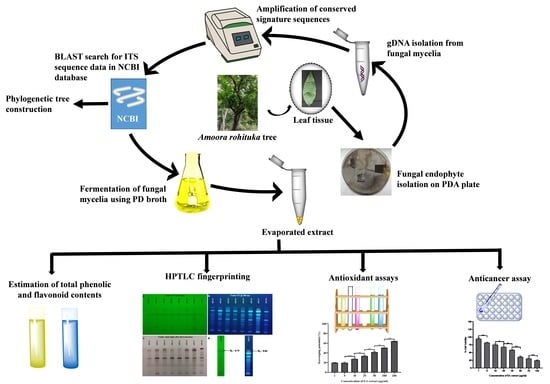
Assessment of Biological Activities of Fungal Endophytes Derived Bioactive Compounds Isolated from Amoora rohituka
Abstract
:1. Introduction
2. Materials and Method
2.1. Collection of Plant Sample and Isolation of Endophytic Fungi
2.2. Morphological and Molecular Identification of Isolated Fungal Strains
2.3. Phylogenetic Tree Construction
2.4. Fermentation Procedure and Extraction of Crude Fungal Extract
2.5. Estimation of Total Flavonoid Content and Total Phenolic Content of EA Extract of Fungal Mycelia
2.6. HPTLC Fingerprinting Analysis-Based Metabolites Profiling of EA Extract of Fungal Mycelia
2.7. Antioxidant Assays
2.7.1. Free Radical Scavenging Assay
2.7.2. Superoxide Anion Scavenging Activity
2.7.3. Hydroxyl Radical Scavenging Assay
2.7.4. Nitric Oxide Scavenging Assay
2.8. Culture Media Preparation and Cell Line Maintenance
Cytotoxicity Assay
2.9. Statistical Analysis
3. Results and Discussion
3.1. A Total of 8 Fungal Endophytes Were Isolated Using Culture-Dependent Approach
3.2. Eight Fungal Endophyte Species Belonging to the Division Ascomycota were Identified
3.3. EA Extract of P. oxalicum Contains Highest Amount of Phenolic and Flavonoid Content
3.4. HPTLC Fingerprint Analysis Showed the Presence of Unique Bioactive Components in P. oxalicum Derived Bioactive Compounds
3.5. EA Extract of A. flavus and P. oxalicum Shows Potential Free Radical Scavenging Activity
3.6. EA Extract of A. flavus and P. oxalicum Display Potential Superoxide Anion Scavenging Activity
3.7. EA Extract of T. longibrachiatum and P. oxalicum Exhibit Significant Hydroxyl Radical Scavenging Activity
3.8. EA Extract of T. longibrachiatum and P. oxalicum Shows Maximum Nitric Oxide Scavenging Activity
3.9. EA Extract of P. oxalicum Showed Promising Cytotoxic Activity against Cancer Cells
3.10. Comparative Analysis between EA Extract of AR-L7 and P. oxalicum (PO-01) Isolated from Rhizospheric Soil of Maize
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oladeji, O. The characteristics and roles of medicinal plants: Some important medicinal plants in Nigeria. Nat. Prod. Ind. J. 2016, 12, 102. [Google Scholar]
- Hamilton, A.C. Medicinal plants, conservation and livelihoods. Biodivers. Conserv. 2004, 13, 1477–1517. [Google Scholar] [CrossRef]
- Kaul, S.; Gupta, S.; Ahmed, M.; Dhar, M.K. Endophytic fungi from medicinal plants: A treasure hunt for bioactive metabolites. Phytochem. Rev. 2012, 11, 487–505. [Google Scholar] [CrossRef]
- Keshri, P.K.; Rai, N.; Verma, A.; Kamble, S.C.; Barik, S.; Mishra, P.; Singh, S.K.; Salvi, P.; Gautam, V. Biological potential of bioactive metabolites derived from fungal endophytes associated with medicinal plants. Mycol. Prog. 2021, 20, 577–594. [Google Scholar] [CrossRef]
- Rai, N.; Kumari Keshri, P.; Verma, A.; Kamble, S.C.; Mishra, P.; Barik, S.; Kumar Singh, S.; Gautam, V. Plant associated fungal endophytes as a source of natural bioactive compounds. Mycology 2021, 12, 139–159. [Google Scholar] [CrossRef]
