Recreating Tissue Structures Representative of Teratomas In Vitro Using a Combination of 3D Cell Culture Technology and Human Embryonic Stem Cells
Abstract
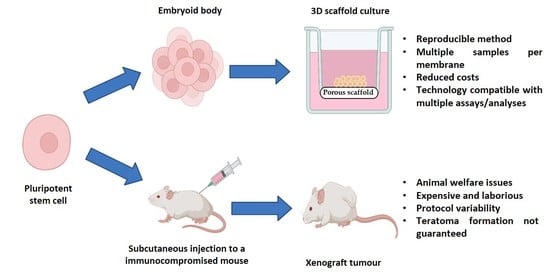
:1. Introduction
2. Materials and Methods
2.1. H9 Routine Culture
2.2. Formation, Maintenance and Differentiation of H9 Embryoid Bodies
2.3. Preparation of Alvetex® Membranes and Seeding/Maintenance of EBs
2.4. Sample Processing and Sectioning
2.5. Haematoxylin and Eosin Staining
2.6. Immunohistochemical Staining
2.7. Teratoma Xenograft Assay
3. Results
3.1. Teratomas Formed from Human Pluripotent Stem Cells Are Composed of Diverse Tissue Derivatives of the Three Embryonic Germ Layers
3.2. A Novel In Vitro Culture Method to Improve the Cellular Microenvironment and Enhance the Differentiation of Human Pluripotent Stem Cell-Derived Tissues
3.3. Improvement of Viability Allows for Increased Time in Culture, Resulting in the Formation of More Complex and Mature Tissue Structures from hPSC-Derived EBs
3.4. Application of Exogenous Morphogens to Direct EB Differentiation Results in the Formation of Tissues from Specific Lineages
3.5. In Vitro Tissues Derived from hESC Are Highly Similar to In Vivo Xenograft Teratomas Derived from the Same Cell Line
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic stem cell lines derived from human blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavaliere, G. A 14-day limit for bioethics: The debate over human embryo research. BMC Med. Ethics 2017, 18, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- UK Government. Human Fertilisation and Embryology Act. 2008. Available online: https://www.legislation.gov.uk/ukpga/2008/22/contents (accessed on 16 March 2022).
- Irie, N.; Kuratani, S. The developmental hourglass model: A predictor of the basic body plan? Development 2014, 141, 4649–4655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossant, J. Mouse and human blastocyst-derived stem cells: Vive les differences. Development 2015, 142, 9–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cahan, P.; Daley, G.Q. Origins and implications of pluripotent stem cell variability and heterogeneity. Nat. Rev. Mol. Cell Biol. 2013, 14, 357–368. [Google Scholar] [CrossRef] [Green Version]
- Ortmann, D.; Vallier, L. Variability of human pluripotent stem cell lines. Curr. Opin. Genet. Dev. 2017, 46, 179–185. [Google Scholar] [CrossRef]
- Bouma, M.J.; van Iterson, M.; Janssen, B.; Mummery, C.L.; Salvatori, D.C.F.; Freund, C. Differentiation-defective human induced pluripotent stem cells reveal strengths and limitations of the teratoma assay and in vitro pluripotency assays. Stem Cell Rep. 2017, 8, 1340–1353. [Google Scholar] [CrossRef] [Green Version]
- Buta, C.; David, R.; Dressel, R.; Emgård, M.; Fuchs, C.; Gross, U.; Healy, L.; Hescheler, J.; Kolar, R.; Martin, U.; et al. Reconsidering pluripotency tests: Do we still need teratoma assays? Stem Cell Res. 2013, 11, 552–562. [Google Scholar] [CrossRef] [Green Version]
- Hentze, H.; Soong, P.L.; Wang, S.T.; Phillips, B.W.; Putti, T.C.; Dunn, N.R. Teratoma formation by human embryonic stem cells: Evaluation of essential parameters for future safety studies. Stem Cell Res. 2009, 2, 198–210. [Google Scholar] [CrossRef] [Green Version]
- Müller, F.-J.; Goldmann, J.; Löser, P.; Loring, J.F. A call to standardize teratoma assays used to define human pluripotent cell lines. Cell Stem Cell 2010, 6, 412–414. [Google Scholar] [CrossRef] [Green Version]
