Anti-Platelet Properties of Apple Must/Skin Yeasts and of Their Fermented Apple Cider Products
Abstract
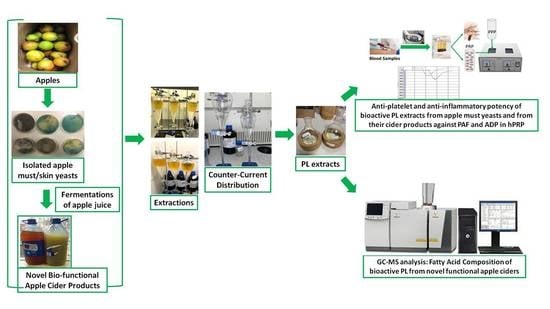
:1. Introduction
2. Materials and Methods
2.1. Isolation of Yeasts Strains from Apple Surface and Musts
2.2. Flow Cytometric Identification and Profiling of Apple Microflora and Apple Cider Fermentation
2.3. Fermentations
2.4. Extraction and Separation of Lipid Bioactives from Apple Musts Yeast Strains and from Their Apple Cider Fermented Products
2.5. Platelet Aggregometry Biological Assays
2.6. Gas Chromatography–Mass Spectrometry (GC–MS)
2.7. Statistical Analysis
3. Results and Discussion
3.1. Anti-Platelet Potency of PL Bioactives from the Apple Surface/Must Yeast Strains and from Their Fermented Apple Cider Products
3.2. Fatty Acid Profile of PL Extracts Apple Ciders Produced from Apple Juice Fermented by Utilizing the Most Bioactive Strains of Apple Cider Must Microflora
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACA, ACB, ACF, ACG and ACI | fermented novel apple cider (AC) products derived from fermentations of apple juice by utilizing the most bioactive and resilient/efficient for fermentations apple must yeast strains A, B, F, G, and I, respectively. |
| PAF | platelet activating factor |
| CVD | cardiovascular diseases |
| ADP | adenosine 5′ diphosphate |
| BSA | bovine serum albumin |
| hPRP | human platelet-rich plasma |
| PL | polar lipids |
| TL | total lipids |
| NL | neutral lipids |
| GCMS | gas chromatography mass spectra |
| FAME | fatty acid methyl esters |
| PC | Phosphatidylcholine |
| PE | Phosphatidylethanolamine |
| SFA | saturated fatty acids |
| MUFA | monounsaturated fatty acids |
| PUFA | polyunsaturated fatty acids |
| n-3 | omega-3 PUFA |
| n-6 | omega-6 PUFA |
| ALA | alpha linolenic acid |
| LA | linoleic acid |
| EPA | eicosapentaenoic acid |
| DPA | docosapentaenoic acid |
| DHA | docosahexaenoic acid |
| NA | nutrient agar |
| WLN | Wallerstein nutrient agar |
| MRS | De man, Rogosa, and Sharpe agar |
| WGA | wheat germ agglutinin |
| PBS | phosphate-buffered saline |
| PI | propidium iodide |
| ND | non-detectable |
References
- Tsoupras, A.; Moran, D.; Pleskach, H.; Durkin, M.; Traas, C.; Zabetakis, I. Beneficial Anti-Platelet and Anti-Inflammatory Properties of Irish Apple Juice and Cider Bioactives. Foods 2021, 10, 412. [Google Scholar] [CrossRef]
- Tsoupras, A.; Moran, D.; Byrne, T.; Ryan, J.; Barrett, L.; Traas, C.; Zabetakis, I. Anti-Inflammatory and Anti-Platelet Properties of Lipid Bioactives from Apple Cider By-Products. Molecules 2021, 26, 2869. [Google Scholar] [CrossRef]
- Fragopoulou, E.; Antonopoulou, S.; Tsoupras, A.; Tsantila, N.; Grypioti, A.; Gribilas, G.; Gritzapi, H.; Konsta, E.; Skandalou, E.; Papadopoulou, A.; et al. Antiatherogenic properties of red/white wine, musts, grape-skins, and yeast. Chem. Phys. Lipids 2004, 130, 66. [Google Scholar]