- Fadiji, A.E.; Babalola, O.O. Elucidating mechanisms of endophytes used in plant protection and other bioactivities with multifunctional prospects. Front. Bioeng. Biotechnol. 2020, 8, 467. [Google Scholar] [CrossRef]
- Ranjan, A.; Singh, R.K.; Khare, S.; Tripathi, R.; Pandey, R.K.; Singh, A.K.; Gautam, V.; Tripathi, J.S.; Singh, S.K. Characterization and evaluation of mycosterol secreted from endophytic strain of Gymnema sylvestre for inhibition of α-glucosidase activity. Sci. Rep. 2019, 9, 17302. [Google Scholar] [CrossRef] [Green Version]
- Gupta, P.; Verma, A.; Rai, N.; Singh, A.K.; Singh, S.K.; Kumar, B.; Kumar, R.; Gautam, V. Mass Spectrometry-Based Technology and Workflows for Studying the Chemistry of Fungal Endophyte Derived Bioactive Compounds. ACS Chem. Biol. 2021, 16, 2068–2086. [Google Scholar] [CrossRef]
- Chopra, R.N. Glossary of Indian Medicinal Plants; Council of Scientific & Industrial Research: Delhi, India, 1956. [Google Scholar]
- Jagetia, G.C.; Venkatesha, V. Preclinical determination of the anticancer activity of rohituka (Aphanamixis polystachya) in Ehrlich ascites tumor-bearing mice. Med. Aromat. Plant Sci. Biotechnol. 2012, 6, 42–51. [Google Scholar]
- Dhar, M.; Dhar, M.; Dhawan, B.; Mehrotra, B.; Ray, C. Screening of Indian plants for biological activity: Part I. Indian J. Exp. Biol. 1968, 6, 232–472. [Google Scholar]
- Jagetia, G.C.; Venkatesha, V.A.K. Enhancement of radiation effect by Aphanamixis polystachya in mice transplanted with Ehrlich ascites carcinoma. Biol. Pharm. Bull. 2005, 28, 69–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jagetia, G.C.; Venkatesha, V. Treatment of mice with stem bark extract of Aphanamixis polystachya reduces radiation-induced chromosome damage. Int. J. Radiat. Biol. 2006, 82, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Hart, A.; Wimalasena, T.; Tucker, G.; Greetham, D. Expression of RCK2 MAPKAP (MAPK-activated protein kinase) rescues yeast cells sensitivity to osmotic stress. Microb. Cell Factories 2015, 14, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boericke, W. New Manual of Homoeopathic Materia Medica and Repertory; B. Jain Publishers: Uttar Pradesh, India, 2001. [Google Scholar]
- Harmon, A.D.; Weiss, U.; Silverton, J. The structure of rohitukine, the main alkaloid of Amoora rohituka (syn. Aphanamixis polystachya) (Meliaceae). Tetrahedron Lett. 1979, 20, 721–724. [Google Scholar] [CrossRef]
- Naik, R.G.; Kattige, S.; Bhat, S.; Alreja, B.; De Souza, N.; Rupp, R. An antiinflammatory cum immunomodulatory piperidinylbenzopyranone from Dysoxylum binectariferum: Isolation, structure and total synthesis. Tetrahedron 1988, 44, 2081–2086. [Google Scholar] [CrossRef]
- Singh, N.; Singh, P.; Shrivastva, S.; Mishra, S.K.; Lakshmi, V.; Sharma, R.; Palit, G. Gastroprotective effect of anti-cancer compound rohitukine: Possible role of gastrin antagonism and H+ K+-ATPase inhibition. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2012, 385, 277–286. [Google Scholar] [CrossRef]
