Analysis of Phytosterols Content in Italian-Standard Espresso Coffee
Abstract
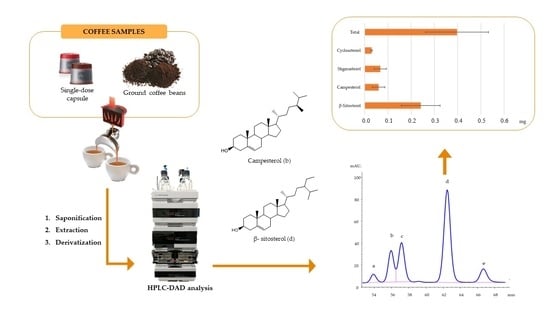
:1. Introduction
2. Materials and Methods
2.1. Reagents and Standards
2.2. Coffee Samples and Espresso Machines
2.3. EC Preparation
2.4. Phytsterols Extraction from EC
2.5. HPLC-DAD Analyses
2.6. Statistical Analyses
3. Results and Discussion
3.1. Extraction of PS in EC
3.2. HPLC Analysis
3.3. Analysis of Phytosterols in EC Samples
3.4. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Illy, A.; Viani, R.; Suggi Liverani, F. Espresso Coffee: The Science of Quality, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- International Coffee Organization World Coffee Consumption, Data as of November 2020. Available online: https://ico.org/ (accessed on 1 August 2021).
- Global Coffee Pods and Capsules Market–Growth, Trends and Forecasts (2020–2025), 2020, Mordor Intelligence. Available online: https://www.prnewswire.com/ (accessed on 1 August 2021).
- Severini, C.; Caporizzi, R.; Fiore, A.G.; Ricci, I.; Onur, O.M.; Derossi, A. Reuse of spent espresso coffee as sustainable source of fibre and antioxidants. A map on functional, microstructure and sensory effects of novel enriched muffins. LWT 2020, 119, 108877. [Google Scholar] [CrossRef]
- Caprioli, G.; Cortese, M.; Cristalli, G.; Maggi, F.; Odello, L.; Ricciutelli, M.; Sagratini, G.; Sirocchi, V.; Tomassoni, G.; Vittori, S. Optimization of espresso machine parameters through the analysis of coffee odorants by HS-SPME–GC/MS. Food Chem. 2012, 135, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Parenti, A.; Guerrini, L.; Masella, P.; Spinelli, S.; Calamai, L.; Spugnoli, P. Comparison of espresso coffee brewing techniques. J. Food Eng. 2014, 121, 112–117. [Google Scholar] [CrossRef]
- Labbe, D.; Sudre, J.; Dugas, V.; Folmer, B. Impact of crema on expected and actual espresso coffee experience. Food Res. Int. 2016, 82, 53–58. [Google Scholar] [CrossRef]
- Navarini, L.; Rivetti, D. Water quality for espresso coffee. Food Chem. 2010, 122, 424–428. [Google Scholar] [CrossRef]
- Caprioli, G.; Cortese, M.; Sagratini, G.; Vittori, S. The influence of different types of preparation (espresso and brew) on coffee aroma and main bioactive constituents. J. Food Compos. Anal. 2015, 66, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Andueza, S.; Maeztu, L.; Pascual, L.; Ibanez, C.; de Pena, M.P.; Cid, C. Influence of extraction temperature on the final quality of espresso coffee. J. Sci. Food Agric. 2003, 322, 190–193. [Google Scholar] [CrossRef]
- Khamitova, G.; Angeloni, S.; Fioretti, L.; Ricciutelli, M.; Sagratini, G.; Torregiani, E.; Vittori, S.; Caprioli, G. The impact of different filter baskets, heights of perforated disc and amount of ground coffee on the extraction of organics acids and the main bioactive compounds in espresso coffee. Int. Food Res. J. 2020, 133, 199–220. [Google Scholar] [CrossRef]
- Pimpley, V.; Patil, S.; Srinivasan, K.; Desai, N.; Murthy, P.S. The chemistry of chlorogenic acid from green coffee and its role in attenuation of obesity and diabetes. Prep. Biochem. Biotechnol. 2020, 50, 969–978. [Google Scholar]
