] [CrossRef]
- Kasuga, T.; Mizuno, T.; Watanabe, M.; Nogami, M.; Niinomi, M. Calcium phosphate invert glass-ceramic coatings joined by self-development of compositionally gradient layers on a titanium alloy. Biomaterials 2001, 22, 577–582. [Google Scholar] [CrossRef]
- Kasuga, T.; Hattori, T.; Niinomi, M. Phosphate Glasses and Glass-Ceramics for Biomedical Applications. Phosphorus Res. Bull. 2012, 26, 8–15. [Google Scholar] [CrossRef]
- Ege, D.; Zheng, K.; Boccaccini, A.R. Borate Bioactive Glasses (BBG): Bone Regeneration, Wound Healing Applications, and Future Directions. ACS Appl. Bio. Mater. 2022, 5, 3608–3622. [Google Scholar] [CrossRef]
- Wright, A.C.; Dalba, G.; Rocca, F.; Vedishcheva, N.M. Borate versus silicate glasses: Why are they so different? Phys. Chem. Glas.-Eur. J. Glass Sci. Technol. Part B 2010, 51, 233–265. [Google Scholar]
- Lepry, W.C.; Nazhat, S.N. The anomaly in bioactive sol–gel borate glasses. Mater. Adv. 2020, 1, 1371–1381. [Google Scholar] [CrossRef]
- Eigen, M. CHAPTER 12-OXIDE GLASSES. In Structural Chemistry of Glasses; Rao, K.J., Ed.; Elsevier Science Ltd.: Oxford, UK, 2002; pp. 463–511. [Google Scholar] [CrossRef]
- Lepry, W.C.; Smith, S.; Nazhat, S.N. Effect of sodium on bioactive sol-gel-derived borate glasses. J. Non-Cryst. Solids 2018, 500, 141–148. [Google Scholar] [CrossRef]
- Fu, H.; Fu, Q.; Zhou, N.; Huang, W.; Rahaman, M.N.; Wang, D.; Liu, X. In vitro evaluation of borate-based bioactive glass scaffolds prepared by a polymer foam replication method. Mater. Sci. Eng. C 2009, 29, 2275–2281. [Google Scholar] [CrossRef]
- Balasubramanian, P.; Grünewald, A.; Detsch, R.; Hupa, L.; Jokic, B.; Tallia, F.; Solanki, A.K.; Jones, J.R.; Boccaccini, A.R. Ion Release, Hydroxyapatite Conversion, and Cytotoxicity of Boron-Containing Bioactive Glass Scaffolds. Int. J. Appl. Glass Sci. 2016, 7, 206–215. [Google Scholar] [CrossRef]
- Deliormanlı, A.M. Size-dependent degradation and bioactivity of borate bioactive glass. Ceram. Int. 2013, 39, 8087–8095. [Google Scholar] [CrossRef]
- Shearer, A.; Montazerian, M.; Mauro, J.C. Modern definition of bioactive glasses and glass-ceramics. J. Non-Cryst. Solids 2023, 608, 122228. [Google Scholar] [CrossRef]
- Deshmukh, K.; Kovářík, T.; Křenek, T.; Docheva, D.; Stich, T.; Pola, J. Recent advances and future perspectives of sol–gel derived porous bioactive glasses: A review. RSC Adv. 2020, 10, 33782–33835. [Google Scholar] [CrossRef]
- Kargozar, S.; Kermani, F.; Mollazadeh Beidokhti, S.; Hamzehlou, S.; Verné, E.; Ferraris, S.; Baino, F. Functionalization and Surface Modifications of Bioactive Glasses (BGs): Tailoring of the Biological Response Working on the Outermost Surface Layer. Materials 2019, 12, 3696. [Google Scholar] [CrossRef]
- Eqtesadi, S.; Motealleh, A.; Pajares, A.; Guiberteau, F.; Miranda, P. Improving mechanical properties of 13–93 bioactive glass robocast scaffold by poly (lactic acid) and poly (ε-caprolactone) melt infiltration. J. Non-Cryst. Solids 2016, 432, 111–119. [Google Scholar] [CrossRef]
- Middleton, J.C.; Tipton, A.J. Synthetic biodegradable polymers as orthopedic devices. Biomaterials 2000, 21, 2335–2346. [Google Scholar] [CrossRef]
- Boccaccini, A.R.; Jones, J.R.; Chen, Q.-Z. Composites Containing Bioactive Glass. In Bio-Glasses; John Wiley & Sons: Hoboken, NJ, USA, 2012; pp. 121–138. [Google Scholar] [CrossRef]
- Sergi, R.; Bellucci, D.; Cannillo, V. A Review of Bioactive Glass/Natural Polymer Composites: State of the Art. Materials 2020, 13, 5560. [Google Scholar] [CrossRef]
- Morgan, E.F.; Barnes, G.L.; Einhorn, T.A. The Bone Organ System: Form and Function. In Fundamentals of Osteoporosis; Marcus, R., Feldman, D., Nelson, D.A., Rosen, C.J., Eds.; Academic Press: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The basic science of articular cartilage: Structure, composition, and function. Sport. Health 2009, 1, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Kim, K.E.; Kwon, I.C.; Ahn, H.J.; Lee, S.H.; Cho, H.; Kim, H.J.; Seong, S.C.; Lee, M.C. Effects of the controlled-released TGF-beta 1 from chitosan microspheres on chondrocytes cultured in a collagen/chitosan/glycosaminoglycan scaffold. Biomaterials 2004, 25, 4163–4173. [Google Scholar] [CrossRef]
- Leite, A.J.; Mano, J.F. Biomedical applications of natural-based polymers combined with bioactive glass nanoparticles. J. Mater. Chem. B 2017, 5, 4555–4568. [Google Scholar] [CrossRef] [PubMed]
- Elzoghby, A.O.; Samy, W.M.; Elgindy, N.A. Protein-based nanocarriers as promising drug and gene delivery systems. J. Control. Release 2012, 161, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Lynn, A.K.; Yannas, I.V.; Bonfield, W. Antigenicity and immunogenicity of collagen. J. Biomed. Mater. Res. B Appl. Biomater. 2004, 71, 343–354. [Google Scholar] [CrossRef]
- Owczarczyk-Saczonek, A.; Zdanowska, N.; Wygonowska, E.; Placek, W. The Immunogenicity of Hyaluronic Fillers and Its Consequences. Clin. Cosmet. Investig. Derm. 2021, 14, 921–934. [Google Scholar] [CrossRef]
- Dunn, A.S.; Campbell, P.G.; Marra, K.G. The influence of polymer blend composition on the degradation of polymer/hydroxyapatite biomaterials. J. Mater. Sci. Mater. Med. 2001, 12, 673–677. [Google Scholar] [CrossRef]