- Ismail, I.S.; Nagakura, Y.; Hirasawa, Y.; Hosoya, T.; Lazim, M.I.M.; Lajis, N.H.; Shiro, M.; Morita, H. Chrotacumines A−D, Chromone Alkaloids from Dysoxylum acutangulum. J. Nat. Prod. 2009, 72, 1879–1883. [Google Scholar] [CrossRef]
- Singh, R.K.; Ranjan, A.; Srivastava, A.K.; Singh, M.; Shukla, A.K.; Atri, N.; Mishra, A.; Singh, A.K.; Singh, S.K. Cytotoxic and apoptotic inducing activity of Amoora rohituka leaf extracts in human breast cancer cells. J. Ayurveda Integr. Med. 2020, 11, 383–390. [Google Scholar] [CrossRef]
- Apu, A.S.; Pathan, A.H.; Jamaluddin, A.T.M.; Ara, F.; Bhuyan, S.H.; Islam, M.R. Phytochemical analysis and bioactivities of Aphanamixis polystachya (Wall.) R. Parker leaves from Bangladesh. J. Biol. Sci. 2013, 13, 393–399. [Google Scholar] [CrossRef] [Green Version]
- Petrini, O.; Sieber, T.N.; Toti, L.; Viret, O. Ecology, metabolite production, and substrate utilization in endophytic fungi. Nat. Toxins 1993, 1, 185–196. [Google Scholar] [CrossRef]
- Nisa, H.; Kamili, A.N.; Nawchoo, I.A.; Bhat, M.S.; Nazir, R. Isolation and identification of endophytic fungi from Artemisia scoparia (Asteraceae). Int. J. Theor. Appl. Sci. 2018, 10, 83–88. [Google Scholar]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Cai, Y.; Luo, Q.; Sun, M.; Corke, H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004, 74, 2157–2184. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [Green Version]
- Singh, N.; Rajini, P. Free radical scavenging activity of an aqueous extract of potato peel. Food Chem. 2004, 85, 611–616. [Google Scholar] [CrossRef]
- Nishikimi, M.; Rao, N.A.; Yagi, K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972, 46, 849–854. [Google Scholar] [CrossRef]
- Gupta, P.; Jain, V.; Pareek, A.; Kumari, P.; Singh, R.; Agarwal, P.; Sharma, V. Evaluation of effect of alcoholic extract of heartwood of Pterocarpus marsupium on in vitro antioxidant, anti-glycation, sorbitol accumulation and inhibition of aldose reductase activity. J. Tradit. Complementary Med. 2017, 7, 307–314. [Google Scholar] [CrossRef] [Green Version]
- Okayo, R.O.; Andika, D.O.; Dida, M.M.; K’Otuto, G.O.; Gichimu, B.M. Morphological and Molecular Characterization of Toxigenic Aspergillus flavus from Groundnut Kernels in Kenya. Int. J. Microbiol. 2020, 2020, 8854718. [Google Scholar] [CrossRef]
- Zhao, R.-B.; Bao, H.-Y.; Liu, Y.-X. Isolation and characterization of Penicillium oxalicum ZHJ6 for biodegradation of methamidophos. Agric. Sci. China 2010, 9, 695–703. [Google Scholar] [CrossRef]
- Gomes, R.; Glienke, C.; Videira, S.; Lombard, L.; Groenewald, J.; Crous, P. Diaporthe: A genus of endophytic, saprobic and plant pathogenic fungi. Pers. Mol. Phylogeny Evol. Fungi 2013, 31, 1–41. [Google Scholar] [CrossRef] [Green Version]