- Sandi, D.; Araújo, J.M.A. Extraction of coffee diterpenes and coffee oil using supercritical carbon dioxide. Food Chem. 2007, 101, 1087–1094. [Google Scholar]
- Jeszka-Skowron, M.; Zgoła-Grześkowiak, A.; Grześkowiak, T. Analytical methods applied for the characterization and the determination of bioactive compounds in coffee. Eur. Food Res. Technol. 2015, 240, 19–31. [Google Scholar] [CrossRef]
- Angeloni, S.; Mustafa, A.; Abouelenein, D.; Alessandroni, L.; Acquaticci, L.; Nzekoue, F.; Petrelli, R.; Sagratini, G.; Vittori, S.; Torregiani, E.; et al. Characterization of the Aroma Profile and Main Key Odorants of Espresso Coffee. Molecules 2021, 26, 3856. [Google Scholar] [CrossRef]
- Voldřich, H.Č.V.S.M.; Ševčík, R. Differentiation of coffee varieties according to their sterolic profile. J. Food Nutr. Res. 2007, 46, 28–34. [Google Scholar]
- Speer, K.; Kölling-Speer, I. The lipid fraction of the coffee bean. Braz. J. Plant Physiol. 2006, 18, 201–216. [Google Scholar] [CrossRef] [Green Version]
- Nzekoue, F.K.; Alesi, A.; Vittori, S.; Sagratini, G.; Caprioli, G. Development of a functional whey cheese (ricotta) enriched in phytosterols: Evaluation of the suitability of whey cheese matrix and processing for phytosterols supplementation. LWT 2021, 139, 110479. [Google Scholar] [CrossRef]
- Feng, S.; Wang, L.; Belwal, T.; Li, L.; Luo, Z. Phytosterols extraction from hickory (Carya cathayensis Sarg.) husk with a green direct citric acid hydrolysis extraction method. Food Chem. 2020, 315, 126217. [Google Scholar] [CrossRef] [PubMed]
- Cornelis, M.C.; El-Sohemy, A. Coffee, caffeine, and coronary heart disease. Curr. Opin. Lipidol. 2007, 18, 13–19. [Google Scholar] [CrossRef]
- Gökcen, B.B.; Şanlier, N. Coffee consumption and disease correlations. Crit. Rev. Food Sci. Nutr. 2019, 59, 336–348. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, J.H.; Di Nicolantonio, J.J.; Lavie, C.J. Coffee for cardioprotection and longevity. Prog. Cardiovasc. Dis. 2018, 61, 38–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Huang, W.; Hu, Y.; Zhang, L.; Shao, Y.; Wang, M.; Zhang, F.; Zhao, Z.; Mei, X.; Li, T.; et al. Phytosterol profiles of common foods and estimated natural intake of different structures and forms in China. J. Sci. Food Agr. 2018, 66, 2669–2676. [Google Scholar] [CrossRef]
- Asl, P.J.; Niazmand, R.; Jahani, M. Theoretical and experimental assessment of supercritical CO2 in the extraction of phytosterols from rapeseed oil deodorizer distillates. J. Food Eng. 2020, 269, 109748. [Google Scholar]
- Vu, D.C.; Lei, Z.; Sumner, L.W.; Coggeshall, M.V.; Lin, C.H. Identification and quantification of phytosterols in black walnut kernels. J. Food Compos. Anal. 2019, 75, 61–69. [Google Scholar] [CrossRef]
- Nzekoue, F.K.; Khamitova, G.; Angeloni, S.; Sempere, A.N.; Tao, J.; Maggi, F.; Xiao, J.; Sagratini, G.; Vittori, S.; Caprioli, G. Spent coffee grounds: A potential commercial source of phytosterols. Food Chem. 2020, 325, 126836. [Google Scholar] [CrossRef]
- Khamitova, G.; Angeloni, S.; Borsetta, G.; Xiao, J.; Maggi, F.; Sagratini, G.; Vittori, S.; Caprioli, G. Optimization of espresso coffee extraction through variation of particle sizes, perforated disk height and filter basket aimed at lowering the amount of ground coffee used. Food Chem. 2020, 314, 126220. [Google Scholar] [CrossRef]
