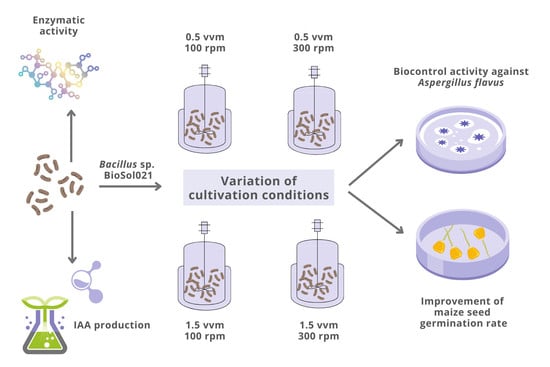
The Effect of Cultivation Conditions on Antifungal and Maize Seed Germination Activity of Bacillus-Based Biocontrol Agent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Producing Microorganism and Phytopathogens
2.2. Screening of Plant Growth Promotion Traits
2.2.1. Protease Activity Screening
2.2.2. Cellulase and Xylanase Activity Screening
2.2.3. Pectinase Activity Screening
2.2.4. Indole Compounds Production
2.3. Media Composition, Cultivation Conditions, and Bioprocess Monitoring
2.4. Antimicrobial Activity Assay
2.5. Seed Germination Activity Assay
2.6. Bioprocess Kinetics Analysis
2.7. Experimental Data Analysis
3. Results
3.1. PGP Traits of the Producing Strain Bacillus sp. BioSol021
3.2. Microbial Growth Kinetics
3.3. Cultivation Course of Bacillus sp. BioSol021—Oxygen Saturation Level, pH Value, and Residual Nutrients (Cellulose, Total Nitrogen) Content
3.4. Antimicrobial Activity of Bacillus sp. BioSol021 Cultivation Broth and Cell-Free Supernatant Samples against Aflatoxigenic A. flavus Isolates
3.5. Maize Seed Germination Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gizaw, Z. Public Health Risks Related to Food Safety Issues in the Food Market: A Systematic Literature Review. Environ. Health Prev. Med. 2019, 24, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vlajkov, V.; Grahovac, M.; Budakov, D.; Loc, M.; Pajčin, I.; Milić, D.; Novaković, T.; Grahovac, J. Distribution, Genetic Diversity and Biocontrol of Aflatoxigenic Aspergillus flavus in Serbian Maize Fields. Toxins 2021, 13, 687. [Google Scholar] [CrossRef]
- Moretti, A.; Pascale, M.; Logrieco, A.F. Mycotoxin Risks under a Climate Change Scenario in Europe. Trends Food Sci. Technol. 2019, 84, 38–40. [Google Scholar] [CrossRef]
- Savić, Z.; Dudaš, T.; Loc, M.; Grahovac, M.; Budakov, D.; Jajić, I.; Krstović, S.; Barošević, T.; Krska, R.; Sulyok, M.; et al. Biological Control of Aflatoxin in Maize Grown in Serbia. Toxins 2020, 12, 162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbas, H.K.; Wilkinson, J.R.; Zablotowicz, R.M.; Accinelli, C.; Abel, C.A.; Bruns, H.A.; Weaver, M.A. Ecology of Aspergillus Flavus, Regulation of Aflatoxin Production, and Management Strategies to Reduce Aflatoxin Contamination of Corn. Toxin Rev. 2009, 28, 142–153. [Google Scholar] [CrossRef]
- Aloo, B.N.; Makumba, B.A.; Mbega, E.R. The Potential of Bacilli Rhizobacteria for Sustainable Crop Production and Environmental Sustainability. Microbiol. Res. 2019, 219, 26–39. [Google Scholar] [CrossRef]
- Ongena, M.; Jacques, P. Bacillus Lipopeptides: Versatile Weapons for Plant Disease Biocontrol. Trends Microbiol. 2008, 16, 115–125. [Google Scholar] [CrossRef]
- Leconte, A.; Tournant, L.; Muchembled, J.; Paucellier, J.; Héquet, A.; Deracinois, B.; Deweer, C.; Krier, F.; Deleu, M.; Oste, S.; et al. Assessment of Lipopeptide Mixtures Produced by Bacillus subtilis as Biocontrol Products against Apple Scab (Venturia inaequalis). Microorganisms 2022, 10, 1810. [Google Scholar] [CrossRef]