- Oberoi, H.; Sandhu, S. Therapeutic and Nutraceutical Potential of Bioactive Compounds Extracted from Fruit Residues AU—Babbar, Neha. Crit. Rev. Food Sci. Nutr 2015, 55, 319–337. [Google Scholar]
- Nermien, H.; Thabet, A.; Markeb, A.; Sayed, D.; El-Maali, N. In vitro-exploration of fungal endophytes of Egyptian Cynara scolymus L. (artichoke) and investigation of some their bioactive potentials. Glob. NEST J. 2019, 21, 296–308. [Google Scholar]
- Gauchan, D.P.; Kandel, P.; Tuladhar, A.; Acharya, A.; Kadel, U.; Baral, A.; Shahi, A.B.; García-Gil, M.R. Evaluation of antimicrobial, antioxidant and cytotoxic properties of bioactive compounds produced from endophytic fungi of Himalayan yew (Taxus wallichiana) in Nepal. FResearch 2020, 9, 379. [Google Scholar]
- Ayob, F.W.; Mohamad, J.; Simarani, K. Antioxidants and Phytochemical Analysis of Endophytic Fungi Isolated from a Medicinal Plant Catharanthus roseus. Borneo J. Sci. Technol. 2019, 1, 62–68. [Google Scholar]
- Agatonovic-Kustrin, S.; Kustrin, E.; Gegechkori, V.; Morton, D.W. High-performance thin-layer chromatography hyphenated with microchemical and biochemical derivatizations in bioactivity profiling of marine species. Mar. Drugs 2019, 17, 148. [Google Scholar] [CrossRef] [Green Version]
- Randhir, R.; Shetty, K. Mung beans processed by solid-state bioconversion improves phenolic content and functionality relevant for diabetes and ulcer management. Innov. Food Sci. Emerg. Technol. 2007, 8, 197–204. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.Y. Oxygen radical absorbing capacity of phenolics in blueberries, cranberries, chokeberries, and lingonberries. J. Agric. Food Chem. 2003, 51, 502–509. [Google Scholar] [CrossRef]
- De Pinedo, A.T.; Peñalver, P.; Morales, J.C. Synthesis and evaluation of new phenolic-based antioxidants: Structure–activity relationship. Food Chem. 2007, 103, 55–61. [Google Scholar] [CrossRef]
- Sousa, B.; Correia, R. Phenolic content, antioxidant activity and antiamylolytic activity of extracts obtained from bioprocessed pineapple and guava wastes. Braz. J. Chem. Eng. 2012, 29, 25–30. [Google Scholar] [CrossRef]
- Lim, S.M.; Agatonovic-Kustrin, S.; Lim, F.T.; Ramasamy, K. High-performance thin layer chromatography-based phytochemical and bioactivity characterisation of anticancer endophytic fungal extracts derived from marine plants. J. Pharm. Biomed. Anal. 2021, 193, 113702. [Google Scholar] [CrossRef]
- Piluzza, G.; Bullitta, S. Correlations between phenolic content and antioxidant properties in twenty-four plant species of traditional ethnoveterinary use in the Mediterranean area. Pharm. Biol. 2011, 49, 240–247. [Google Scholar] [CrossRef]
- Kaur, R.; Kaur, J.; Kaur, M.; Kalotra, V.; Chadha, P.; Kaur, A.; Kaur, A. An endophytic Penicillium oxalicum isolated from Citrus limon possesses antioxidant and genoprotective potential. J. Appl. Microbiol. 2020, 128, 1400–1413. [Google Scholar] [CrossRef]
- Abrol, V.; Kushwaha, M.; Arora, D.; Mallubhotla, S.; Jaglan, S. Mutation, Chemoprofiling, Dereplication, and Isolation of Natural Products from Penicillium oxalicum. ACS Omega 2021, 6, 16266–16272. [Google Scholar] [CrossRef]