- Caprioli, G.; Cortese, M.; Odello, L.; Ricciutelli, M.; Sagratini, G.; Tomassoni, G.; Torregiani, E.; Vittori, S. Importance of espresso coffee machine parameters on the extraction of chlorogenic acids in a certified Italian espresso by using SPE-HPLC-DAD. J. Food Res. 2013, 2, 55. [Google Scholar] [CrossRef] [Green Version]
- Nzekoue, F.K.; Caprioli, G.; Ricciutelli, M.; Cortese, M.; Alesi, A.; Vittori, S.; Sagratini, G. Development of an innovative phytosterol derivatization method to improve the HPLC-DAD analysis and the ESI-MS detection of plant sterols/stanols. Int. Food Res. J. 2020, 131, 108998. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Nyström, L. Phytosterols. In Whole Grains and Their Bioactives: Composition and Health; Johnson, J., Wallace, T., Eds.; Wiley: Hoboken, NJ, USA, 2019; pp. 427–466. [Google Scholar]
- Duong, S.; Strobel, N.; Buddhadasa, S.; Stockham, K.; Auldist, M.J.; Wales, W.J.; Moate, P.J.; Orbell, J.D.; Cran, M.J. Influence of acid hydrolysis, saponification and sample clean-up on the measurement of phytosterols in dairy cattle feed using GC–MS and GC with flame ionization detection. J. Sep. Sci. 2018, 41, 3467–3476. [Google Scholar] [CrossRef] [PubMed]
- Moreau, R.A.; Nyström, L.; Whitaker, B.D.; Winkler-Moser, J.K.; Baer, D.J.; Gebauer, S.K.; Hicks, K.B. Phytosterols and their derivatives: Structural diversity, distribution, metabolism, analysis, and health-promoting uses. Prog. Lipid Res. 2018, 70, 35–61. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Ishimaru, M.; Shibata, T.; Hatate, H.; Tanaka, R. High-performance liquid chromatography with fluorescence detection for simultaneous analysis of phytosterols (stigmasterol, β-sitosterol, campesterol, ergosterol, and fucosterol) and cholesterol in plant foods. Food Anal. Methods 2017, 10, 2692–2699. [Google Scholar] [CrossRef]
- Speer, K.; Kölling-Speer, I. Lipids: Production, quality and chemistry. In Coffee; Farah, A., Ed.; Royal Society of Chemistry: London, UK, 2019; pp. 458–504. [Google Scholar]
- Shahzad, N.; Khan, W.; Shadab, M.D.; Ali, A.; Saluja, S.S.; Sharma, S.; Al-Allaf, F.A.; Abduljaleel, Z.; Ibrahim, I.A.A.; Abdel-Wahab, A.F.; et al. Phytosterols as a natural anticancer agent: Current status and future perspective. Biomed. Pharm. 2017, 88, 786–794. [Google Scholar] [CrossRef]
- Yang, R.; Xue, L.; Zhang, L.; Wang, X.; Qi, X.; Jiang, J.; Yu, L.; Wang, X.; Zhang, W.; Zhang, Q.; et al. Phytosterol contents of edible oils and their contributions to estimated phytosterol intake in the Chinese diet. Foods 2019, 8, 334. [Google Scholar] [CrossRef] [Green Version]
- Martins, C.M.; Fonseca, F.A.; Ballus, C.A.; Figueiredo-Neto, A.M.; Meinhart, A.D.; de Godoy, H.T.; Izar, M.C. Common sources and composition of phytosterols and their estimated intake by the population in the city of São Paulo, Brazil. Nutrition 2013, 29, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Ratnayake, W.M.N.; Hollywood, R.; O’grady, E.; Stavric, B. Lipid content and composition of coffee brews prepared by different methods. Food Chem. Toxicol. 1993, 31, 263–269. [Google Scholar] [CrossRef]