- Allison, T.F.; Andrews, P.W.; Avior, Y.; Barbaric, I.; Benvenisty, N.; Bock, C.; Brehm, J.; Brüstle, O.; Damjanov, I.; Elefanty, A.; et al. Assessment of established techniques to determine developmental and malignant potential of human pluripotent stem cells. Nat. Commun. 2018, 9, 1925. [Google Scholar] [CrossRef]
- Bulic-Jakus, F.; Bojanac, A.K.; Juric-Lekic, G.; Vlahovic, M.; Sincic, N. Teratoma: From spontaneous tumors to the pluripotency/malignancy assay. Wiley Interdiscip. Rev. Dev. Biol. 2016, 5, 186–209. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Aranda, I.; Ramos-Mejia, V.; Bueno, C.; Munoz-Lopez, M.; Real, P.J.; Mácia, A.; Sanchez, L.; Ligero, G.; Garcia-Parez, J.L.; Menendez, P. Human induced pluripotent stem cells develop teratoma more efficiently and faster than human embryonic stem cells regardless the site of injection. Stem Cells 2010, 28, 1568–1570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aleckovic, M.; Simón, C. Is teratoma formation in stem cell research a characterization tool or a window to developmental biology? Reprod. Biomed. Online 2008, 17, 270–280. [Google Scholar] [CrossRef]
- Al-Jomard, R.; Amarin, Z. The use of human mature cystic ovarian teratoma as a model to study the development of human lymphatics. Anat. Cell Biol. 2017, 50, 104–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hultman, I.; Björk, L.; Blomberg, E.; Sandstedt, B.; Ährlund-Richter, L. Experimental teratoma: At the crossroad of fetal- and onco-development. Semin. Cancer Biol. 2014, 29, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Jamil, S.; Cedervall, J.; Hultman, I.; Ali, R.; Margaryan, N.V.; Rasmuson, A.; Johnsen, J.I.; Sveinbjörnsson, B.; Dalianis, T.; Kanter, L.; et al. Neuroblastoma cells injected into experimental mature teratoma reveal a tropism for embryonic loose mesenchyme. Int. J. Oncol. 2013, 43, 831–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooke, M.J.; Stojkovic, M.; Przyborski, S.A. Growth of teratomas derived from human pluripotent stem cells is influenced by the graft site. Stem Cells Dev. 2006, 15, 254–259. [Google Scholar] [CrossRef]
- Prokhorova, T.A.; Harkness, L.M.; Frandsen, U.; Ditzel, N.; Schrøder, H.D.; Burns, J.S.; Kassem, M. teratoma formation by human embryonic stem cells is site dependent and enhanced by the presence of matrigel. Stem Cells Dev. 2009, 18, 47–54. [Google Scholar] [CrossRef]
- Lee, A.S.; Tang, C.; Cao, F.; Xie, X.; Van Der Bogt, K.; Hwang, A.; Connolly, A.J.; Robbins, R.C.; Wu, J.C. Effects of cell number on teratoma formation by human embryonic stem cells. Cell Cycle 2009, 8, 2608–2612. [Google Scholar] [CrossRef]
- Gropp, M.; Shilo, V.; Vainer, G.; Gov, M.; Gil, Y.; Khaner, H.; Matzrafi, L.; Idelson, M.; Kopolovic, J.; Zak, N.B.; et al. Standardization of the teratoma assay for analysis of pluripotency of human ES cells and biosafety of their differentiated progeny. PLoS ONE 2012, 7, e45532. [Google Scholar] [CrossRef] [PubMed]
- Tannenbaum, J.; Bennett, B.T. Russell and burch’s 3Rs then and now: The need for clarity in definition and purpose. J. Am. Assoc. Lab. Anim. Sci. 2015, 54, 120–132. [Google Scholar] [PubMed]
- Flecknell, P. Replacement, reduction and refinement. Altern. Anim. Exp. 2002, 19, 73–78. [Google Scholar]
- Lang, A.; Jirkof, P.; Mocho, J.-P.; Sanchez-Morgado, J.; Tremoleda, J.L. Report from the 11th edition of the World Congress on Alternatives and Animal Use in the Life Sciences (WC11). Lab. Anim. 2022, 56, 101–102. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.A.; Hidalgo Aguilar, A.; Owens, D.D.G.; Quelch, R.H.; Knight, E.; Przyborski, S.A. Using advanced cell culture techniques to differentiate pluripotent stem cells and recreate tissue structures representative of teratoma xenografts. Front. Cell Dev. Biol. 2021, 9, 667246. [Google Scholar] [CrossRef]