- Maddu, N. Diseases related to types of free radicals. In Antioxidants; IntechOpen: London, UK, 2019. [Google Scholar]
- Chen, Y.; Mao, W.; Tao, H.; Zhu, W.; Qi, X.; Chen, Y.; Li, H.; Zhao, C.; Yang, Y.; Hou, Y. Structural characterization and antioxidant properties of an exopolysaccharide produced by the mangrove endophytic fungus Aspergillus sp. Y16. Bioresour. Technol. 2011, 102, 8179–8184. [Google Scholar] [CrossRef]
- Tang, Z.; Qin, Y.; Chen, W.; Zhao, Z.; Lin, W.; Xiao, Y.; Chen, H.; Liu, Y.; Bu, T.; Li, Q. Diversity, Chemical Constituents, and Biological Activities of Endophytic Fungi Isolated from Ligusticum chuanxiong Hort. Front. Microbiol. 2021, 12, 771000–771000. [Google Scholar] [CrossRef]
- Kandasamy, S.; Kandasamy, K. Antioxidant activity of the mangrove endophytic fungus (Trichoderma sp.). J. Coast. Life Med. 2014, 2, 566–570. [Google Scholar]
- Bonekamp, N.A.; Völkl, A.; Fahimi, H.D.; Schrader, M.J.B. Reactive oxygen species and peroxisomes: Struggling for balance. Biofactors 2009, 35, 346–355. [Google Scholar] [CrossRef]
- Yang, X.; Kang, M.-C.; Li, Y.; Kim, E.-A.; Kang, S.-M.; Jeon, Y.-J. Asperflavin, an anti-inflammatory compound produced by a marine-derived fungus, Eurotium amstelodami. Molecules 2017, 22, 1823. [Google Scholar] [CrossRef] [Green Version]
- Freeman, B.L.; Eggett, D.L.; Parker, T.L. Synergistic and antagonistic interactions of phenolic compounds found in navel oranges. J. Food Sci. 2010, 75, C570–C576. [Google Scholar] [CrossRef]
- Hidalgo, M.; Sánchez-Moreno, C.; de Pascual-Teresa, S. Flavonoid–flavonoid interaction and its effect on their antioxidant activity. Food Chem. 2010, 121, 691–696. [Google Scholar] [CrossRef]
- Tapfuma, K.I.; Uche-Okereafor, N.; Sebola, T.E.; Hussan, R.; Mekuto, L.; Makatini, M.M.; Green, E.; Mavumengwana, V. Cytotoxic activity of crude extracts from Datura stramonium’s fungal endophytes against A549 lung carcinoma and UMG87 glioblastoma cell lines and LC-QTOF-MS/MS based metabolite profiling. BMC Complementary Altern. Med. 2019, 19, 330. [Google Scholar] [CrossRef]
- Kumar, V.S.; Kumaresan, S.; Tamizh, M.M.; Islam, M.I.H.; Thirugnanasambantham, K. Anticancer potential of NF-κB targeting apoptotic molecule “flavipin” isolated from endophytic Chaetomium globosum. Phytomedicine 2019, 61, 152830. [Google Scholar] [CrossRef]
- Deshmukh, S.K.; Mishra, P.D.; Kulkarni-Almeida, A.; Verekar, S.; Sahoo, M.R.; Periyasamy, G.; Goswami, H.; Khanna, A.; Balakrishnan, A.; Vishwakarma, R. Anti-inflammatory and anticancer activity of ergoflavin isolated from an endophytic fungus. Chem. Biodivers. 2009, 6, 784–789. [Google Scholar] [CrossRef]
- Parthasarathy, R.; Chandrika, M.; Rao, H.Y.; Kamalraj, S.; Jayabaskaran, C.; Pugazhendhi, A. Molecular profiling of marine endophytic fungi from green algae: Assessment of antibacterial and anticancer activities. Process Biochem. 2020, 96, 11–20. [Google Scholar] [CrossRef]