- Tsoupras, A.; Lordan, R.; O’Keefe, E.; Shiels, K.; Saha, S.K.; Zabetakis, I. Structural Elucidation of Irish Ale Bioactive Polar Lipids with Antithrombotic Properties. Biomolecules 2020, 10, 1075. [Google Scholar] [CrossRef]
- Tsoupras, A.B.; Demopoulos, C.A.; Pappas, K.M. Platelet-activating factor detection, metabolism and inhibitors in the ethanologenic bacterium Zymomonas mobilis. Eur. J. Lipid Sci. Technol. 2012, 114, 123–133. [Google Scholar] [CrossRef]
- Le Quere, J.-M.; Husson, F.; Renard, C.M.G.C.; Primault, J. French cider charakterization by sensory, technological and chemical evaluations. LWT Food Sci. Technol. 2006, 9, 1033–1044. [Google Scholar] [CrossRef]
- Al Daccache, M.; Koubaa, M.; Maroun, R.G.; Salameh, D.; Louka, N.; Vorobiev, E. Impact of the Physicochemical Composition and Microbial Diversity in Apple Juice Fermentation Process: A Review. Molecules 2020, 25, 3698. [Google Scholar] [CrossRef]
- Ye, M.; Yue, T.; Yuan, Y. Effects of sequential mixed cultures of Wickerhamomyces anomalus and Saccharomyces cerevisiae on apple cider fermentation. FEMS Yeast Res. 2014, 14, 873–882. [Google Scholar] [CrossRef] [Green Version]
- Morrissey, W.F.; Davenport, B.; Querol, A.; Dobson, A.D. The role of indigenous yeasts in traditional Irish cider fermentations. J. Appl. Microbiol. 2004, 97, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Lorenzini, M.; Simonato, B.; Slaghenaufi, D.; Ugliano, M.; Zapparoli, G. Assessment of yeasts for apple juice fermentation and production of cider volatile compounds. LWT 2019, 99, 224–230. [Google Scholar] [CrossRef]
- Suarez Valles, B.; Bedrinana, R.P.; Tascon, N.F.; Simon, A.Q.; Madrera, R.R. Yeast species associated with the spontaneous fermentation of cider. Food Microbiol. 2007, 24, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.; Cronin, U.P.; Wilkinson, M.G. Responses of Escherichia coli, Listeria monocytogenes, and Staphylococcus aureus to simulated food processing treatments, determined using fluorescence-activated cell sorting and plate counting. Appl. Environ. Microbiol. 2011, 77, 4657–4668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrero, M.; Quiros, C.; Garcia, L.A.; Diaz, M. Use of flow cytometry to follow the physiological states of microorganisms in cider fermentation processes. Appl. Environ. Microbiol. 2006, 72, 6725–6733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambriz-Aviña, V.; Contreras-Garduño, J.A.; Pedraza-Reyes, M. Applications of flow cytometry to characterize bacterial physiological responses. Biomed. Res. Int. 2014, 2014, 461941. [Google Scholar] [CrossRef]