- Van de Velde, K.; Kiekens, P. Biopolymers: Overview of several properties and consequences on their applications. Polym. Test. 2002, 21, 433–442. [Google Scholar] [CrossRef]
- Tyler, B.; Gullotti, D.; Mangraviti, A.; Utsuki, T.; Brem, H. Polylactic acid (PLA) controlled delivery carriers for biomedical applications. Adv. Drug Deliv. Rev. 2016, 107, 163–175. [Google Scholar] [CrossRef]
- Lucas, S. Poly Lactic-Co-Glycolic Acid [P(L/G)A] Safety Profile. In Medical Device Material Performance Study; ECRI Institute: Plymouth Meeting, PA, USA, 2020. [Google Scholar]
- Schabowsky, C.N. Polycaprolactone (PCL) Safety Profile. In Medical Device Material Performance Study; ECRI Institute: Plymouth Meeting, PA, USA, 2021. [Google Scholar]
- Arun, Y.; Ghosh, R.; Domb, A.J. Biodegradable Hydrophobic Injectable Polymers for Drug Delivery and Regenerative Medicine. Adv. Funct. Mater. 2021, 31, 2010284. [Google Scholar] [CrossRef]
- Hiasa, M.; Okui, T.; Allette, Y.M.; Ripsch, M.S.; Sun-Wada, G.H.; Wakabayashi, H.; Roodman, G.D.; White, F.A.; Yoneda, T. Bone Pain Induced by Multiple Myeloma Is Reduced by Targeting V-ATPase and ASIC3. Cancer Res. 2017, 77, 1283–1295. [Google Scholar] [CrossRef]
- Song, R.; Murphy, M.; Li, C.; Ting, K.; Soo, C.; Zheng, Z. Current development of biodegradable polymeric materials for biomedical applications. Drug Des. Devel. 2018, 12, 3117–3145. [Google Scholar] [CrossRef]
- Arnett, T.R. Extracellular pH regulates bone cell function. J. Nutr. 2008, 138, 415S–418S. [Google Scholar] [CrossRef] [PubMed]
- Cai, F.; Hong, X.; Tang, X.; Liu, N.C.; Wang, F.; Zhu, L.; Xie, X.H.; Xie, Z.Y.; Wu, X.T. ASIC1a activation induces calcium-dependent apoptosis of BMSCs under conditions that mimic the acidic microenvironment of the degenerated intervertebral disc. Biosci. Rep. 2019, 39, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Rezwan, K.; Chen, Q.Z.; Blaker, J.J.; Boccaccini, A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef] [PubMed]
- Costantini, M.; Barbetta, A. Gas foaming technologies for 3D scaffold engineering. In Functional 3D Tissue Engineering Scaffolds; Woodhead Publishing: Sawston, UK, 2018; pp. 127–149. [Google Scholar] [CrossRef]
- O’Brien, F.J.; Harley, B.A.; Yannas, I.V.; Gibson, L. Influence of freezing rate on pore structure in freeze-dried collagen-GAG scaffolds. Biomaterials 2004, 25, 1077–1086. [Google Scholar] [CrossRef]
- Akbarzadeh, R.; Yousefi, A.M. Effects of processing parameters in thermally induced phase separation technique on porous architecture of scaffolds for bone tissue engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 1304–1315. [Google Scholar] [CrossRef]
- Prasad, A.; Sankar, M.R.; Katiyar, V. State of Art on Solvent Casting Particulate Leaching Method for Orthopedic ScaffoldsFabrication. Mater. Today Proc. 2017, 4, 898–907. [Google Scholar] [CrossRef]
- Ohman, C.; Baleani, M.; Pani, C.; Taddei, F.; Alberghini, M.; Viceconti, M.; Manfrini, M. Compressive behaviour of child and adult cortical bone. Bone 2011, 49, 769–776. [Google Scholar] [CrossRef]
- Giesen, E.B.; Ding, M.; Dalstra, M.; van Eijden, T.M. Mechanical properties of cancellous bone in the human mandibular condyle are anisotropic. J. Biomech. 2001, 34, 799–803. [Google Scholar] [CrossRef]
- Vastel, L.; Meunier, A.; Siney, H.; Sedel, L.; Courpied, J.P. Effect of different sterilization processing methods on the mechanical properties of human cancellous bone allografts. Biomaterials 2004, 25, 2105–2110. [Google Scholar] [CrossRef]
- Jurvelin, J.S.; Buschmann, M.D.; Hunziker, E.B. Mechanical anisotropy of the human knee articular cartilage in compression. Proc. Inst. Mech. Eng. H 2003, 217, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Thompson, I.D.; Hench, L.L. Mechanical properties of bioactive glasses, glass-ceramics and composites. Proc. Inst. Mech. Eng. H 1998, 212, 127–136. [Google Scholar] [CrossRef]
- Gerhardt, L.C.; Boccaccini, A.R. Bioactive Glass and Glass-Ceramic Scaffolds for Bone Tissue Engineering. Materials 2010, 3, 3867–3910. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Jinsong, L.; Songlin, H.; Rudd, C.; Liu, X. The Structure, Degradation and Fibre Drawing Properties of Phosphate Based Glasses Fibre:The Effects of Fe2O3 and B2O3 Addition. Ceram.-Silik. 2018, 62, 111–120. [Google Scholar] [CrossRef]
- Rodenas-Rochina, J.; Ribelles, J.L.; Lebourg, M. Comparative study of PCL-HAp and PCL-bioglass composite scaffolds for bone tissue engineering. J. Mater. Sci. Mater. Med. 2013, 24, 1293–1308. [Google Scholar] [CrossRef]
- Niu, Y.; Guo, L.; Liu, J.; Shen, H.; Su, J.; An, X.; Yu, B.; Wei, J.; Shin, J.W.; Guo, H.; et al. Bioactive and degradable scaffolds of the mesoporous bioglass and poly(l-lactide) composite for bone tissue regeneration. J. Mater. Chem. B 2015, 3, 2962–2970. [Google Scholar] [CrossRef]
- Blaker, J.J.; Nazhat, S.N.; Maquet, V.; Boccaccini, A.R. Long-term in vitro degradation of PDLLA/bioglass bone scaffolds in acellular simulated body fluid. Acta Biomater. 2011, 7, 829–840. [Google Scholar] [CrossRef]
- Shah Mohammadi, M.; Rezabeigi, E.; Bertram, J.; Marelli, B.; Gendron, R.; Nazhat, S.N.; Bureau, M.N. Poly(d,l-Lactic acid) Composite Foams Containing Phosphate Glass Particles Produced via Solid-State Foaming Using CO2 for Bone Tissue Engineering Applications. Polymers 2020, 12, 231. [Google Scholar] [CrossRef]