- Platel, R.; Sawicki, M.; Esmaeel, Q.; Randoux, B.; Trapet, P.; El Guilli, M.; Chtaina, N.; Arnauld, S.; Bricout, A.; Rochex, A.; et al. Isolation and Identification of Lipopeptide-Producing Bacillus velezensis Strains from Wheat Phyllosphere with Antifungal Activity against the Wheat Pathogen Zymoseptoria tritici. Agronomy 2022, 12, 95. [Google Scholar] [CrossRef]
- He, C.-N.; Ye, W.-Q.; Zhu, Y.-Y.; Zhou, W.-W. Antifungal Activity of Volatile Organic Compounds Produced by Bacillus methylotrophicus and Bacillus thuringiensis against Five Common Spoilage Fungi on Loquats. Molecules 2020, 25, 3360. [Google Scholar] [CrossRef]
- Lahlali, R.; Ezrari, S.; Radouane, N.; Kenfaoui, J.; Esmaeel, Q.; El Hamss, H.; Belabess, Z.; Barka, E.A. Biological Control of Plant Pathogens: A Global Perspective. Microorganisms 2022, 10, 596. [Google Scholar] [CrossRef]
- Choub, V.; Won, S.-J.; Ajuna, H.B.; Moon, J.-H.; Choi, S.-I.; Lim, H.-I.; Ahn, Y.S. Antifungal Activity of Volatile Organic Compounds from Bacillus velezensis CE 100 against Colletotrichum gloeosporioides. Horticulturae 2022, 8, 557. [Google Scholar] [CrossRef]
- Stamenković, S.; Beškoski, V.; Karabegović, I.; Lazić, M.; Nikolić, N. Microbial Fertilizers: A Comprehensive Review of Current Findings and Future Perspectives. Span. J. Agric. Res. 2018, 16, e09R01. [Google Scholar] [CrossRef] [Green Version]
- Vejan, P.; Abdullah, R.; Khadiran, T.; Ismail, S.; Nasrulhaq Boyce, A. Role of Plant Growth Promoting Rhizobacteria in Agricultural Sustainability-A Review. Molecules 2016, 21, 573. [Google Scholar] [CrossRef] [Green Version]
- Shafi, J.; Tian, H.; Ji, M. Bacillus Species as Versatile Weapons for Plant Pathogens: A Review. Biotechnol. Biotechnol. Equip. 2017, 31, 446–459. [Google Scholar] [CrossRef] [Green Version]
- Cardarelli, M.; Woo, S.L.; Rouphael, Y.; Colla, G. Seed Treatments with Microorganisms Can Have a Biostimulant Effect by Influencing Germination and Seedling Growth of Crops. Plants 2022, 11, 259. [Google Scholar] [CrossRef]
- Miljaković, D.; Marinković, J.; Balešević-Tubić, S. The Significance of Bacillus spp. In Disease Suppression and Growth Promotion of Field and Vegetable Crops. Microorganisms 2020, 8, 1037. [Google Scholar] [CrossRef]
- Crater, J.S.; Lievense, J.C. Scale-up of Industrial Microbial Processes. FEMS Microbiol. Lett. 2018, 365, fny138. [Google Scholar] [CrossRef]
- Dmitrović, S.; Pajčin, I.; Vlajkov, V.; Grahovac, M.; Jokić, A.; Grahovac, J. Dairy and Wine Industry Effluents as Alternative Media for the Production of Bacillus-Based Biocontrol Agents. Bioengineering 2022, 9, 663. [Google Scholar] [CrossRef]
- Grahovac, J.; Pajcin, I.; Vlajkov, V.; Roncevic, Z.; Dodic, J.; Cvetkovic, D.; Jokic, A. Xanthomonas campestris Biocontrol Agent: Selection, Medium Formulation and Bioprocess Kinetic Analysis. Chem. Ind. Chem. Eng. Q. 2020, 27, 131–142. [Google Scholar] [CrossRef]
- Montesinos, E. Development, Registration and Commercialization of Microbial Pesticides for Plant Protection. Int. Microbiol. 2003, 6, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Baras, J.; Veljković, V.; Popov, S.; Povrenović, D.; Lazić, M.; Zlatković, B. Osnovi Bioprocesnog Inženjerstva; Tehnološki Fakultet: Leskovac, Serbia, 2009; p. 218. [Google Scholar]
