Mycotherapy: Potential of Fungal Bioactives for the Treatment of Mental Health Disorders and Morbidities of Chronic Pain
Abstract
:1. Introduction
2. Mental Health Disorders
Mental Health and Co-Morbidities of Chronic Pain
3. Fungal Biologics: Unlocking the Potential of Eastern Practice into Western Medicine
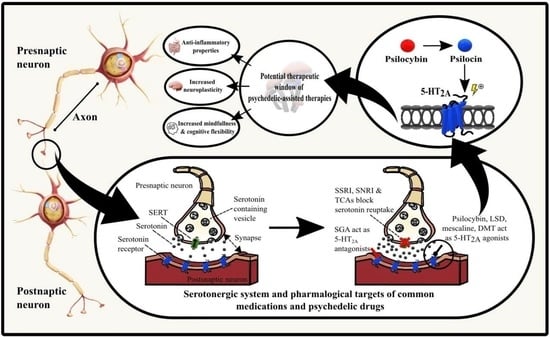
3.1. Psilocybe Mushrooms
3.2. Claviceps purpurea
3.3. Amanita muscaria
3.4. Hericium erinaceus (H. erinaceus)
3.5. Pleurotus cornucopiae
4. Pharmacological Consideration of Mycotherapy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association. DSM 5 Diagnostic and Statistical Manual of Mental Disorders; American Psychiatric Association: Washington, DC, USA, 2013; 947p. [Google Scholar]
- Ebneter, D.S.; Latner, J.D. Stigmatizing attitudes differ across mental health disorders: A comparison of stigma across eating disorders, obesity, and major depressive disorder. J. Nerv. Ment. Dis. 2013, 201, 281–285. [Google Scholar] [CrossRef]
- Adam, D. Mental health: On the spectrum. Nature 2013, 496, 416–418. [Google Scholar] [CrossRef] [Green Version]
- Kessler, R.C.; Angermeyer, M.; Anthony, J.C.; De Graaf, R.; Demyttenaere, K.; Gasquet, I.; De Girolamo, G.; Gluzman, S.; Gureje, O.; Haro, J.M.; et al. Lifetime prevalence and age-of-onset distributions of mental disorders in the World Health Organization’s World Mental Health Survey Initiative. World Psychiatry 2007, 6, 168–176. [Google Scholar] [PubMed]
- World Health Organisation. Mental Health. Available online: https://www.who.int/health-topics/mental-health#tab=tab_12021 (accessed on 23 February 2022).
- Trautmann, S.; Rehm, J.; Wittchen, H.-U. The economic costs of mental disorders: Do our societies react appropriately to the burden of mental disorders? EMBO Rep. 2016, 17, 1245–1249. [Google Scholar] [CrossRef] [PubMed]
- Chong, P.S.; Fung, M.-L.; Wong, K.H.; Lim, L.W. Therapeutic Potential of Hericium erinaceus for Depressive Disorder. Int. J. Mol. Sci. 2019, 21, 163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keller, M.B. Issues in treatment-resistant depression. J. Clin. Psychiatry 2005, 66 (Suppl. 8), 5–12. [Google Scholar]
- Al-Harbi, K.S. Treatment-resistant depression: Therapeutic trends, challenges, and future directions. Patient Prefer. Adherence 2012, 6, 369–388. [Google Scholar] [CrossRef] [Green Version]
- Johansen, P.; Krebs, T.S. Psychedelics not linked to mental health problems or suicidal behavior: A population study. J. Psychopharmacol. 2015, 29, 270–279. [Google Scholar] [CrossRef]
- Davis, A.K.; Agin-Liebes, G.; España, M.; Pilecki, B.; Luoma, J. Attitudes and Beliefs about the Therapeutic Use of Psychedelic Drugs among Psychologists in the United States. J. Psychoact. Drugs 2021, 53, 1–10. [Google Scholar] [CrossRef]
- Strawn, J.R.; Geracioti, L.; Rajdev, N.; Clemenza, K.; Levine, A. Pharmacotherapy for generalized anxiety disorder in adult and pediatric patients: An evidence-based treatment review. Expert Opin. Pharmacother. 2018, 19, 1057–1070. [Google Scholar] [CrossRef]
- Garakani, A.; Murrough, J.W.; Freire, R.C.; Thom, R.P.; Larkin, K.; Buono, F.D.; Iosifescu, D.V. Pharmacotherapy of Anxiety Disorders: Current and Emerging Treatment Options. Review. Front. Psychiatry 2020, 11, 1412. [Google Scholar] [CrossRef]
- Moraczewski, J.; Aedma, K.K. Tricyclic Antidepressants; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Schneider, J.; Patterson, M.; Jimenez, X.F. Beyond depression: Other uses for tricyclic antidepressants. Clevel. Clin. J. Med. 2019, 86, 807–814. [Google Scholar] [CrossRef]
- Chamberlain, S.R.; Baldwin, D.S. Monoamine oxidase inhibitors (MAOIs) in psychiatric practice: How to use them safely and effectively. CNS Drugs 2021, 35, 703–716. [Google Scholar] [CrossRef]