- Sun, Z.; Wu, F.; Gao, H.; Cui, K.; Xian, M.; Zhong, J.; Tian, Y.; Fan, S.; Wu, G. A Dexamethasone-Eluting Porous Scaffold for Bone Regeneration Fabricated by Selective Laser Sintering. ACS Appl. Bio. Mater. 2020, 3, 8739–8747. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Saiz, E.; Tomsia, A.P. Direct ink writing of highly porous and strong glass scaffolds for load-bearing bone defects repair and regeneration. Acta Biomater. 2011, 7, 3547–3554. [Google Scholar] [CrossRef]
- Liu, X.; Rahaman, M.N.; Hilmas, G.E.; Bal, B.S. Mechanical properties of bioactive glass (13–93) scaffolds fabricated by robotic deposition for structural bone repair. Acta Biomater. 2013, 9, 7025–7034. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Luo, Y.; Cuniberti, G.; Xiao, Y.; Gelinsky, M. Three-dimensional printing of hierarchical and tough mesoporous bioactive glass scaffolds with a controllable pore architecture, excellent mechanical strength and mineralization ability. Acta Biomater. 2011, 7, 2644–2650. [Google Scholar] [CrossRef]
- Gao, F.; Xu, Z.; Liang, Q.; Li, H.; Peng, L.; Wu, M.; Zhao, X.; Cui, X.; Ruan, C.; Liu, W. Osteochondral Regeneration with 3D-Printed Biodegradable High-Strength Supramolecular Polymer Reinforced-Gelatin Hydrogel Scaffolds. Adv. Sci. 2019, 6, 1900867. [Google Scholar] [CrossRef]
- Baier, R.V.; Contreras Raggio, J.I.; Giovanetti, C.M.; Palza, H.; Burda, I.; Terrasi, G.; Weisse, B.; De Freitas, G.S.; Nystrom, G.; Vivanco, J.F.; et al. Shape fidelity, mechanical and biological performance of 3D printed polycaprolactone-bioactive glass composite scaffolds. Biomater. Adv. 2022, 134, 112540. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, D.; Wang, M.; Mao, R.; Zhao, H.; Huang, X.; Gf Shen, S. Seamless route of self-assembly and 3D printing to fabricate hierarchical mesoporous bioactive glass scaffold for customized bone regeneration with enhanced efficacy. Chem. Eng. J. 2022, 446, 137270. [Google Scholar] [CrossRef]
- Distler, T.; Fournier, N.; Grunewald, A.; Polley, C.; Seitz, H.; Detsch, R.; Boccaccini, A.R. Polymer-Bioactive Glass Composite Filaments for 3D Scaffold Manufacturing by Fused Deposition Modeling: Fabrication and Characterization. Front. Bioeng. Biotechnol. 2020, 8, 552. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yang, Y.; Chi, G.; Yuan, K.; Zhou, F.; Dong, L.; Liu, H.; Zhou, Q.; Gong, W.; Yang, S.; et al. A 3D printed Ga containing scaffold with both anti-infection and bone homeostasis-regulating properties for the treatment of infected bone defects. J. Mater. Chem. B 2021, 9, 4735–4745. [Google Scholar] [CrossRef]
- Ma, Z.; Xie, J.; Shan, X.Z.; Zhang, J.; Wang, Q. High solid content 45S5 Bioglass®-based scaffolds using stereolithographic ceramic manufacturing: Process, structural and mechanical properties. J. Mech. Sci. Technol. 2021, 35, 823–832. [Google Scholar] [CrossRef]
- Bidgoli, M.R.; Alemzadeh, I.; Tamjid, E.; Khafaji, M.; Vossoughi, M. Fabrication of hierarchically porous silk fibroin-bioactive glass composite scaffold via indirect 3D printing: Effect of particle size on physico-mechanical properties and in vitro cellular behavior. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 103, 109688. [Google Scholar] [CrossRef]
- Marsh, A.C.; Zhang, Y.; Poli, L.; Hammer, N.; Roch, A.; Crimp, M.; Chatzistavrou, X. 3D printed bioactive and antibacterial silicate glass-ceramic scaffold by fused filament fabrication. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111516. [Google Scholar] [CrossRef]
- Qian, G.; Zhang, L.; Liu, X.; Wu, S.; Peng, S.; Shuai, C. Silver-doped bioglass modified scaffolds: A sustained antibacterial efficacy. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 129, 112425. [Google Scholar] [CrossRef]
- Mousavi Nejad, Z.; Zamanian, A.; Saeidifar, M.; Vanaei, H.R.; Salar Amoli, M. 3D Bioprinting of Polycaprolactone-Based Scaffolds for Pulp-Dentin Regeneration: Investigation of Physicochemical and Biological Behavior. Polymers 2021, 13, 4442. [Google Scholar] [CrossRef]
- He, L.; Liu, X.; Rudd, C. Additive-Manufactured Gyroid Scaffolds of Magnesium Oxide, Phosphate Glass Fiber and Polylactic Acid Composite for Bone Tissue Engineering. Polymers 2021, 13, 270. [Google Scholar] [CrossRef]
- Luo, G.; Ma, Y.; Cui, X.; Jiang, L.; Wu, M.; Hu, Y.; Luo, Y.; Pan, H.; Ruan, C. 13-93 bioactive glass/alginate composite scaffolds 3D printed under mild conditions for bone regeneration. RSC Adv. 2017, 7, 11880–11889. [Google Scholar] [CrossRef]
- Salmoria, G.V.; Pereira, R.V.; Fredel, M.C.; Casadei, A.P.M. Properties of PLDLA/bioglass scaffolds produced by selective laser sintering. Polym. Bull. 2017, 75, 1299–1309. [Google Scholar] [CrossRef]
- Liao, M.; Zhu, S.; Guo, A.; Han, X.; Li, Q.; Chen, Y.; Liu, Y.; Chen, D.; Chen, X.; Mo, S.; et al. 3D printed bioactive glasses porous scaffolds with high strength for the repair of long-bone segmental defects. Compos. Part B Eng. 2023, 254, 110582. [Google Scholar] [CrossRef]
- International Standard Organization. Additive Manufacturing-General Principles-Fundamentals and Vocabulary; International Standard Organization: Geneva, Switzerland, 2022.