- Mitrović, I.; Grahovac, J.A.; Dodić, J.M.; Grahovac, M.S.; Dodić, S.N.; Vučurović, D.G.; Vlajkov, V.R. Effect of Agitation Rate on the Production of Antifungal Metabolites by Streptomyces hygroscopicus in a Lab-Scale Bioreactor. Acta Period. Technol. 2017, 48, 231–244. [Google Scholar] [CrossRef]
- Zhou, Y.; Han, L.R.; He, H.W.; Sang, B.; Yu, D.L.; Feng, J.T.; Zhang, X. Effects of Agitation, Aeration and Temperature on Production of a Novel Glycoprotein Gp-1 by Streptomyces kanasenisi Zx01 and Scale-up Based on Volumetric Oxygen Transfer Coefficient. Molecules 2018, 23, 125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahajan, P.M.; Gokhale, S.V.; Lele, S.S. Production of Nattokinase Using Bacillus natto NRRL 3666: Media Optimization, Scale Up, and Kinetic Modeling. Food Sci. Biotechnol. 2010, 19, 1593–1603. [Google Scholar] [CrossRef]
- Bonaterra, A.; Badosa, E.; Daranas, N.; Francés, J.; Roselló, G.; Montesinos, E. Bacteria as Biological Control Agents of Plant Diseases. Microorganisms 2022, 10, 1759. [Google Scholar] [CrossRef]
- Pajčin, I.; Vlajkov, V.; Dodić, J.; Loc, M.; Grahovac, M.; Grahovac, J. Bacillus velezensis—Biocontrol activity of cells and extracellular compounds against Xanthomonas spp. J. Process. Energy Agric 2022, 25, 15–18. [Google Scholar] [CrossRef]
- Pla, M.L.; Oltra, S.; Esteban, M.D.; Andreu, S.; Palop, A. Comparison of Primary Models to Predict Microbial Growth by the Plate Count and Absorbance Methods. Biomed Res. Int. 2015, 2015, 365025. [Google Scholar] [CrossRef] [Green Version]
- Dmitrović, S.; Pajčin, I.; Lukić, N.; Vlajkov, V.; Grahovac, M.; Grahovac, J.; Jokić, A. Taguchi Grey Relational Analysis for Multi-Response Optimization of Bacillus Bacteria Flocculation Recovery from Fermented Broth by Chitosan to Enhance Biocontrol Efficiency. Polymers 2022, 14, 3282. [Google Scholar] [CrossRef]
- Adinarayana, K.; Ellaiah, P.; Prasad, D.S. Purification and partial characterization of thermostable serine alkaline protease from a newly isolated Bacillus subtilis PE-11. AAPS PharmSciTech 2003, 4, E56. [Google Scholar] [CrossRef] [Green Version]
- Amore, A.; Parameswaran, B.; Kumar, R.; Birolo, L.; Vinciguerra, R.; Marcolongo, L.; Ionata, E.; La Cara, F.; Pandey, A.; Faraco, V. Application of a New Xylanase Activity from Bacillus amyloliquefaciens XR44A in Brewer’s Spent Grain Saccharification. J. Chem. Technol. Biotechnol. 2015, 90, 573–581. [Google Scholar] [CrossRef]
- Mohandas, A.; Raveendran, S.; Parameswaran, B.; Abraham, A.; Athira, R.S.R.; Mathew, A.K.; Pandey, A. Production of Pectinase from Bacillus Sonorensis MPTD1. Food Technol. Biotechnol. 2018, 56, 110–116. [Google Scholar] [CrossRef]
- Syed Ab Rahman, S.F.; Singh, E.; Pieterse, C.M.J.; Schenk, P.M. Emerging Microbial Biocontrol Strategies for Plant Pathogens. Plant Sci. 2018, 267, 102–111. [Google Scholar] [CrossRef] [Green Version]
- Malbaša, R.; Vitas, J.; Vukmanović, S. Analytical Chemistry: A Practicum with a Workbook; University of Technology Novi Sad: Novi Sad, Serbia, 2021. [Google Scholar]
- Cunniff, P. Association of Official Analytical Chemists. Official Methods of Analysis of AOAC International, 16th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 1995; Available online: http://www.worldcat.org/oclc/421897987 (accessed on 5 October 2022).