- Sub Laban, T.; Saadabadi, A. Monoamine Oxidase Inhibitors (MAOI); StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Ansara, E.D. Management of treatment-resistant generalized anxiety disorder. Ment. Health Clin. 2020, 10, 326–334. [Google Scholar] [CrossRef]
- Sartori, S.B.; Singewald, N. Novel pharmacological targets in drug development for the treatment of anxiety and anxiety-related disorders. Pharmacol. Ther. 2019, 204, 107402. [Google Scholar] [CrossRef]
- Shmuts, R.; Kay, A.; Beck, M. Buspirone: A forgotten friend. Curr. Psychiatry 2020, 19, 20. [Google Scholar]
- Wilson, T.K.; Tripp, J. Buspirone; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Schloesser, R.J.; Martinowich, K.; Manji, H.K. Mood-stabilizing drugs: Mechanisms of action. Trends Neurosci. 2012, 35, 36–46. [Google Scholar] [CrossRef]
- Iannaccone, T.; Sellitto, C.; Manzo, V.; Colucci, F.; Giudice, V.; Stefanelli, B.; Iuliano, A.; Corrivetti, G.; Filippelli, A. Pharmacogenetics of carbamazepine and valproate: Focus on polymorphisms of drug metabolizing enzymes and transporters. Pharmaceuticals 2021, 14, 204. [Google Scholar] [CrossRef]
- Pérez de Mendiola, X.; Hidalgo-Mazzei, D.; Vieta, E.; González-Pinto, A. Overview of lithium’s use: A nationwide survey. Int. J. Bipolar Disord. 2021, 9, 10. [Google Scholar] [CrossRef]
- Murru, A.; Popovic, D.; Pacchiarotti, I.; Hidalgo, D.; León-Caballero, J.; Vieta, E. Management of Adverse Effects of Mood Stabilizers. Curr. Psychiatry Rep. 2015, 17, 66. [Google Scholar] [CrossRef]
- Bourin, M. Mechanism of action of valproic acid and its derivatives. SOJ Pharm. Sci. 2020, 7, 1–4. [Google Scholar]
- Grunze, A.; Amann, B.L.; Grunze, H. Efficacy of Carbamazepine and Its Derivatives in the Treatment of Bipolar Disorder. Medicina 2021, 57, 433. [Google Scholar] [CrossRef]
- Chokhawala, K.; Stevens, L. Antipsychotic Medications; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Stroup, T.S.; Gray, N. Management of common adverse effects of antipsychotic medications. World Psychiatry Off. J. World Psychiatr. Assoc. 2018, 17, 341–356. [Google Scholar] [CrossRef]
- Lally, J.; MacCabe, J.H. Antipsychotic medication in schizophrenia: A review. Br. Med. Bull. 2015, 114, 169–179. [Google Scholar] [CrossRef] [Green Version]
- Norris, M.L.; Spettigue, W.; Buchholz, A.; Henderson, K.A.; Gomez, R.; Maras, D.; Gaboury, I.; Ni, A. Olanzapine use for the adjunctive treatment of adolescents with anorexia nervosa. J. Child Adolesc. Psychopharmacol. 2011, 21, 213–220. [Google Scholar] [CrossRef] [Green Version]
- Çöpür, S.; Çöpür, M. Olanzapine in the treatment of anorexia nervosa: A systematic review. Egypt. J. Neurol. Psychiatry Neurosurg. 2020, 56, 60. [Google Scholar] [CrossRef]
- Milano, W.; De Rosa, M.; Milano, L.; Riccio, A.; Sanseverino, B.; Capasso, A. The Pharmacological Options in the Treatment of Eating Disorders. ISRN Pharmacol. 2013, 2013, 352865. [Google Scholar] [CrossRef]
- Fariba, K.; Saadabadi, A. Topiramate; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Grant, J.E.; Schreiber, L.; Odlaug, B.L. Impulse control disorders: Updated review of clinical characteristics and pharmacological management. Front. Psychiatry 2011, 2, 1. [Google Scholar]
- Douaihy, A.B.; Kelly, T.M.; Sullivan, C. Medications for substance use disorders. Soc. Work Public Health 2013, 28, 264–278. [Google Scholar] [CrossRef] [Green Version]
- Fontenelle, L.F.; Oostermeijer, S.; Harrison, B.J.; Pantelis, C.; Yücel, M. Obsessive-compulsive disorder, impulse control disorders and drug addiction. Drugs 2011, 71, 827–840. [Google Scholar] [CrossRef]
- Kayser, R.R. Pharmacotherapy for treatment-resistant obsessive-compulsive disorder. J. Clin. Psychiatry 2020, 81, 14428. [Google Scholar] [CrossRef] [PubMed]