- Gibson, I.; Rosen, D.; Stucker, B.; Khorasani, M. Material Extrusion. In Additive Manufacturing Technologies; Gibson, I., Rosen, D., Stucker, B., Khorasani, M., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 171–201. [Google Scholar]
- Petretta, M.; Gambardella, A.; Boi, M.; Berni, M.; Cavallo, C.; Marchiori, G.; Maltarello, M.C.; Bellucci, D.; Fini, M.; Baldini, N.; et al. Composite Scaffolds for Bone Tissue Regeneration Based on PCL and Mg-Containing Bioactive Glasses. Biology 2021, 10, 398. [Google Scholar] [CrossRef]
- Daskalakis, E.; Huang, B.; Vyas, C.; Acar, A.A.; Fallah, A.; Cooper, G.; Weightman, A.; Koc, B.; Blunn, G.; Bartolo, P. Novel 3D Bioglass Scaffolds for Bone Tissue Regeneration. Polymers 2022, 14, 445. [Google Scholar] [CrossRef]
- Gold, S.A.; Strong, R.; Turner, B.N. A review of melt extrusion additive manufacturing processes: I. Process design and modeling. Rapid. Prototyp. J. 2014, 20, 192–204. [Google Scholar] [CrossRef]
- Ming, Y.; Hannah Jia Hui, N.; Vincent, D.W.N.; Chou, N.; Tseng Tsai, Y. Cranial reconstruction using a polycaprolactone implant after burr hole trephination. J. 3D Print. Med. 2020, 4, 9–16. [Google Scholar] [CrossRef]
- Auriemma, G.; Tommasino, C.; Falcone, G.; Esposito, T.; Sardo, C.; Aquino, R.P. Additive Manufacturing Strategies for Personalized Drug Delivery Systems and Medical Devices: Fused Filament Fabrication and Semi Solid Extrusion. Molecules 2022, 27, 2784. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, W.; Ba, Z.; Cui, S.; Wei, J.; Li, H. 3D-printed scaffolds of mesoporous bioglass/gliadin/polycaprolactone ternary composite for enhancement of compressive strength, degradability, cell responses and new bone tissue ingrowth. Int. J. Nanomed. 2018, 13, 5433–5447. [Google Scholar] [CrossRef]
- Wang, C.; Meng, C.; Zhang, Z.; Zhu, Q. 3D printing of polycaprolactone/bioactive glass composite scaffolds for in situ bone repair. Ceram. Int. 2022, 48, 7491–7499. [Google Scholar] [CrossRef]
- Lee, D.; Lee, Y.; Kim, I.; Hwang, K.; Kim, N. Thermal and Mechanical Degradation of Recycled Polylactic Acid Filaments for Three-Dimensional Printing Applications. Polymers 2022, 14, 5385. [Google Scholar] [CrossRef]
- Liu, W.; Wang, D.; Huang, J.; Wei, Y.; Xiong, J.; Zhu, W.; Duan, L.; Chen, J.; Sun, R.; Wang, D. Low-temperature deposition manufacturing: A novel and promising rapid prototyping technology for the fabrication of tissue-engineered scaffold. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 70, 976–982. [Google Scholar] [CrossRef]
- Du, X.; Wei, D.; Huang, L.; Zhu, M.; Zhang, Y.; Zhu, Y. 3D printing of mesoporous bioactive glass/silk fibroin composite scaffolds for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 103, 109731. [Google Scholar] [CrossRef]
- Gomez-Cerezo, M.N.; Lozano, D.; Arcos, D.; Vallet-Regi, M.; Vaquette, C. The effect of biomimetic mineralization of 3D-printed mesoporous bioglass scaffolds on physical properties and in vitro osteogenicity. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 109, 110572. [Google Scholar] [CrossRef]
- Mei, N.; Wu, Y.; Chen, B.; Zhuang, T.; Yu, X.; Sui, B.; Ding, T.; Liu, X. 3D-printed mesoporous bioactive glass/GelMA biomimetic scaffolds for osteogenic/cementogenic differentiation of periodontal ligament cells. Front. Bioeng. Biotechnol. 2022, 10, 950970. [Google Scholar] [CrossRef]
- Schuhladen, K.; Bednarzig, V.; Rembold, N.; Boccaccini, A.R. The effect of borate bioactive glass on the printability of methylcellulose-manuka honey hydrogels. J. Mater. Res. 2021, 36, 3843–3850. [Google Scholar] [CrossRef]
- Montalbano, G.; Borciani, G.; Cerqueni, G.; Licini, C.; Banche-Niclot, F.; Janner, D.; Sola, S.; Fiorilli, S.; Mattioli-Belmonte, M.; Ciapetti, G.; et al. Collagen Hybrid Formulations for the 3D Printing of Nanostructured Bone Scaffolds: An Optimized Genipin-Crosslinking Strategy. Nanomaterials 2020, 10, 1681. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Huang, Y.; Qin, J.; Honiball, J.R.; Wen, D.; Xie, X.; Shi, Z.; Cui, X.; Li, B. Development of a borosilicate bioactive glass scaffold incorporating calcitonin gene-related peptide for tissue engineering. Biomater. Adv. 2022, 138, 212949. [Google Scholar] [CrossRef] [PubMed]
- del-Mazo-Barbara, L.; Ginebra, M.-P. Rheological characterisation of ceramic inks for 3D direct ink writing: A review. J. Eur. Ceram. Soc. 2021, 41, 18–33. [Google Scholar] [CrossRef]
- M’Barki, A.; Bocquet, L.; Stevenson, A. Linking Rheology and Printability for Dense and Strong Ceramics by Direct Ink Writing. Sci. Rep. 2017, 7, 6017. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.S.L.; Sesso, M.L.; Franks, G.V. Direct ink writing of hierarchical porous alumina-stabilized emulsions: Rheology and printability. J. Am. Ceram. Soc. 2020, 103, 5554–5566. [Google Scholar] [CrossRef]
- Bento, R.; Gaddam, A.; Oskoei, P.; Oliveira, H.; Ferreira, J.M.F. 3D Printing of Macro Porous Sol-Gel Derived Bioactive Glass Scaffolds and Assessment of Biological Response. Materials 2021, 14, 5946. [Google Scholar] [CrossRef]