- Vlajkov, V.; Anđelić, S.; Pajčin, I.; Grahovac, M.; Budakov, D.; Jokić, A.; Grahovac, J. Medium for the Production of Bacillus-Based Biocontrol Agent Effective against Aflatoxigenic Aspergillus flavus: Dual Approach for Modelling and Optimization. Microorganisms 2022, 10, 1165. [Google Scholar] [CrossRef]
- Caballero, P.; Macías-Benítez, S.; Moya, A.; Rodríguez-Morgado, B.; Martín, L.; Tejada, M.; Castaño, A.; Parrado Rubio, J. Biochemical and Microbiological Soil Effects of a Biostimulant Based on Bacillus Licheniformis-Fermented Sludge. Agronomy 2022, 12, 1743. [Google Scholar] [CrossRef]
- Shahid, I.; Han, J.; Hanooq, S.; Malik, K.A.; Borchers, C.H.; Mehnaz, S. Profiling of Metabolites of Bacillus spp. and Their Application in Sustainable Plant Growth Promotion and Biocontrol. Front. Sustain. Food Syst. 2021, 5, 605195. [Google Scholar] [CrossRef]
- Raddadi, N.; Cherif, A.; Boudabous, A.; Daffonchio, D. Screening of Plant Growth Promoting Traits of Bacillus Thuringiensis. Ann. Microbiol. 2008, 58, 47–52. [Google Scholar] [CrossRef]
- Ali, Q.; Ayaz, M.; Mu, G.; Hussain, A.; Yuanyuan, Q.; Yu, C.; Xu, Y.; Manghwar, H.; Gu, Q.; Wu, H.; et al. Revealing Plant Growth-Promoting Mechanisms of Bacillus Strains in Elevating Rice Growth and Its Interaction with Salt Stress. Front. Plant Sci. 2022, 13, 994902. [Google Scholar] [CrossRef]
- Sharma, A.; Kashyap, P.L.; Srivastava, A.K.; Bansal, Y.K.; Kaushik, R. Isolation and characterization of halotolerant bacilli from chickpea (Cicer arietinum L.) rhizosphere for plant growth promotion and biocontrol traits. Eur. J. Plant Pathol. 2019, 153, 787–800. [Google Scholar] [CrossRef]
- Idris, E.S.E.; Iglesias, D.J.; Talon, M.; Borriss, R. Tryptophan-Dependent Production of Indole-3-Acetic Acid (IAA) Affects Level of Plant Growth Promotion by Bacillus amyloliquefaciens FZB42. Mol. Plant-Microbe Interact. 2007, 20, 619–626. [Google Scholar] [CrossRef] [Green Version]
- Asari, S.; Tarkowská, D.; Rolčík, J.; Novák, O.; Palmero, D.V.; Bejai, S.; Meijer, J. Analysis of Plant Growth-Promoting Properties of Bacillus amyloliquefaciens UCMB5113 Using Arabidopsis Thaliana as Host Plant. Planta 2017, 245, 15–30. [Google Scholar] [CrossRef]
- Khan, M.S.; Gao, J.; Chen, X.; Zhang, M.; Yang, F.; Du, Y.; Moe, T.S.; Munir, I.; Xue, J.; Zhang, X. The Endophytic Bacteria Bacillus velezensis Lle-9, Isolated from Lilium Leucanthum, Harbors Antifungal Activity and Plant Growth-Promoting Effects. J. Microbiol. Biotechnol. 2020, 30, 668–680. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.K.; Kumar, M.; Chakdar, H.; Anuroopa, N.; Bagyaraj, D.J. Bacillus Species in Soil as a Natural Resource for Plant Health and Nutrition. J. Appl. Microbiol. 2020, 128, 1583–1594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, N.; Martínez-Hidalgo, P.; Ice, T.A.; Maymon, M.; Humm, E.A.; Nejat, N.; Sanders, E.R.; Kaplan, D.; Hirsch, A.M. Antifungal Activity of Bacillus Species against Fusarium and Analysis of the Potential Mechanisms Used in Biocontrol. Front. Microbiol. 2018, 9, 2363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pathak, E.; Sanjyal, A.; Regmi, C.R.; Paudel, S.; Shrestha, A. Screening of Potential Plant Growth Promoting Properties of Bacillus Species Isolated from Different Regions of Nepal. Nepal J. Biotechnol. 2021, 9, 79–84. [Google Scholar] [CrossRef]
- Suberu, Y.; Akande, I.; Samuel, T.; Lawal, A.; Olaniran, A. Optimization of Protease Production in Indigenous Bacillus Species Isolated from Soil Samples in Lagos, Nigeria Using Response Surface Methodology. Biocatal. Agric. Biotechnol. 2019, 18, 101011. [Google Scholar] [CrossRef]