- Dvash, T.; Mayshar, Y.; Darr, H.; McElhaney, M.; Barker, D.; Yanuka, O.; Kotkow, K.J.; Rubin, L.L.; Benvenisty, N.; Eiges, R. Temporal gene expression during differentiation of human embryonic stem cells and embryoid bodies. Hum. Reprod. 2004, 19, 2875–2883. [Google Scholar] [CrossRef]
- Keller, G. Embryonic stem cell differentiation: Emergence of a new era in biology and medicine. Genes Dev. 2005, 19, 1129–1155. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.; Huangfu, D. Human pluripotent stem cells: An emerging model in developmental biology. Development 2013, 140, 705–717. [Google Scholar] [CrossRef] [Green Version]
- Van Winkle, A.P.; Gates, I.D.; Kallos, M.S. mass transfer limitations in embryoid bodies during human embryonic stem cell differentiation. Cells Tissues Organs 2012, 196, 34–47. [Google Scholar] [CrossRef]
- Gothard, D.; Roberts, S.J.; Shakesheff, K.M.; Buttery, L.D. Controlled embryoid body formation via surface modification and avidin-biotin cross-linking. Cytotechnology 2009, 61, 135–144. [Google Scholar] [CrossRef] [Green Version]
- Son, M.-Y.; Kim, H.-J.; Kim, M.-J.; Cho, Y.S. Physical passaging of embryoid bodies generated from human pluripotent stem cells. PLoS ONE 2011, 6, e19134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soman, P.; Tobe, B.T.D.; Lee, J.W.; Winquist, A.M.; Singec, I.; Vecchio, K.S.; Snyder, E.Y.; Chen, S. Three-dimensional scaffolding to investigate neuronal derivatives of human embryonic stem cells. Biomed. Microdevices 2012, 14, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, N.; Hashemi, S.M.; Salehi, M.; Jahani, H.; Mowla, S.J.; Soleimani, M.; Hosseinkhani, H. Influence of oriented nanofibrous PCL scaffolds on quantitative gene expression during neural differentiation of mouse embryonic stem cells. J. Biomed. Mater. Res. Part A 2016, 104, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Giandomenico, S.L.; Sutcliffe, M.; Lancaster, M.A. Generation and long-term culture of advanced cerebral organoids for studying later stages of neural development. Nat. Protoc. 2021, 16, 579–602. [Google Scholar] [CrossRef] [PubMed]
- Itskovitz-Eldor, J.; Schuldiner, M.; Karsenti, D.; Eden, A.; Yanuka, O.; Amit, M.; Soreq, H.; Benvenisty, N. Differentiation of human embryonic stem cells into embryoid bodies compromising the three embryonic germ layers. Mol. Med. 2000, 6, 88–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khoo, M.L.M.; McQuade, L.R.; Smith, M.S.R.; Lees, J.G.; Sidhu, K.S.; Tuch, B.E. Growth and differentiation of embryoid bodies derived from human embryonic stem cells: Effect of glucose and basic fibroblast growth factor. Biol. Reprod. 2005, 73, 1147–1156. [Google Scholar] [CrossRef]
- Stachelscheid, H.; Wulf-Goldenberg, A.; Eckert, K.; Jensen, J.; Edsbagge, J.; Björquist, P.; Rivero, M.; Strehl, R.; Jozefczuk, J.; Prigione, A.; et al. Teratoma formation of human embryonic stem cells in three-dimensional perfusion culture bioreactors. J. Tissue Eng. Regen. Med. 2013, 7, 729–741. [Google Scholar] [CrossRef] [Green Version]
- Vallier, L.; Reynolds, D.; Pedersen, R.A. Nodal inhibits differentiation of human embryonic stem cells along the neuroectodermal default pathway. Dev. Biol. 2004, 275, 403–421. [Google Scholar] [CrossRef] [Green Version]