- Chen, Q.; Schmidt, F.; Gorke, O.; Asif, A.; Weinhold, J.; Aghaei, E.; Rehman, I.U.; Gurlo, A.; Shah, A.T. Ceramic Stereolithography of Bioactive Glasses: Influence of Resin Composition on Curing Behavior and Green Body Properties. Biomedicines 2022, 10, 395. [Google Scholar] [CrossRef]
- Kim, Y.; Lim, J.Y.; Yang, G.H.; Seo, J.-H.; Ryu, H.-S.; Kim, G. 3D-printed PCL/bioglass (BGS-7) composite scaffolds with high toughness and cell-responses for bone tissue regeneration. J. Ind. Eng. Chem. 2019, 79, 163–171. [Google Scholar] [CrossRef]
- Ojansivu, M.; Rashad, A.; Ahlinder, A.; Massera, J.; Mishra, A.; Syverud, K.; Finne-Wistrand, A.; Miettinen, S.; Mustafa, K. Wood-based nanocellulose and bioactive glass modified gelatin-alginate bioinks for 3D bioprinting of bone cells. Biofabrication 2019, 11, 035010. [Google Scholar] [CrossRef]
- Bertuola, M.; Aráoz, B.; Gilabert, U.; Gonzalez-Wusener, A.; Pérez-Recalde, M.; Arregui, C.O.; Hermida, É.B. Gelatin–alginate–hyaluronic acid inks for 3D printing: Effects of bioglass addition on printability, rheology and scaffold tensile modulus. J. Mater. Sci. 2021, 56, 15327–15343. [Google Scholar] [CrossRef]
- Fermani, M.; Platania, V.; Kavasi, R.-M.; Karavasili, C.; Zgouro, P.; Fatouros, D.; Chatzinikolaidou, M.; Bouropoulos, N. 3D-Printed Scaffolds from Alginate/Methyl Cellulose/Trimethyl Chitosan/Silicate Glasses for Bone Tissue Engineering. Appl. Sci. 2021, 11, 8677. [Google Scholar] [CrossRef]
- Gomez-Cerezo, N.; Casarrubios, L.; Saiz-Pardo, M.; Ortega, L.; de Pablo, D.; Diaz-Guemes, I.; Fernandez-Tome, B.; Enciso, S.; Sanchez-Margallo, F.M.; Portoles, M.T.; et al. Mesoporous bioactive glass/varepsilon-polycaprolactone scaffolds promote bone regeneration in osteoporotic sheep. Acta Biomater. 2019, 90, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Chen, J.; Lin, B.; Li, J.; Wang, H.; Wang, D.; Pang, L.; Zeng, X.; Wang, H.; Zhang, Y. A novel 3D printed bioactive scaffolds with enhanced osteogenic inspired by ancient Chinese medicine HYSA for bone repair. Exp. Cell Res. 2020, 394, 112139. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zeng, X.; Pang, L.; Wang, H.; Lin, B.; Deng, Z.; Qi, E.L.X.; Miao, N.; Wang, D.; Huang, P.; et al. Integrative treatment of anti-tumor/bone repair by combination of MoS2 nanosheets with 3D printed bioactive borosilicate glass scaffolds. Chem. Eng. J. 2020, 396, 125081. [Google Scholar] [CrossRef]
- Qi, X.; Wang, H.; Zhang, Y.; Pang, L.; Xiao, W.; Jia, W.; Zhao, S.; Wang, D.; Huang, W.; Wang, Q. Mesoporous bioactive glass-coated 3D printed borosilicate bioactive glass scaffolds for improving repair of bone defects. Int. J. Biol. Sci. 2018, 14, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Deng, Z.; Chen, J.; Qi, X.; Pang, L.; Lin, B.; Adib, Y.T.Y.; Miao, N.; Wang, D.; Zhang, Y.; et al. A novel vehicle-like drug delivery 3D printing scaffold and its applications for a rat femoral bone repairing in vitro and in vivo. Int. J. Biol. Sci. 2020, 16, 1821–1832. [Google Scholar] [CrossRef]
- Zhu, H.; Monavari, M.; Zheng, K.; Distler, T.; Ouyang, L.; Heid, S.; Jin, Z.; He, J.; Li, D.; Boccaccini, A.R. 3D Bioprinting of Multifunctional Dynamic Nanocomposite Bioinks Incorporating Cu-Doped Mesoporous Bioactive Glass Nanoparticles for Bone Tissue Engineering. Small 2022, 18, e2104996. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Enhe, J.; Yao, B.; Wang, Y.; Zhu, D.; Li, Z.; Song, W.; Duan, X.; Yuan, X.; et al. Bioactive nanoparticle reinforced alginate/gelatin bioink for the maintenance of stem cell stemness. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 126, 112193. [Google Scholar] [CrossRef]
- Wei, L.; Li, Z.; Li, J.; Zhang, Y.; Yao, B.; Liu, Y.; Song, W.; Fu, X.; Wu, X.; Huang, S. An approach for mechanical property optimization of cell-laden alginate-gelatin composite bioink with bioactive glass nanoparticles. J. Mater. Sci. Mater. Med. 2020, 31, 103. [Google Scholar] [CrossRef]
- Li, X.; Zhang, H.; Shen, Y.; Xiong, Y.; Dong, L.; Zheng, J.; Zhao, S. Fabrication of porous β-TCP/58S bioglass scaffolds via top-down DLP printing with high solid loading ceramic-resin slurry. Mater. Chem. Phys. 2021, 267, 124587. [Google Scholar] [CrossRef]
- Gibson, I.; Rosen, D.; Stucker, B.; Khorasani, M. Vat Photopolymerization. In Additive Manufacturing Technologies; Gibson, I., Rosen, D., Stucker, B., Khorasani, M., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 77–124. [Google Scholar]
- Su, J.; Hua, S.; Chen, A.; Chen, P.; Yang, L.; Yuan, X.; Qi, D.; Zhu, H.; Yan, C.; Xiao, J.; et al. Three-dimensional printing of gyroid-structured composite bioceramic scaffolds with tuneable degradability. Biomater. Adv. 2022, 133, 112595. [Google Scholar] [CrossRef]
- Kleinfehn, A.P.; Lammel Lindemann, J.A.; Razvi, A.; Philip, P.; Richardson, K.; Nettleton, K.; Becker, M.L.; Dean, D. Modulating Bioglass Concentration in 3D Printed Poly(propylene fumarate) Scaffolds for Post-Printing Functionalization with Bioactive Functional Groups. Biomacromolecules 2019, 20, 4345–4352. [Google Scholar] [CrossRef]