- Pertiwiningrum, A.; Anggraini, F.D.; Fitrianto, N.A.; Rochijan, R. Isolation and Identification of Bacterial Protease Enzyme of Leather Waste. J. Indones. Trop. Anim. Agric. 2017, 42, 33–41. [Google Scholar] [CrossRef] [Green Version]
- Alnahdi, H.S. Isolation and Screening of Extracellular Proteases Produced by New Isolated Bacillus Sp. J. Appl. Pharm. Sci. 2012, 2, 71–74. [Google Scholar] [CrossRef] [Green Version]
- Rz, S. Hydrolytic Enzymes of Rhizospheric Microbes in Crop Protection. MOJ Cell Sci. Rep. 2016, 3, 135–136. [Google Scholar] [CrossRef]
- Gaur, R.; Tiwari, S. Isolation, production, purification and characterization of an organic-solvent-thermostable alkalophilic cellulase from Bacillus vallismortis RG-07. BMC Biotechnol. 2015, 15, 19. [Google Scholar] [CrossRef] [Green Version]
- Balla, A.; Silini, A.; Cherif-Silini, H.; Bouket, A.C.; Boudechicha, A.; Luptakova, L.; Alenezi, F.N.; Belbahri, L. Screening of Cellulolytic Bacteria from Various Ecosystems and Their Cellulases Production under Multi-Stress Conditions. Catalysts 2022, 12, 769. [Google Scholar] [CrossRef]
- Torimiro, N.; Okonji, E.R. A Comparative Study of Pectinolytic Enzyme Production by Bacillus Species. African J. Biotechnol. 2013, 12, 6498–6503. [Google Scholar] [CrossRef] [Green Version]
- Alqahtani, Y.S.; More, S.S.; R., K.; Shaikh, I.A.; K.J., A.; More, V.S.; Niyonzima, F.N.; Muddapur, U.M.; Khan, A.A. Production and Purification of Pectinase from Bacillus subtilis 15A-B92 and Its Biotechnological Applications. Molecules 2022, 27, 4195. [Google Scholar] [CrossRef]
- Kabir, M.S.; Tasmim, T. Isolation of Pectinase Producing Bacteria from the Rhizosphere of Andrographis paniculata Nees and 16S RRNA Gene Sequence Comparison of Some Potential Strains. Adv. Microbiol. 2019, 9, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Oumer, O.J.; Abate, D. Screening and Molecular Identification of Pectinase Producing Microbes from Coffee Pulp. Biomed. Res. Int. 2018, e2961767. [Google Scholar] [CrossRef]
- Ammoneh, H.; Harba, M.; Akeed, Y.; Al-Halabi, M.; Bakri, Y. Isolation and Identification of Local Bacillus Isolates for Xylanase Biosynthesis. Iran. J. Microbiol. 2014, 6, 127–132. [Google Scholar]
- Tamariz-Angeles, C.; Lázaro-Palomino, J.; Olivera-Gonzales, P.; Castañeda-Barreto, A.; Villena, G.K. Isolation of Thermotolerant Bacillus Subtilis DCH4 From The Chancos Hot Spring (Carhuaz, Peru) With Potential To Degrade Agricultural Lignocellulosic Residues. Peruv. J. Biol. 2020, 27, 67–78. [Google Scholar] [CrossRef] [Green Version]
- Mohite, B. Isolation and Characterization of Indole Acetic Acid (IAA) Producing Bacteria from Rhizospheric Soil and Its Effect on Plant Growth. J. Soil Sci. Plant Nutr. 2013, 13, 638–649. [Google Scholar] [CrossRef]
- Wagi, S.; Ahmed, A. Bacillus Spp.: Potent Microfactories of Bacterial IAA. PeerJ 2019, 7, e7258. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.A.; Hamayun, M.; Asaf, S.; Khan, M.; Yun, B.-W.; Kang, S.-M.; Lee, I.-J. Rhizospheric Bacillus spp. Rescues Plant Growth Under Salinity Stress via Regulating Gene Expression, Endogenous Hormones, and Antioxidant System of Oryza sativa L. Front. Plant Sci. 2021, 12, 665590. [Google Scholar] [CrossRef]
- Kumar, P.; Dubey, R.C.; Maheshwari, D.K. Bacillus Strains Isolated from Rhizosphere Showed Plant Growth Promoting and Antagonistic Activity against Phytopathogens. Microbiol. Res. 2012, 167, 493–499. [Google Scholar] [CrossRef]