- Chaudhari, R.D.; Stenson, J.D.; Overton, T.W.; Thomas, C.R. Effect of bud scars on the mechanical properties of Saccharomyces cerevisiae cell walls. Chem. Eng. Sci. 2012, 84, 188–196. [Google Scholar] [CrossRef] [Green Version]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [Green Version]
- Galanos, D.S.; Kapoulas, V.M. Isolation of polar lipids from triglyceride mixtures. J. Lipid Res. 1962, 3, 134–136. [Google Scholar] [CrossRef]
- Tsoupras, A.; Lordan, R.; Demuru, M.; Shiels, K.; Saha, S.K.; Nasopoulou, C.; Zabetakis, I. Structural elucidation of irish organic farmed salmon (Salmo salar) polar lipids with antithrombotic activities. Mar. Drugs 2018, 16, 176. [Google Scholar] [CrossRef] [Green Version]
- Tsoupras, A.; O’Keeffe, E.; Lordan, R.; Redfern, S.; Zabetakis, I. Bioprospecting for Antithrombotic Polar Lipids from Salmon, Herring, and Boarfish By-Products. Foods 2019, 8, 416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsoupras, A.; Zabetakis, I.; Lordan, R. Platelet aggregometry assay for evaluating the effects of platelet agonists and antiplatelet compounds on platelet function in vitro. MethodsX 2019, 6, 63–70. [Google Scholar] [CrossRef]
- Tsoupras, A.; Lordan, R.; Harrington, J.; Pienaar, R.; Devaney, K.; Heaney, S.; Koidis, A.; Zabetakis, I. The Effects of Oxidation on the Antithrombotic Properties of Tea Lipids Against PAF, Thrombin, Collagen, and ADP. Foods 2020, 9, 385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koukouraki, P.; Tsoupras, A.; Sotiroudis, G.; Demopoulos, C.A.; Sotiroudis, T.G. Antithrombotic properties of Spirulina extracts against platelet-activating factor and thrombin. Food Biosci. 2020, 37, 100686. [Google Scholar] [CrossRef]
- Tsoupras, A.; Lordan, R.; Zabetakis, I. Inflammation, not Cholesterol, Is a Cause of Chronic Disease. Nutrients 2018, 10, 604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simopoulos, A.P. The Importance of the Omega-6/Omega-3 Fatty Acid Ratio in Cardiovascular Disease and Other Chronic Diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef]


| Samples | TL (g/100 g) | NL (g/100 g) | PL (g/100 g) |
|---|---|---|---|
| ACA | 0.031 ± 0.021 | 0.006 ± 0.003 | 0.025 ± 0.011 |
| ACB | 0.036 ± 0.015 | 0.005 ± 0.002 | 0.031 ± 0.009 |
| ACF | 0.046 ± 0.022 | 0.004 ± 0.001 | 0.042 ± 0.005 |
| ACG | 0.051 ± 0.025 | 0.007 ± 0.002 | 0.044 ± 0.008 |
| ACI | 0.042 ± 0.009 | 0.006 ± 0.002 | 0.036 ± 0.011 |
| Fatty Acid | ACA | ACB | ACF | ACG | ACI |
|---|---|---|---|---|---|
| C13:0 | 0.43 ± 0.13 | 0.44 ± 0.09 | 0.23 ± 0.13 | 0.22 ± 0.11 | 0.43 ± 0.09 |
| C14:0 | 4.73 ± 0.40 | 4.60 ± 0.20 | 4.26 ± 0.70 | 4.27 ± 0.72 | 4.40 ± 0.20 |
| C15:0 | 0.91 ± 0.17 | 0.78 ± 0.18 | 0.78 ± 0.18 | 0.73 ± 0.10 | 0.77 ± 0.13 |
| C16:0 | 29.56 ± 0.82 | 29.66 ± 0.85 | 29.50 ± 0.69 | 29.25 ± 0.79 | 29.42 ± 0.86 |
| C16:1 c9 | 1.69 ± 0.11 | 1.69 ± 0.11 | 1.30 ± 0.21 | 1.33 ± 0.18 | 1.61 ± 0.14 |