- Kang, J.-H.; Jang, K.-J.; Sakthiabirami, K.; Oh, G.-J.; Jang, J.-G.; Park, C.; Lim, H.-P.; Yun, K.-D.; Park, S.-W. Mechanical properties and optical evaluation of scaffolds produced from 45S5 bioactive glass suspensions via stereolithography. Ceram. Int. 2020, 46, 2481–2488. [Google Scholar] [CrossRef]
- Par, M.; Spanovic, N.; Mohn, D.; Attin, T.; Taubock, T.T.; Tarle, Z. Curing potential of experimental resin composites filled with bioactive glass: A comparison between Bis-EMA and UDMA based resin systems. Dent. Mater. 2020, 36, 711–723. [Google Scholar] [CrossRef]
- Par, M.; Tarle, Z.; Hickel, R.; Ilie, N. Polymerization kinetics of experimental bioactive composites containing bioactive glass. J. Dent. 2018, 76, 83–88. [Google Scholar] [CrossRef]
- Xu, F.; Ren, H.; Zheng, M.; Shao, X.; Dai, T.; Wu, Y.; Tian, L.; Liu, Y.; Liu, B.; Gunster, J.; et al. Development of biodegradable bioactive glass ceramics by DLP printed containing EPCs/BMSCs for bone tissue engineering of rabbit mandible defects. J. Mech. Behav. Biomed. Mater. 2020, 103, 103532. [Google Scholar] [CrossRef]
- Gibson, I.; Rosen, D.; Stucker, B.; Khorasani, M. Powder Bed Fusion. In Additive Manufacturing Technologies; Gibson, I., Rosen, D., Stucker, B., Khorasani, M., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 125–170. [Google Scholar]
- Shuai, C.; Xu, Y.; Feng, P.; Wang, G.; Xiong, S.; Peng, S. Antibacterial polymer scaffold based on mesoporous bioactive glass loaded with in situ grown silver. Chem. Eng. J. 2019, 374, 304–315. [Google Scholar] [CrossRef]
- Karl, D.; Jastram, B.; Kamm, P.H.; Schwandt, H.; Gurlo, A.; Schmidt, F. Evaluating porous polylactide-co-glycolide/bioactive glass composite microsphere powders for laser sintering of scaffolds. Powder Technol. 2019, 354, 289–300. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, P.; Feng, P.; Guo, W.; Yang, W.; Shuai, C. Interfacial reinforcement in a poly-l-lactic acid/mesoporous bioactive glass scaffold via polydopamine. Colloids Surf. B Biointerfaces 2018, 170, 45–53. [Google Scholar] [CrossRef]
- Shuai, C.; Xu, Y.; Feng, P.; Zhao, Z.; Deng, Y. Hybridization of graphene oxide and mesoporous bioactive glass: Micro-space network structure enhance polymer scaffold. J. Mech. Behav. Biomed. Mater. 2020, 109, 103827. [Google Scholar] [CrossRef]
- Cao, Y.; Yang, B.; Gao, C.; Feng, P.; Shuai, C. Laser sintering of nano 13-93 glass scaffolds: Microstructure, mechanical properties and bioactivity. Sci. Sinter. 2015, 47, 31–39. [Google Scholar] [CrossRef]
- Liu, J.; Hu, H.; Li, P.; Shuai, C.; Peng, S. Fabrication and Characterization of Porous 45S5 Glass Scaffolds via Direct Selective Laser Sintering. Mater. Manuf. Process. 2013, 28, 610–615. [Google Scholar] [CrossRef]
- Gao, C.; Liu, T.; Shuai, C.; Peng, S. Enhancement mechanisms of graphene in nano-58S bioactive glass scaffold: Mechanical and biological performance. Sci. Rep. 2014, 4, 4712. [Google Scholar] [CrossRef]
- Filho, O.P.; La Torre, G.P.; Hench, L.L. Effect of crystallization on apatite-layer formation of bioactive glass 45S5. J. Biomed. Mater. Res. 1996, 30, 509–514. [Google Scholar] [CrossRef]
- El-Ghannam, A.; Hamazawy, E.; Yehia, A. Effect of thermal treatment on bioactive glass microstructure, corrosion behavior, ζ potential, and protein adsorption. J. Biomed. Mater. Res. 2001, 55, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Fagerlund, S.; Hupa, L. Melt-derived Bioactive Silicate Glasses. In Bioactive Glasses: Fundamentals, Technology and Applications; Boccaccini, A.R., Brauer, D.S., Hupa, L., Eds.; The Royal Society of Chemistry: London, UK, 2016. [Google Scholar]
- Fagerlund, S.; Massera, J.; Hupa, M.; Hupa, L. T–T–T behaviour of bioactive glasses 1–98 and 13–93. J. Eur. Ceram. Soc. 2012, 32, 2731–2738. [Google Scholar] [CrossRef]
- Hupa, L.; Karlsson, K.H. Tailoring of Bioactive Glasses. In Bioactive Glasses: Fundamentals, Technology and Applications; Boccaccini, A.R., Brauer, D.S., Hupa, L., Eds.; The Royal Society of Chemistry: London, UK, 2016. [Google Scholar]
- Rodrigo-Vázquez, C.S.; Kamboj, N.; Aghayan, M.; Sáez, A.; De Aza, A.H.; Rodríguez, M.A.; Hussainova, I. Manufacturing of silicon–Bioactive glass scaffolds by selective laser melting for bone tissue engineering. Ceram. Int. 2020, 46, 26936–26944. [Google Scholar] [CrossRef]
- Sara Rodrigo-Vázquez, C.; Rodríguez, M.A.; De Aza, A.H. Devitrification study of a novel bioactive glass designed on the CaSiO3–Ca3(PO4)2–MgCa(SiO3)2 system. J. Non-Cryst. Solids 2020, 528, 119705. [Google Scholar] [CrossRef]
- Han, J.; Wu, J.; Xiang, X.; Xie, L.; Chen, R.; Li, L.; Ma, K.; Sun, Q.; Yang, R.; Huang, T.; et al. Biodegradable BBG/PCL composite scaffolds fabricated by selective laser sintering for directed regeneration of critical-sized bone defects. Mater. Des. 2023, 225, 111543. [Google Scholar] [CrossRef]
- Wei, W.; Dai, H. Articular cartilage and osteochondral tissue engineering techniques: Recent advances and challenges. Bioact. Mater. 2021, 6, 4830–4855. [Google Scholar] [CrossRef]