- Reetha, S.; Bhuvaneswari, G.; Thamizhiniyan, P.; Mycin, T.R. Isolation of Indole Acetic Acid (IAA) Producing Rhizobacteria of Pseudomonas Fluorescens and Bacillus Subtilis and Enhance Growth of Onion (Allium Cepa L.). Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 568–574. [Google Scholar]
- Grover, M.; Bodhankar, S.; Sharma, A.; Sharma, P.; Singh, J.; Nain, L. PGPR Mediated Alterations in Root Traits: Way Toward Sustainable Crop Production. Front. Sustain. Food Syst. 2021, 4, 287. [Google Scholar] [CrossRef]
- Wu, R.; Chen, G.; Pan, S.; Zeng, J.; Liang, Z. Cost-Effective Fibrinolytic Enzyme Production by Bacillus subtilis WR350 Using Medium Supplemented with Corn Steep Powder and Sucrose. Sci. Rep. 2019, 9, 6824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shih, I.L.; Lin, C.Y.; Wu, J.Y.; Hsieh, C. Production of Antifungal Lipopeptide from Bacillus subtilis in Submerged Fermentation Using Shake Flask and Fermentor. Korean J. Chem. Eng. 2009, 26, 1652–1661. [Google Scholar] [CrossRef]
- Pajčin, I.; Vlajkov, V.; Frohme, M.; Grebinyk, S.; Grahovac, M.; Mojićević, M.; Grahovac, J. Pepper Bacterial Spot Control by Bacillus velezensis: Bioprocess Solution. Microorganisms 2020, 8, 1463. [Google Scholar] [CrossRef]
- Dey, A.; Bhunia, B.; Dutta, S. Studies on the Effect of Agitation and Aeration for the Improved Protease Production by Bacillus licheniformis NCIM-2042. Mater. Today Proc. 2016, 3, 3444–3449. [Google Scholar] [CrossRef]
- Tzeng, Y.M.; Rao, Y.K.; Tsay, K.J.; Wu, W.S. Effect of Cultivation Conditions on Spore Production from Bacillus amyloliquefaciens B128 and Its Antagonism to Botrytis Elliptica. J. Appl. Microbiol. 2008, 104, 1275–1282. [Google Scholar] [CrossRef]
- Pokhrel, C.; Ohga, S. Submerged culture conditions for mycelial yield and polysaccharides production by Lyophyllum decastes. Food Chem. 2007, 105, 641–646. [Google Scholar] [CrossRef]
- Satapute, P.; Hiremath, G.; Satapute, P.P.; Shetti, H.S.; Kulkarni, A.A.; Hiremath, A.G.; Shivsharan, G.B.; Kaliwal, B.B. Isolation and Characterization of Nitrogen Fixing Bacillus Subtilis Strain As-4 from Agricultural Soil Pesticides Effect on Soil Microbiology and Biochemistry. Int. J. Recent Sci. Res. 2012, 3, 762–765. [Google Scholar]
- Yaseen, Y.; Gancel, F.; Béchet, M.; Drider, D.; Jacques, P. Study of the Correlation between Fengycin Promoter Expression and Its Production by Bacillus Subtilis under Different Culture Conditions and the Impact on Surfactin Production. Arch. Microbiol. 2017, 199, 1371–1382. [Google Scholar] [CrossRef]
- Mounsef, J.R.; Salameh, D.; Louka, N.; Brandam, C.; Lteif, R. The Effect of Aeration Conditions, Characterized by the Volumetric Mass Transfer Coefficient KLa, on the Fermentation Kinetics of Bacillus Thuringiensis Kurstaki. J. Biotechnol. 2015, 210, 100–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, S.; Kim, T.Y.; Won, S.J.; Moon, J.H.; Ajuna, H.B.; Kim, K.Y.; Ahn, Y.S. Control of Fungal Diseases and Fruit Yield Improvement of Strawberry Using Bacillus Velezensis CE 100. Microorganisms 2022, 10, 365. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liang, L.; Shao, H.; Ye, X.; Yang, X.; Chen, X.; Shi, Y.; Zhang, L.; Xu, L.; Wang, J. Isolation of the Novel Strain Bacillus Amyloliquefaciens F9 and Identification of Lipopeptide Extract Components Responsible for Activity against Xanthomonas Citri Subsp. Citri. Plants 2022, 11, 457. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.F.A.; Seleiman, M.F.; Al-Saif, A.M.; Alshiekheid, M.A.; Battaglia, M.L.; Taha, R.S. Biological Control of Celery Powdery Mildew Disease Caused by Erysiphe Heraclei Dc in Vitro and in Vivo Conditions. Plants 2021, 10, 2342. [Google Scholar] [CrossRef] [PubMed]