- Alves, R.C.; Casal, S.; Oliveira, M.B.P. Tocopherols in coffee brews: Influence of coffee species, roast degree and brewing procedure. J. Food Compos. Anal. 2010, 23, 802–808. [Google Scholar] [CrossRef]
- Rendón, M.Y.; dos Santos Scholz, M.B.; Bragagnolo, N. Physical characteristics of the paper filter and low cafestol content filter coffee brews. Int. Food Res. J. 2018, 108, 280–285. [Google Scholar] [CrossRef]
- Pacetti, D.; Boselli, E.; Balzano, M.; Frega, N.G. Authentication of Italian Espresso coffee blends through the GC peak ratio between kahweol and 16-O-methylcafestol. Food Chem. 2012, 135, 1569–1574. [Google Scholar] [CrossRef] [PubMed]
- Moeenfard, M.; Silva, J.A.; Borges, N.; Santos, A.; Alves, A. Diterpenes in espresso coffee: Impact of preparation parameters. Eur. Food Res. Technol. 2015, 240, 763–773. [Google Scholar] [CrossRef] [Green Version]
- Urgert, R.; Katan, M.B. The cholesterol-raising factor from coffee beans. Ann. Rev. Nutr. 1997, 17, 305–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novaes, F.J.M.; Bayan, F.C.; Neto, F.R.A.; Resende, C.M. The occurrence of cafestol and kahweol diterpenes in different coffee brews. Coffee Sci. 2019, 14, 265–280. [Google Scholar] [CrossRef]
- Naidoo, N.; Chen, C.; Rebello, S.A.; Speer, K.; Tai, E.S.; Lee, J.; Buchmann, S.; Koelling-Speer, I.; van Dam, R.M. Cholesterol-raising diterpenes in types of coffee commonly consumed in Singapore, Indonesia and India and associations with blood lipids: A survey and cross sectional study. Nutr. J. 2011, 10, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Tavani, A.; Bertuzzi, M.; Negri, E.; Sorbara, L.; La Vecchia, C. Alcohol, smoking, coffee and risk of non-fatal acute myocardial infarction in Italy. Eur. J. Epidemiol. 2001, 17, 1131–1137. [Google Scholar] [CrossRef]
- Rosner, S.A.; Åkesson, A.; Stampfer, M.J.; Wolk, A. Coffee consumption and risk of myocardial infarction among older Swedish women. Am. J. Epidemiol. 2007, 165, 288–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleemola, P.; Jousilahti, P.; Pietinen, P.; Vartiainen, E.; Tuomilehto, J. Coffee consumption and the risk of coronary heart disease and death. Arch. Intern. Med. 2000, 160, 3393–3400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez-Garcia, E.; Willett, W.C.; Rimm, E.B.; van Dam, R.; Manson, J.E.; Stampfer, M.J.; Hu, F.B. Coffee consumption and coronary heart disease in men and women: A prospective cohort study. Circulation 2006, 111, E210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grioni, S.; Agnoli, C.; Sieri, S.; Pala, V.; Ricceri, F.; Masala, G.; Saieva, C.; Panico, S.; Mattiello, A.; Chiodini, P.; et al. Espresso coffee consumption and risk of coronary heart disease in a large Italian cohort. PLoS ONE 2015, 10, e0126550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williamson, K.; Hatzakis, E. NMR analysis of roasted coffee lipids and development of a spent ground coffee application for the production of bioplastic precursors. Food Res. Int. 2019, 119, 683–692. [Google Scholar] [CrossRef]