- Unterseher, M.; Schnittler, M. Species richness analysis and ITS rDNA phylogeny revealed the majority of cultivable foliar endophytes from beech (Fagus sylvatica). Fungal Ecol. 2010, 3, 366–378. [Google Scholar] [CrossRef]
- Moricca, S.; Ragazzi, A. Fungal endophytes in Mediterranean oak forests: A lesson from Discula quercina. Phytopathology 2008, 98, 380–386. [Google Scholar] [CrossRef] [Green Version]
- Redman, R.S.; Dunigan, D.D.; Rodriguez, R.J. Fungal symbiosis from mutualism to parasitism: Who controls the outcome, host or invader? New Phytol. 2001, 151, 705–716. [Google Scholar] [CrossRef] [Green Version]
- Kogel, K.-H.; Franken, P.; Hückelhoven, R. Endophyte or parasite–what decides? Curr. Opin. Plant Biol. 2006, 9, 358–363. [Google Scholar] [CrossRef]
- Steyn, P. The isolation, structure and absolute configuration of secalonic acid D, the toxic metabolite of Penicillium oxalicum. Tetrahedron 1970, 26, 51–57. [Google Scholar] [CrossRef]
- Sun, Y.-L.; Bao, J.; Liu, K.-S.; Zhang, X.-Y.; He, F.; Wang, Y.-F.; Nong, X.-H.; Qi, S.-H. Cytotoxic dihydrothiophene-condensed chromones from the marine-derived fungus Penicillium oxalicum. Planta Med. 2013, 79, 1474–1479. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Xie, J.; Jiang, D.; Fu, Y.; Li, G.; Lin, F. Antifungal substances produced by Penicillium oxalicum strain PY-1—Potential antibiotics against plant pathogenic fungi. World J. Microbiol. Biotechnol. 2008, 24, 909–915. [Google Scholar] [CrossRef]
- Shen, S.; Li, W.; Wang, J. Antimicrobial and antitumor activities of crude secondary metabolites from a marine fungus Penicillium oxalicum 0312F1. Afr. J. Microbiol. Res. 2014, 8, 1480–1485. [Google Scholar]
- Nyongesa, B.W.; Okoth, S.; Ayugi, V. Identification key for Aspergillus species isolated from maize and soil of Nandi County, Kenya. Adv. Microbiol. 2015, 5, 205. [Google Scholar] [CrossRef] [Green Version]
- Wickerham, L.J. Validation of the species Pichia guilliermondii. J. Bacteriol. 1966, 92, 1269. [Google Scholar] [CrossRef] [Green Version]
- Prameeladevi, T.; Prabhakaran, N.; Kamil, D.; Toppo, R.S.; Tyagi, A. Trichoderma pseudokoningii identified based on morphology was re-identified as T. longibrachiatum through molecular characterization. Indian Phytopathol. 2018, 71, 579–587. [Google Scholar] [CrossRef]
- Noonim, P.; Mahakarnchanakul, W.; Varga, J.; Frisvad, J.C.; Samson, R.A. Two novel species of Aspergillus section Nigri from Thai coffee beans. Int. J. Syst. Evol. Microbiol. 2008, 58, 1727–1734. [Google Scholar] [CrossRef]
- Moubasher, A.; Soliman, Z. Aspergillus assiutensis, a new species from the air of grapevine plantation, Egypt. J. Basic Appl. Mycol. Egypt 2011, 2, 83–90. [Google Scholar]
 ), Spores (
), Spores (  ), Conidia (
), Conidia (  ) Conidiophore (
) Conidiophore (  ).
).
 ), Spores (
), Spores (  ), Conidia (
), Conidia (  ) Conidiophore (
) Conidiophore (  ).
).