- Hong, P.; Hao, W.; Luo, J.; Chen, S.; Hu, M.; Zhong, G. Combination of Hot Water, Bacillus Amyloliquefaciens HF-01 and Sodium Bicarbonate Treatments to Control Postharvest Decay of Mandarin Fruit. Postharvest Biol. Technol. 2014, 88, 96–102. [Google Scholar] [CrossRef]
- Saravanan, R.; Nakkeeran, S.; Saranya, N.; Senthilraja, C.; Renukadevi, P.; Krishnamoorthy, A.S.; El Enshasy, H.A.; El-Adawi, H.; Malathi, V.G.; Salmen, S.H.; et al. Mining the Genome of Bacillus Velezensis VB7 (CP047587) for MAMP Genes and Non-Ribosomal Peptide Synthetase Gene Clusters Conferring Antiviral and Antifungal Activity. Microorganisms 2021, 9, 511. [Google Scholar] [CrossRef]
- Lai, K.; Chen, S.; Hu, M.; Hu, Q.; Geng, P.; Weng, Q.; Jia, J. Control of Postharvest Green Mold of Citrus Fruit by Application of Endophytic Paenibacillus Polymyxa Strain SG-6. Postharvest Biol. Technol. 2012, 69, 40–48. [Google Scholar] [CrossRef]
- Oerke, E.C.; Dehne, H.W. Safeguarding production—Losses in major crops and the role of crop protection. Crop. Prot. 2004, 23, 275–285. [Google Scholar] [CrossRef]
- Pal, G.; Kumar, K.; Verma, A.; Kumar Verma, S. Seed inhabiting bacterial endophytes of maize promote seedling establishment and provide protection against fungal disease. Microbiol. Res. 2022, 255, 126926. [Google Scholar] [CrossRef]
- Bodhankar, S.; Grover, M.; Hemanth, S.; Reddy, G.; Rasul, S.; Yadav, S.K.; Desai, S.; Mallappa, M.; Mandapaka, M.; Srinivasarao, C. Maize seed endophytic bacteria: Dominance of antagonistic, lytic enzyme-producing Bacillus spp. 3 Biotech 2017, 7, 232. [Google Scholar] [CrossRef]
- Pitzschke, A. Developmental peculiarities and seed-borne endophytes in quinoa: Omnipresent, robust Bacilli contribute to plant fitness. Front. Microbiol. 2016, 7, 2. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Parmar, S.; Sharma, V.K.; White, J.F. Seed endophytes and their potential applications. In Seed Endophytes; Verma, S.K., White, J.F., Jr., Eds.; Springer: Cham, Switzerland, 2019; pp. 35–54. [Google Scholar]
- Verma, S.K.; Kharwar, R.N.; White, J.F. The role of seed-vectored endophytes in seedling development and establishment. Symbiosis 2019, 78, 107–113. [Google Scholar] [CrossRef]
- White, J.F.; Kingsley, K.L.; Butterworth, S.; Brindisi, L.; Gatei, J.W.; Elmore, M.T.; Verma, S.K.; Yao, X.; Kowalski, K.P. Seed-vectored microbes: Their roles in improving seedling fitness and competitor plant suppression. In Seed Endophytes; Springer: Cham, Switzerland, 2019; pp. 3–20. [Google Scholar]
- Yaghoubian, I.; Msimbira, L.A.; Smith, D.L. Cell-Free Supernatant of Bacillus Strains can Improve Seed Vigor Index of Corn (Zea mays L.) Under Salinity Stress. Front. Sustain. Food Syst. 2022, 6, 857643. [Google Scholar] [CrossRef]
- Delshadi, S.; Ebrahimi, M.; Shirmohammadi, E. Influence of plantgrowth-promoting bacteria on germination, growth and nutrients’ uptake of Onobrychis sativa L. under drought stress. J. Plant Interact. 2017, 12, 200–208. [Google Scholar] [CrossRef] [Green Version]
- Nuncio-Orta, G.; Mendoza-Villarreal, R.; Robledo-Torres, V.; Vazquez Badillo, M.; Almaraz Suarez, J.J. Influence of rhizobacteria on seed germination and vigor of seeds chili jalapeno (Capsicum annuum L. ‘var. Grande’). ITEA Inform. Tecn. Econ Agraria. 2015, 111, 18–33. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, H.J.; Sun, Q.Q.; Cao, Y.Y.; Li, X.; Zhao, B.; Wu, P.; Guo, Y.-D. Proteomic analysis reveals a role of melatonin in promoting cucumber seed germination under high salinity by regulating energy production. Sci. Rep. 2017, 7, 503. [Google Scholar] [CrossRef]