- Guercia, E.; Berti, F.; Navarini, L.; Demitri, N.; Forzato, C. Isolation and characterization of major diterpenes from C. canephora roasted coffee oil. Tetrahedron Asymmetry 2016, 27, 649–656. [Google Scholar] [CrossRef]
- Wuerges, K.L.; Santos, A.C.F.D.; Mori, A.L.B.; Benassi, M.D.T. Contents of diterpenes in espresso coffee brews prepared from commercial capsules. Coffee Sci. 2016, 11, 276–284. [Google Scholar]
- European Commission. Commission Regulation (EU) No 432/2012. Off. J. Eur. Union 2012, 55, 136/1–136/40. [Google Scholar]






| Sample N°. | Sample Origin | |
|---|---|---|
| 1 | C | Brazil |
| 2 | C | Ethiopia (1) |
| 3 | C | Colombia |
| 4 | C | Costa Rica |
| 5 | R | Guatemala |
| 6 | R | EI Salvador |
| 7 | R | Yemen |
| 8 | R | Dominican Rep. |
| 9 | R | Ethiopia (2) |
| 10 | R | Rwanda |
| 11 | R | Burundi |
| 12 | R | Uganda |
| 13 | R | Kenya |
| 14 | R | Timor-Leste |
| Samples | Stigmasterol | Campesterol | β-Sitosterol | Cycloartenol | Total |
|---|---|---|---|---|---|
| 1 | 1.5 ± 0.1 | 1.0 ± 0.0 | 5.8 ± 0.2 | 0.3 ± 0.1 | 8.6 ± 0.3 a |
| 2 | 4.3 ± 0.3 | 3.2 ± 0.1 | 13.3 ± 0.4 | 1.2 ± 0.1 | 22.0 ± 1.0 bc |
| 3 | 1.9 ± 0.3 | 1.6 ± 0.3 | 8.4 ± 0.1 | 0.5 ± 0.0 | 12.4 ± 3.8 ad |
| 4 | 4.9 ± 0.1 | 4.6 ± 0.1 | 18.0 ± 0.3 | 2.0 ± 0.1 | 29.5 ± 0.6 c |
| 5 | 1.8 ± 0.1 | 1.5 ± 0.0 | 6.3 ± 0.3 | 0.7 ± 0.1 | 10.3 ± 0.2 ad |
| 6 | 1.1 ± 0.1 | 1.0 ± 0.1 | 4.8 ± 0.1 | 0.5 ± 0.0 | 7.5 ± 1.4 a |
| 7 | 2.0 ± 0.4 | 2.0 ± 0.6 | 8.8 ± 0.2 | 1.0 ± 0.3 | 13.7 ± 3.6 abd |
| 8 | 2.1 ± 0.0 | 1.8 ± 0.2 | 8.1 ± 0.1 | 0.8 ± 0.1 | 12.8 ± 0.4 ad |
| 9 | 3.0 ± 0.1 | 2.6 ± 0.0 | 9.4 ± 0.2 | 1.0 ± 0.0 | 16.0 ± 0.2 abd |
| 10 | 3.1 ± 0.2 | 2.8 ± 0.4 | 10.9 ± 0.9 | 1.4 ± 0.3 | 18.2 ± 0.2 bd |
| 11 | 2.8 ± 0.1 | 2.7 ± 0.1 | 10.5 ± 0.5 | 1.1 ± 0.3 | 16.4 ± 1.0 abd |
| 12 | 3.4 ± 1.0 | 3.3 ± 0.2 | 10.4 ± 2.2 | 1.5 ± 0.4 | 18.6 ± 2.8 bd |
| 13 | 2.9 ± 0.1 | 2.6 ± 0.2 | 11.0 ± 0.5 | 1.1 ± 0.0 | 17.5 ± 1.0 bd |
| 14 | 3.0 ± 0.8 | 2.5 ± 1.0 | 9.7 ± 2.0 | 1.2 ± 0.4 | 16.4 ± 2.2 abd |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nzekoue, F.K.; Alessandroni, L.; Caprioli, G.; Khamitova, G.; Navarini, L.; Ricciutelli, M.; Sagratini, G.; Sempere, A.N.; Vittori, S. Analysis of Phytosterols Content in Italian-Standard Espresso Coffee. Beverages 2021, 7, 61. https://doi.org/10.3390/beverages7030061
Nzekoue FK, Alessandroni L, Caprioli G, Khamitova G, Navarini L, Ricciutelli M, Sagratini G, Sempere AN, Vittori S. Analysis of Phytosterols Content in Italian-Standard Espresso Coffee. Beverages. 2021; 7(3):61. https://doi.org/10.3390/beverages7030061
Chicago/Turabian StyleNzekoue, Franks Kamgang, Laura Alessandroni, Giovanni Caprioli, Gulzhan Khamitova, Luciano Navarini, Massimo Ricciutelli, Gianni Sagratini, Alba Nácher Sempere, and Sauro Vittori. 2021. "Analysis of Phytosterols Content in Italian-Standard Espresso Coffee" Beverages 7, no. 3: 61. https://doi.org/10.3390/beverages7030061
APA StyleNzekoue, F. K., Alessandroni, L., Caprioli, G., Khamitova, G., Navarini, L., Ricciutelli, M., Sagratini, G., Sempere, A. N., & Vittori, S. (2021). Analysis of Phytosterols Content in Italian-Standard Espresso Coffee. Beverages, 7(3), 61. https://doi.org/10.3390/beverages7030061