- Osafune, K.; Caron, L.; Borowiak, M.; Martinez, R.J.; Fitz-gerald, C.S.; Sato, Y.; Cowan, C.A.; Chien, K.R.; Melton, D.A. Marked differences in differentiation propensity among human embryonic stem cell lines. Nat. Biotechnol. 2008, 26, 313–315. [Google Scholar] [CrossRef]
- Ebisuya, M.; Briscoe, J. What does time mean in development? Development 2018, 145, 164368. [Google Scholar] [CrossRef] [Green Version]
- Paavilainen, T.; Pelkonen, A.; Mäkinen, M.E.L.; Peltola, M.; Huhtala, H.; Fayuk, D.; Narkilahti, S. Effect of prolonged differentiation on functional maturation of human pluripotent stem cell-derived neuronal cultures. Stem Cell Res. 2018, 27, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.E.; Anzai, T.; Chanthra, N.; Uosaki, H. A brief review of current maturation methods for human induced pluripotent stem cells-derived cardiomyocytes. Front. Cell Dev. Biol. 2020, 8, 178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fowler, J.L.; Ang, L.T.; Loh, K.M. A critical look: Challenges in differentiating human pluripotent stem cells into desired cell types and organoids. Wiley Interdiscip. Rev. Dev. Biol. 2020, 9, e368. [Google Scholar] [CrossRef]





| Morphogen | Supplier | Final Concentration |
|---|---|---|
| SB431542 | Peprotech, London, UK | 10 µM |
| LDN193189 | Peprotech | 0.25 µM |
| Basic Fibroblast Growth Factor (bFGF) | Peprotech | 100 ng/mL |
| Antibody | Species | Dilution | Product Code | Supplier |
|---|---|---|---|---|
| Classs III β tubulin | Rabbit | 1:100 | ab18207 | Abcam UK |
| E-cadherin | Mouse | 1:200 | 610,181 | BD Biosciences UK |
| Nestin | Mouse | 1:200 | ab22035 | Abcam UK |
| Vimentin | Mouse | 1:100 | sc6260 | Santa Cruz Biotechnology UK |
| Fibronectin | Rabbit | 1:100 | ab17808 | Abcam UK |
| Antibody | Dilution | Product Code | Supplier |
|---|---|---|---|
| Goat Anti-Mouse Alexa Fluor 594 | 1:1000 | A11005 | Invitrogen, Fisher Scientific UK |
| Goat Anti-Rabbit Alexa Fluor 488 | 1:1000 | A11034 | Invitrogen, Fisher Scientific UK |
| Donkey Anti-Rabbit Alexa Fluor 594 | 1:1000 | A21207 | Invitrogen, Fisher Scientific UK |
| Donkey Anti-Mouse Alexa Fluor 488 | 1:1000 | A21202 | Invitrogen, Fisher Scientific UK |
| Hoechst 33342 | 1:10,000 | H3570 | Fisher Scientific UK |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hidalgo Aguilar, A.; Smith, L.; Owens, D.; Quelch, R.; Przyborski, S. Recreating Tissue Structures Representative of Teratomas In Vitro Using a Combination of 3D Cell Culture Technology and Human Embryonic Stem Cells. Bioengineering 2022, 9, 185. https://doi.org/10.3390/bioengineering9050185
Hidalgo Aguilar A, Smith L, Owens D, Quelch R, Przyborski S. Recreating Tissue Structures Representative of Teratomas In Vitro Using a Combination of 3D Cell Culture Technology and Human Embryonic Stem Cells. Bioengineering. 2022; 9(5):185. https://doi.org/10.3390/bioengineering9050185
Chicago/Turabian StyleHidalgo Aguilar, Alejandro, Lucy Smith, Dominic Owens, Rebecca Quelch, and Stefan Przyborski. 2022. "Recreating Tissue Structures Representative of Teratomas In Vitro Using a Combination of 3D Cell Culture Technology and Human Embryonic Stem Cells" Bioengineering 9, no. 5: 185. https://doi.org/10.3390/bioengineering9050185
APA StyleHidalgo Aguilar, A., Smith, L., Owens, D., Quelch, R., & Przyborski, S. (2022). Recreating Tissue Structures Representative of Teratomas In Vitro Using a Combination of 3D Cell Culture Technology and Human Embryonic Stem Cells. Bioengineering, 9(5), 185. https://doi.org/10.3390/bioengineering9050185