| Medium Component | Concentration (g/L) |
|---|---|
| Skim milk | 28 |
| Tryptone | 5 |
| Yeast extract | 2.5 |
| Glucose | 1 |
| Agar | 15 |
| Medium Component | Concentration (g/L) |
|---|---|
| CMC/xylan | 5 |
| Glucose | 1 |
| Yeast extract | 0.5 |
| KCl | 1 |
| K2HPO4 | 1 |
| NaNO3 | 1 |
| MgSO4·7H2O | 0.5 |
| Agar | 17 |
| pH value | 7.0 ± 0.2 |
| Medium Component | Concentration (g/L) |
|---|---|
| Pectin | 10 |
| Yeast extract | 0.5 |
| KCl | 1 |
| K2HPO4 | 1 |
| NaNO3 | 1 |
| MgSO4·7H2O | 0.5 |
| Agar | 20 |
| pH value | 7.0 ± 0.2 |
| Parameter | B1 | B2 | B3 | B4 |
|---|---|---|---|---|
| N0 (log CFU/mL) | ||||
| Gompertz | 7.240 | 7.265 | 7.291 | 7.237 |
| Logistic | 7.241 | 7.268 | 7.293 | 7.240 |
| Exponential plateau | 7.239 | 7.263 | 7.288 | 7.234 |
| Nmax (log CFU/mL) | ||||
| Gompertz | 7.792 | 8.143 | 8.208 | 8.351 |
| Logistic | 7.792 | 8.139 | 8.204 | 8.347 |
| Exponential plateau | 7.793 | 8.147 | 8.212 | 8.355 |
| µ (1/h) | ||||
| Gompertz | 0.0541 | 0.0372 | 0.0382 | 0.0482 |
| Logistic | 0.0554 | 0.0387 | 0.0398 | 0.0505 |
| Exponential plateau | 0.0528 | 0.0357 | 0.0367 | 0.0459 |
| R2 | ||||
| Gompertz | 0.9838 | 0.9719 | 0.9861 | 0.9902 |
| Logistic | 0.9835 | 0.9710 | 0.9854 | 0.9908 |
| Exponential plateau | 0.9842 | 0.9728 | 0.9867 | 0.9895 |
| Parameter | B1 | B2 | B3 | B4 | DW |
|---|---|---|---|---|---|
| Germination rate (%) | 96.00 | 96.00 | 96.00 | 100.00 | 88.00 |
| Root length | |||||
| Mean value (mm) | 21.32 | 21.60 | 36.64 | 68.24 | 17.96 |
| Standard deviation (mm) | 11.07 | 7.62 | 15.91 | 16.06 | 10.49 |
| Maximal value (mm) | 38.00 | 36.00 | 62.00 | 95.00 | 39.00 |
| >75% values larger than… (mm) | 14.00 | 17.00 | 24.00 | 52.00 | 12.00 |
| >50% values larger than… (mm) | 22.00 | 21.00 | 39.00 | 71.00 | 21.00 |
| >25% values larger than… (mm) | 31.00 | 27.00 | 47.00 | 80.00 | 24.00 |
| Shoot length | |||||
| Mean value (mm) | 5.60 | 5.72 | 9.32 | 11.16 | 3.60 |
| Standard deviation (mm) | 2.47 | 5.54 | 8.35 | 7.89 | 4.78 |
| Maximal value (mm) | 9.00 | 16.00 | 24.00 | 27.00 | 19.00 |
| >25% values larger than… (mm) | 4.00 | 0.00 | 2.00 | 3.00 | 0.00 |
| >50% values larger than… (mm) | 5.00 | 4.00 | 7.00 | 12.00 | 2.00 |
| >75% values larger than… (mm) | 7.00 | 10.00 | 15.00 | 17.00 | 6.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vlajkov, V.; Pajčin, I.; Loc, M.; Budakov, D.; Dodić, J.; Grahovac, M.; Grahovac, J. The Effect of Cultivation Conditions on Antifungal and Maize Seed Germination Activity of Bacillus-Based Biocontrol Agent. Bioengineering 2022, 9, 797. https://doi.org/10.3390/bioengineering9120797
Vlajkov V, Pajčin I, Loc M, Budakov D, Dodić J, Grahovac M, Grahovac J. The Effect of Cultivation Conditions on Antifungal and Maize Seed Germination Activity of Bacillus-Based Biocontrol Agent. Bioengineering. 2022; 9(12):797. https://doi.org/10.3390/bioengineering9120797
Chicago/Turabian StyleVlajkov, Vanja, Ivana Pajčin, Marta Loc, Dragana Budakov, Jelena Dodić, Mila Grahovac, and Jovana Grahovac. 2022. "The Effect of Cultivation Conditions on Antifungal and Maize Seed Germination Activity of Bacillus-Based Biocontrol Agent" Bioengineering 9, no. 12: 797. https://doi.org/10.3390/bioengineering9120797
APA StyleVlajkov, V., Pajčin, I., Loc, M., Budakov, D., Dodić, J., Grahovac, M., & Grahovac, J. (2022). The Effect of Cultivation Conditions on Antifungal and Maize Seed Germination Activity of Bacillus-Based Biocontrol Agent. Bioengineering, 9(12), 797. https://doi.org/10.3390/bioengineering9120797