| C17:0 | 0.85 ± 0.12 | 0.85 ± 0.12 | 0.69 ± 0.17 | 0.37 ± 0.16 | 0.85 ± 0.08 |
| C18:0 | 21.33 ± 0.67 | 22.17 ± 0.35 | 19.73 ± 0.70 | 19.13 ± 1.19 | 22.88 ± 0.69 |
| C18:1 c9 | 16.73 ± 0.47 | 16.80 ± 0.92 | 17.08 ± 1.19 | 17.77 ± 0.55 | 16.67 ± 0.40 |
| C18:2 c9,12 (LA) | 12.30 ± 0.50 | 11.69 ± 0.38 | 10.79 ± 0.30 | 12.37 ± 0.24 | 11.36 ± 0.51 |
| C18:3 c9,12,15 (ALA) | 5.18 ± 0.41 | 5.15 ± 0.28 | 6.17 ± 0.48 | 6.69 ± 0.40 | 5.50 ± 0.40 |
| C20:0 | 1.15 ± 0.05 | 1.19 ± 0.08 | 1.77 ± 0.15 | 1.11 ± 0.12 | 1.20 ± 0.10 |
| C20:1 c11 | 0.98 ± 0.13 | 0.97 ± 0.15 | 1.37 ± 0.07 | 1.22 ± 0.13 | 0.96 ± 0.09 |
| C20:3 c8,11,14 | 0.24 ± 0.07 | 0.22 ± 0.06 | 0.35 ± 0.07 | 0.21 ± 0.06 | 0.26 ± 0.05 |
| C20:4 c5,8,11,14 | 0.37 ± 0.08 | 0.37 ± 0.08 | 0.57 ± 0.09 | 0.27 ± 0.05 | 0.35 ± 0.07 |
| C20:5 c5,11,14,17 (EPA) | 0.86 ± 0.09 | 0.83 ± 0.12 | 1.29 ± 0.15 | 1.09 ± 0.11 | 0.81 ± 0.12 |
| C21:0 | 0.74 ± 0.15 | 0.74 ± 0.15 | 1.03 ± 0.24 | 0.74 ± 0.08 | 0.68 ± 0.11 |
| C22:1 c13 | 0.90 ± 0.20 | 0.82 ± 0.15 | 1.51 ± 0.31 | 1.18 ± 0.18 | 0.80 ± 0.13 |
| C22:5 c7,10,13,16,19 (DPA) | 0.44 ± 0.11 | 0.41 ± 0.11 | 0.63 ± 0.20 | 0.84 ± 0.12 | 0.41 ± 0.12 |
| C22:6 c4,7,10,13,16,19 (DHA) | 0.63 ± 0.10 | 0.60 ± 0.14 | 0.94 ± 0.18 | 1.20 ± 0.10 | 0.62 ± 0.15 |
| SFA | 59.71 ± 0.61 | 60.43 ± 0.95 | 58.00 ± 1.64 | 55.82 ± 1.12 | 60.64 ± 0.27 |
| MUFA | 20.67 ± 0.08 | 20.28 ± 0.65 | 21.26 ± 1.29 | 21.50 ± 0.98 | 20.04 ± 0.29 |
| PUFA | 20.02 ± 0.53 | 19.29 ± 0.31 | 20.74 ± 0.47 | 22.68 ± 0.56 | 19.32 ± 0.27 |
| n6 | 12.91 ± 0.44 | 12.30 ± 0.55 | 11.71 ± 0.18 | 12.85 ± 0.23 | 11.97 ± 0.46 |
| n3 | 7.11 ± 0.29 | 6.99 ± 0.09 | 9.03 ± 0.51 * | 9.83 ± 0.34 * | 7.34 ± 0.42 |
| n6/n3 | 1.82 ± 0.09 | 1.76 ± 0.07 | 1.30 ± 0.08 * | 1.31 ± 0.02 * | 1.64 ± 0.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moran, D.; Fleming, M.; Daly, E.; Gaughan, N.; Zabetakis, I.; Traas, C.; Tsoupras, A. Anti-Platelet Properties of Apple Must/Skin Yeasts and of Their Fermented Apple Cider Products. Beverages 2021, 7, 54. https://doi.org/10.3390/beverages7030054
Moran D, Fleming M, Daly E, Gaughan N, Zabetakis I, Traas C, Tsoupras A. Anti-Platelet Properties of Apple Must/Skin Yeasts and of Their Fermented Apple Cider Products. Beverages. 2021; 7(3):54. https://doi.org/10.3390/beverages7030054
Chicago/Turabian StyleMoran, Donal, Mary Fleming, Eimear Daly, Natasha Gaughan, Ioannis Zabetakis, Con Traas, and Alexandros Tsoupras. 2021. "Anti-Platelet Properties of Apple Must/Skin Yeasts and of Their Fermented Apple Cider Products" Beverages 7, no. 3: 54. https://doi.org/10.3390/beverages7030054
APA StyleMoran, D., Fleming, M., Daly, E., Gaughan, N., Zabetakis, I., Traas, C., & Tsoupras, A. (2021). Anti-Platelet Properties of Apple Must/Skin Yeasts and of Their Fermented Apple Cider Products. Beverages, 7(3), 54. https://doi.org/10.3390/beverages7030054