- Wang, M.; Li, H.; Yang, Y.; Yuan, K.; Zhou, F.; Liu, H.; Zhou, Q.; Yang, S.; Tang, T. A 3D-bioprinted scaffold with doxycycline-controlled BMP2-expressing cells for inducing bone regeneration and inhibiting bacterial infection. Bioact. Mater. 2021, 6, 1318–1329. [Google Scholar] [CrossRef]
- Tsai, W.B.; Ting, Y.C.; Yang, J.Y.; Lai, J.Y.; Liu, H.L. Fibronectin modulates the morphology of osteoblast-like cells (MG-63) on nano-grooved substrates. J. Mater. Sci. Mater. Med. 2009, 20, 1367–1378. [Google Scholar] [CrossRef]
- Kotz, F.; Quick, A.S.; Risch, P.; Martin, T.; Hoose, T.; Thiel, M.; Helmer, D.; Rapp, B.E. Two-Photon Polymerization of Nanocomposites for the Fabrication of Transparent Fused Silica Glass Microstructures. Adv. Mater. 2021, 33, e2006341. [Google Scholar] [CrossRef]
- Wen, X.; Zhang, B.; Wang, W.; Ye, F.; Yue, S.; Guo, H.; Gao, G.; Zhao, Y.; Fang, Q.; Nguyen, C.; et al. 3D-printed silica with nanoscale resolution. Nat. Mater. 2021, 20, 1506–1511. [Google Scholar] [CrossRef]
- Bae, C.-J.; Halloran, J.W. Influence of Residual Monomer on Cracking in Ceramics Fabricated by Stereolithography. Int. J. Appl. Ceram. Technol. 2011, 8, 1289–1295. [Google Scholar] [CrossRef]
- Masia, S.; Calvert, P.D.; Rhine, W.E.; Bowen, H.K. Effect of oxides on binder burnout during ceramics processing. J. Mater. Sci. 1989, 24, 1907–1912. [Google Scholar] [CrossRef]
- Zaki, R.M.; Strutynski, C.; Kaser, S.; Bernard, D.; Hauss, G.; Faessel, M.; Sabatier, J.; Canioni, L.; Messaddeq, Y.; Danto, S.; et al. Direct 3D-printing of phosphate glass by fused deposition modeling. Mater. Des. 2020, 194, 108957. [Google Scholar] [CrossRef]
- Liu, C.; Oriekhov, T.; Fokine, M. Investigation of glass bonding and multi-layer deposition during filament-based glass 3D printing. Front. Mater. 2022, 9, 978861. [Google Scholar] [CrossRef]
- Klein, J.; Stern, M.; Franchin, G.; Kayser, M.; Inamura, C.; Dave, S.; Weaver, J.C.; Houk, P.; Colombo, P.; Yang, M.; et al. Additive Manufacturing of Optically Transparent Glass. 3D Print. Addit. Manuf. 2015, 2, 92–105. [Google Scholar] [CrossRef]
- Spirrett, F.; Datsiou, K.C.; Magallanes, M.; Ashcroft, I.; Goodridge, R. Powder-fed directed energy deposition of soda lime silica glass on glass substrates. J. Am. Ceram. Soc. 2022, 106, 227–240. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, Y.; Zhang, W.; Wang, H.; Li, J.; Pan, L.; Han, F.; Li, B. Biomimetic periosteum-bone substitute composed of preosteoblast-derived matrix and hydrogel for large segmental bone defect repair. Acta Biomater. 2020, 113, 317–327. [Google Scholar] [CrossRef]
- Niu, X.; Li, N.; Du, Z.; Li, X. Integrated gradient tissue-engineered osteochondral scaffolds: Challenges, current efforts and future perspectives. Bioact. Mater. 2023, 20, 574–597. [Google Scholar] [CrossRef]
- Smith, L.; Xia, Y.; Galatz, L.M.; Genin, G.M.; Thomopoulos, S. Tissue-engineering strategies for the tendon/ligament-to-bone insertion. Connect. Tissue Res. 2012, 53, 95–105. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, P.; Li, Q.; Li, J.; Li, X.; Liu, X.; Kang, Y.; Ren, L. Engineering biomimetic periosteum with beta-TCP scaffolds to promote bone formation in calvarial defects of rats. Stem. Cell Res. 2017, 8, 134. [Google Scholar] [CrossRef]
- Li, Y.; Jahr, H.; Pavanram, P.; Bobbert, F.S.L.; Paggi, U.; Zhang, X.Y.; Pouran, B.; Leeflang, M.A.; Weinans, H.; Zhou, J.; et al. Additively manufactured functionally graded biodegradable porous iron. Acta Biomater. 2019, 96, 646–661. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Shanjani, Y.; Fazeli, S.; Behn, A.W.; Okuzu, Y.; Goodman, S.B.; Yang, Y.P. Customized, degradable, functionally graded scaffold for potential treatment of early stage osteonecrosis of the femoral head. J. Orthop. Res. 2018, 36, 1002–1011. [Google Scholar] [CrossRef]
- Loh, G.H.; Pei, E.; Harrison, D.; Monzón, M.D. An overview of functionally graded additive manufacturing. Addit. Manuf. 2018, 23, 34–44. [Google Scholar] [CrossRef]
- Xu, H.; Zhu, Y.; Hsiao, A.W.; Xu, J.; Tong, W.; Chang, L.; Zhang, X.; Chen, Y.F.; Li, J.; Chen, W.; et al. Bioactive glass-elicited stem cell-derived extracellular vesicles regulate M2 macrophage polarization and angiogenesis to improve tendon regeneration and functional recovery. Biomaterials 2023, 294, 121998. [Google Scholar] [CrossRef]
- Gogele, C.; Wiltzsch, S.; Lenhart, A.; Civilleri, A.; Weiger, T.M.; Schafer-Eckart, K.; Minnich, B.; Forchheimer, L.; Hornfeck, M.; Schulze-Tanzil, G. Highly porous novel chondro-instructive bioactive glass scaffolds tailored for cartilage tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 130, 112421. [Google Scholar] [CrossRef]






| Materials | Category | BG Weight Fraction | Structural Properties (D = Dense, P = Porous) | Fabrication Methods | Maximum Stress (MPa) | Elastic Modulus (MPa) | Strain at Maximum Stress (%) | Significance as Biomaterials for Bone Tissue Engineering | Refs. |
|---|---|---|---|---|---|---|---|---|---|
| Cortical Bones | Native Tissue | - | - | - | 120–240 (C, human femurs and tibias) | 10,000–22,000 (C, human femurs and tibias) | - | - | [102] |