| S. No. | Species Identified | Total Phenolic Content (µg GAE/mg of EA Extract) | Total Flavonoid Content (µg QE/mg of EA Extract) |
|---|---|---|---|
| 1. | A. flavus | 45.65 | 17.70 |
| 2. | A. aculeatus | 33.68 | 34.16 |
| 3. | M. guilliermondii | 23.40 | 60.08 |
| 4. | T. longibrachiatum | 32.42 | 78.88 |
| 5. | A. aculeatinus | 69.36 | 32.65 |
| 6. | A. assiutensis | 72.19 | 83.40 |
| 7. | P. oxalicum | 120.99 | 155.69 |
| 8. | Diaporthe sp. SAUCC194 | 78.91 | 55.14 |
| S. No. | Species | EC50 Value (µg/mL) | |||
|---|---|---|---|---|---|
| DPPH Free Radical Scavenging Assay | Superoxide Anion Scavenging Activity | Hydroxyl Radical Scavenging Assay | Nitric Oxide Scavenging Assay | ||
| 1. | A. flavus | 178.30 ± 1.446 | 157.52 ± 1.118 | >200 | >200 |
| 2. | A. aculeatus | >200 | >200 | >200 | >200 |
| 3. | M. guilliermondii | >200 | >200 | >200 | >200 |
| 4. | T. longibrachiatum | >200 | 170.43 ± 1.405 | 113.30 ± 1.206 | 89.14 ± 0.894 |
| 5. | A. aculeatinus | >200 | >200 | >200 | >200 |
| 6. | A. assiutensis | >200 | >200 | >200 | >200 |
| 7. | P. oxalicum | 96.98 ± 0.270 | 169.28 ± 0.402 | 126.12 ± 0.636 | 75.79 ± 0.692 |
| 8. | Diaporthe sp. SAUCC194 | >200 | >200 | >200 | >200 |
| Positive control | Ascorbic Acid | 10.60 ± 0.257 | 33.36 ± 1.186 | 24.37 ± 1.116 | 21.75 ± 0.566 |
| Isolated Strains | Species | IC50 Value (µg/mL) | ||
| T-Cell Lymphoma Cancer Cell (HuT-78) | Human Breast Cancer Cell (MDA-MB-231) | Human Breast Cancer Cell (MCF-7) | ||
| AR-L7 | P. oxalicum | 56.81 ± 0.617 | 37.24 ± 1.26 | 260.627 ± 5.415 |
| Fungal Strains | Host Plant | EC50 Value (µg/mL) | |||
|---|---|---|---|---|---|
| DPPH Free Radical Scavenging Assay | Superoxide Anion Scavenging Assay | Hydroxyl Radical Scavenging Assay | Nitric Oxide Scavenging Activity | ||
| AR-L7 (P. oxalicum) | Leaf of A. rohituka | >200 | >200 | >200 | >200 |
| PO-01 (P. oxalicum) | Rhizospheric soil of maize | 83.760 ± 1.14 µg/mL | 162.12 ± 1.185 µg/mL | 123.62 ± 2.01 µg/mL | 75.60 ± 1.31 µg/mL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verma, A.; Gupta, P.; Rai, N.; Tiwari, R.K.; Kumar, A.; Salvi, P.; Kamble, S.C.; Singh, S.K.; Gautam, V. Assessment of Biological Activities of Fungal Endophytes Derived Bioactive Compounds Isolated from Amoora rohituka. J. Fungi 2022, 8, 285. https://doi.org/10.3390/jof8030285
Verma A, Gupta P, Rai N, Tiwari RK, Kumar A, Salvi P, Kamble SC, Singh SK, Gautam V. Assessment of Biological Activities of Fungal Endophytes Derived Bioactive Compounds Isolated from Amoora rohituka. Journal of Fungi. 2022; 8(3):285. https://doi.org/10.3390/jof8030285
Chicago/Turabian StyleVerma, Ashish, Priyamvada Gupta, Nilesh Rai, Rajan Kumar Tiwari, Ajay Kumar, Prafull Salvi, Swapnil C. Kamble, Santosh Kumar Singh, and Vibhav Gautam. 2022. "Assessment of Biological Activities of Fungal Endophytes Derived Bioactive Compounds Isolated from Amoora rohituka" Journal of Fungi 8, no. 3: 285. https://doi.org/10.3390/jof8030285
APA StyleVerma, A., Gupta, P., Rai, N., Tiwari, R. K., Kumar, A., Salvi, P., Kamble, S. C., Singh, S. K., & Gautam, V. (2022). Assessment of Biological Activities of Fungal Endophytes Derived Bioactive Compounds Isolated from Amoora rohituka. Journal of Fungi, 8(3), 285. https://doi.org/10.3390/jof8030285