- Mouaffak, F.; Leite, C.; Hamzaoui, S.; Benyamina, A.; Laqueille, X.; Kebir, O. Naltrexone in the treatment of broadly defined behavioral addictions: A review and meta-analysis of randomized controlled trials. Eur. Addict. Res. 2017, 23, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Bolton, M.; Hodkinson, A.; Boda, S.; Mould, A.; Panagioti, M.; Rhodes, S.; Riste, L.; van Marwijk, H. Serious adverse events reported in placebo randomised controlled trials of oral naltrexone: A systematic review and meta-analysis. BMC Med. 2019, 17, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timäus, C.; Meiser, M.; Bandelow, B.; Engel, K.R.; Paschke, A.M.; Wiltfang, J.; Wedekind, D. Pharmacotherapy of borderline personality disorder: What has changed over two decades? A retrospective evaluation of clinical practice. BMC Psychiatry 2019, 19, 393. [Google Scholar] [CrossRef] [Green Version]
- Bozzatello, P.; Ghirardini, C.; Uscinska, M.; Rocca, P.; Bellino, S. Pharmacotherapy of personality disorders: What we know and what we have to search for. Future Neurol. 2017, 12, 199–222. [Google Scholar] [CrossRef]
- Uher, R.; Zwicker, A. Etiology in psychiatry: Embracing the reality of poly-gene-environmental causation of mental illness. World Psychiatry Off. J. World Psychiatr. Assoc. 2017, 16, 121–129. [Google Scholar] [CrossRef] [Green Version]
- Wu, N.S.; Schairer, L.C.; Dellor, E.; Grella, C. Childhood trauma and health outcomes in adults with comorbid substance abuse and mental health disorders. Addict. Behav. 2010, 35, 68–71. [Google Scholar] [CrossRef] [Green Version]
- Gold, P.W.; Wong, M.-L. Re-assessing the catecholamine hypothesis of depression: The case of melancholic depression. Mol. Psychiatry 2021, 26, 6121–6124. [Google Scholar] [CrossRef]
- Albert, P.R.; Benkelfat, C.; Descarries, L. The neurobiology of depression—Revisiting the serotonin hypothesis. I. Cellular and molecular mechanisms. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2012, 367, 2378–2381. [Google Scholar] [CrossRef] [Green Version]
- Baumeister, D.; Barnes, G.; Giaroli, G.; Tracy, D. Classical hallucinogens as antidepressants? A review of pharmacodynamics and putative clinical roles. Ther. Adv. Psychopharmacol. 2014, 4, 156–169. [Google Scholar] [CrossRef] [Green Version]
- Malaca, S.; Lo Faro, A.F.; Tamborra, A.; Pichini, S.; Busardò, F.P.; Huestis, M.A. Toxicology and Analysis of Psychoactive Tryptamines. Int. J. Mol. Sci. 2020, 21, 9279. [Google Scholar] [CrossRef]
- Lew, S.Y.; Lim, S.H.; Lim, L.W.; Wong, K.H. Neuroprotective effects of Hericium erinaceus (Bull.: Fr.) Pers. against high-dose corticosterone-induced oxidative stress in PC-12 cells. BMC Complement. Med. Ther. 2020, 20, 340. [Google Scholar] [CrossRef]
- Conrad, C.D. Chronic stress-induced hippocampal vulnerability: The glucocorticoid vulnerability hypothesis. Rev. Neurosci. 2008, 19, 395–412. [Google Scholar] [CrossRef] [Green Version]
- Crocker, L.; Heller, W.; Warren, S.; O’Hare, A.; Infantolino, Z.; Miller, G. Relationships among cognition, emotion, and motivation: Implications for intervention and neuroplasticity in psychopathology. Review. Front. Hum. Neurosci. 2013, 7, 261. [Google Scholar] [CrossRef] [Green Version]
- Volpi-Abadie, J.; Kaye, A.M.; Kaye, A.D. Serotonin syndrome. Ochsner J. 2013, 13, 533–540. [Google Scholar]
- Vargas, M.V.; Meyer, R.; Avanes, A.A.; Rus, M.; Olson, D.E. Psychedelics and Other Psychoplastogens for Treating Mental Illness. Review. Front. Psychiatry 2021, 12, 1691. [Google Scholar] [CrossRef]
- Thompson, C.; Szabo, A. Psychedelics as a novel approach to treating autoimmune conditions. Immunol. Lett. 2020, 228, 45–54. [Google Scholar] [CrossRef]
- Janssens, K.A.; Zijlema, W.L.; Joustra, M.L.; Rosmalen, J.G. Mood and Anxiety Disorders in Chronic Fatigue Syndrome, Fibromyalgia, and Irritable Bowel Syndrome: Results from the LifeLines Cohort Study. Psychosom. Med. 2015, 77, 449–457. [Google Scholar] [CrossRef]