| Trabecular Bones | Native Tissue | - | - | - | 13.57 ± 3.1 (C, human femoral head) 1.6–4.5 (C, human mandibular condyle) | 876.8 ± 331.6 (C, human femoral head) 127–431 (C, human mandibular condyle) | - | - | [103,104] |
| Cartilage (human patellofemoral groove) | Native Tissue | - | - | - | - | 0.581 ± 0.168 (normal to the articular surface) 0.854 ± 0.348 (parallel to the articular surface) | - | - | [105] |
| 45S5 | Glass | 100 | D | Melt casting | 500 (C) 42 (T) | 60,000 (C) | - | - | [106,107] |
| Phosphate-based Glass Fiber | Glass | 100 | D | Melt drawing | 1021–1253 (T) | 59,000–62,000 (T) | ~2 |
| [108] |
| Bioglass®/PCL | Glass/Polymer Composites | 5 |
| Porogen leaching | 0.12 ± 0.02 (C, yield strength) | 1.15 ± 0.32 (C) | - |
| [109] |
| BPSG (Si80-P5-Ca15)/PLLA | Glass/Polymer Composites | 30 |
| Porogen leaching | 4.2 ± 2 (C) | 81 ± 4 (C) | - |
| [110] |
| Bioglass®/PDLLA | Glass/Polymer Composites | 30 |
| TIPS | 0.06 ± 0.03 (C) | 2 ± 1 (C) | - |
| [111] |
| Phosphate-based BG (P50-Ca40-Ti10)/PLLA | Glass/Polymer Composites | 30 |
| Solid-state gas foaming | ~1.2 (C) | 6.19 ± 0.45 (F) | - |
| [112] |
| MBG (Si70-Ca30)/PLLA | Glass/Polymer Composites | 10 |
| AM-SLS | 1.5 (C) | 25 (C) | ~18 (C) |
| [113] |
| 6P53B (Si-based BG) | Glass | 100 |
| AM-DIW | 136 (C, parallel to pore channels) 55 (C, vertical to pore channels) | ~2000 (C) | - |
| [114] |
| 13–93 (Si-based BG) | Glass | 100 |
| AM-DIW | 86 ± 9 (C) | 13,000 ± 2000 (C) | ~0.8 (C) |
| [115] |
| MBG (Si80-P5-Ca15)/PVA | Glass/Polymer Composites | 86 |
| AM-DIW | 16.1 ± 1.53 (C) | 155.13 ± 14.89 (C) | ~11 (C) |
| [116] |
| BG/PACG-GelMA (BG: Si27-B27-P2-Na6-Mg8-K8-Ca16-Sr6) | Glass/Polymer Composites | 1 |
| AM-DIW | 2.51 (C) | 0.249 (C) | ~90 (C) |
| [117] |
| 45S5/PCL | Glass/Polymer Composites | 20 |
| AM-FDM | 2.99 ± 0.63 (yield stress) | 46 ± 4 (C) | - |
| [118] |
| MBG (Si85-P5-Ca15) | Glass | 100 |
| AM-DIW or polymer foam templating | ~2.5 (C, AM) ~1.5 (C, foam) | - | ~0.75 (C, AM) ~0.65 (C, foam) |
| [119] |
| 45S5/PLA | Glass/Polymer Composites | 1 |
| AM-FDM | 12 ± 4 (C) | 700 ± 100 (C) | ~11 (C) |
| [120] |
| MBG + Ga (NO3)3/PCL | Glass/Polymer Composites | 30 |
| AM-DIW | 6.96 ± 1.58 (C) | 79.82 ± 16.03 (C) | - |
| [121] |
| 45S5 (Partially crystallized) | Glass–Ceramic | 100 |
| AM-DLP | 6.8–22.5 (C) | - | 2.5–4.5 (C) |
| [122] |
| 13–93 | Glass | 100 |
| AM-DIW | 86 ± 4 (C) | 16,000 ± 4000 (F) | ~3 (C) |
| [76] |
| 45S5 (microparticles)/silk fibroin | Glass/Polymer Composites | 20 w/v% in feedstock |
| Cast onto additive-manufactured polymer template | 1.21 ± 0.08 (C) | 10.35 ± 0.62 (C) | - |
| [123] |
| Silver-doped BG (Si58.6-P7.2-Na1.5-Al4.2-K1.5-Ca24.9-Ag2.1, partially crystallized) | Glass–Ceramic | 100 |
| AM-FDM followed by thermal debinding | 2.84 ± 0.75 (C) | 110 ± 60 (C) | ~3 (C) |
| [124] |
| Silver-doped MBG/PLLA | Glass/Polymer Composites | 29 |
| AM-SLS | 15.91 (C) | 1204.9 (C) | ~11 (C) |
| [125] |
| 45S5/PCL | Glass–Ceramic | 100 |
| AM-FDM | 9.16 (C, yield stress) | 67.4 ± 0.54 (C) | - |
| [126] |
| Phosphate-based BG fibers + MgO/PLA (BG: P48-B12-Na1-Mg17-Ca14-Fe8) | Glass/Polymer Composites | 18 |
| AM-FDM | 17.59 ± 3.75 (C) | 648.14 ± 81.12 (C) | ~7 (C) |
| [127] |
| 13–93/sodium alginate | Glass/Polymer Composites | 33 |
| AM-DIW | 16.74 ± 1.78 (C) | 79.49 ± 7.38 (C) | ~70 (C) |
| [128] |
| 58S/PLDLA | Glass/Polymer Composites | 10 |
| AM-SLS | 2.4 ± 0.6 (F) | 79 ± 24 (F) | 6.9 ± 3.9 (F) |
| [129] |
| Copper- and magnesium-doped BG (Si54-Ca22-P2-K8-Na6-Mg7-Cu1) | Glass | 100 |
| AM-DIW | 109.27 ± 8.18 (C) | - | - |
| [130] |
| AM Technology | Feedstock | Advantages | Limitations | Refs. |
|---|---|---|---|---|
| Melt extrusion (FDM) | A solid powdery mixture or composite filaments of BG particles and thermoplastic polymer |
|
| [37,120,138,139] |
| Direct ink writing (including bioprinting) | Liquid ink homogenized with BG particles |
|
| [117,119,123,162] |
| Vat photopolymerization (e.g., SLA and DLP) | Liquid photopolymerizable resin homogenized with BG particles |
|
| [165,172] |
| Powder Bed Fusion (e.g., SLS) | Solid powders of mixtures or a composite of BG particles and thermoplastic polymer |
|
| [125,178,188] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, L.; Yin, J.; Gao, X. Additive Manufacturing of Bioactive Glass and Its Polymer Composites as Bone Tissue Engineering Scaffolds: A Review. Bioengineering 2023, 10, 672. https://doi.org/10.3390/bioengineering10060672
He L, Yin J, Gao X. Additive Manufacturing of Bioactive Glass and Its Polymer Composites as Bone Tissue Engineering Scaffolds: A Review. Bioengineering. 2023; 10(6):672. https://doi.org/10.3390/bioengineering10060672
Chicago/Turabian StyleHe, Lizhe, Jun Yin, and Xiang Gao. 2023. "Additive Manufacturing of Bioactive Glass and Its Polymer Composites as Bone Tissue Engineering Scaffolds: A Review" Bioengineering 10, no. 6: 672. https://doi.org/10.3390/bioengineering10060672
APA StyleHe, L., Yin, J., & Gao, X. (2023). Additive Manufacturing of Bioactive Glass and Its Polymer Composites as Bone Tissue Engineering Scaffolds: A Review. Bioengineering, 10(6), 672. https://doi.org/10.3390/bioengineering10060672