- Üçeyler, N.; Sommer, C. Fibromyalgiesyndrom. Z. Rheumatol. 2015, 74, 490–495. [Google Scholar] [CrossRef]
- Albrecht, D.S.; MacKie, P.J.; Kareken, D.A.; Hutchins, G.D.; Chumin, E.J.; Christian, B.T.; Yoder, K.K. Differential dopamine function in fibromyalgia. Brain Imaging Behav. 2016, 10, 829–839. [Google Scholar] [CrossRef] [Green Version]
- Moret, C.; Briley, M. Antidepressants in the treatment of fibromyalgia. Neuropsychiatr. Dis. Treat. 2006, 2, 537–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Natelson, B.H.; Lin, J.S.; Lange, G.; Khan, S.; Stegner, A.; Unger, E.R. The effect of comorbid medical and psychiatric diagnoses on chronic fatigue syndrome. Ann. Med. 2019, 51, 371–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fadgyas-Stanculete, M.; Buga, A.-M.; Popa-Wagner, A.; Dumitrascu, D.L. The relationship between irritable bowel syndrome and psychiatric disorders: From molecular changes to clinical manifestations. J. Mol. Psychiatry 2014, 2, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarrett, M.E.; Kohen, R.; Cain, K.C.; Burr, R.L.; Poppe, A.; Navaja, G.P.; Heitkemper, M.M. Relationship of SERT polymorphisms to depressive and anxiety symptoms in irritable bowel syndrome. Biol. Res. Nurs. 2007, 9, 161–169. [Google Scholar] [CrossRef]
- Fukudo, S.; Kanazawa, M.; Mizuno, T.; Hamaguchi, T.; Kano, M.; Watanabe, S.; Sagami, Y.; Shoji, T.; Endo, Y.; Hongo, M.; et al. Impact of serotonin transporter gene polymorphism on brain activation by colorectal distention. Neuroimage 2009, 47, 946–951. [Google Scholar] [CrossRef]
- Smith, J.E.; Rowan, N.J.; Sullivan, R. Medicinal mushrooms: A rapidly developing area of biotechnology for cancer therapy and other bioactivities. Biotechnol. Lett. 2002, 24, 1839–1845. [Google Scholar] [CrossRef]
- Sullivan, R.; Smith, J.E.; Rowan, N.J. Medicinal mushrooms and cancer therapy: Translating a traditional practice into Western medicine. Perspect. Biol. Med. 2006, 49, 159–170. [Google Scholar] [CrossRef]
- Murphy, E.J.; Rezoagli, E.; Major, I.; Rowan, N.J.; Laffey, J.G. β-glucan metabolic and immunomodulatory properties and potential for clinical application. J. Fungi 2020, 6, 356. [Google Scholar] [CrossRef]
- Murphy, E.J.; Masterson, C.; Rezoagli, E.; O’Toole, D.; Major, I.; Stack, G.D.; Lynch, M.; Laffey, J.G.; Rowan, N.J. β-Glucan extracts from the same edible shiitake mushroom Lentinus edodes produce differential in-vitro immunomodulatory and pulmonary cytoprotective effects—Implications for coronavirus disease (COVID-19) immunotherapies. Sci. Total Environ. 2020, 732, 139330. [Google Scholar] [CrossRef]
- Murphy, E.J.; Rezoagli, E.; Pogue, R.; Simonassi-Paiva, B.; Izwani, I.; Abidin, Z.; Waltzer Fehrenbach, G.; O’Neil, E.; Major, I.; Laffey, J.G.; et al. Immunomodulatory activity of β-glucan polysaccharides isolated from different species of mushroom–A potential treatment for inflammatory lung conditions. Sci. Total Environ. 2022, 809, 152177. [Google Scholar] [CrossRef]
- Rowan, N.J.; Galanakis, C.M. Unlocking challenges and opportunities presented by COVID-19 pandemic for cross-cutting disruption in agri-food and green deal innovations: Quo Vadis? Sci. Total Environ. 2020, 748, 141362. [Google Scholar] [CrossRef] [PubMed]
- Rowan, N.J.; Casey, O. Empower Eco multiactor HUB: A triple helix ‘academia-industry-authority’ approach to creating and sharing potentially disruptive tools for addressing novel and emerging new Green Deal opportunities under a United Nations Sustainable Development Goals framework. Curr. Opin. Environ. Sci. Health 2021, 21, 100254. [Google Scholar] [CrossRef]
- Allam, Z.; Sharifi, A.; Giurco, D.; Sharpe, S.A. On the theoretical conceptualisations, knowledge structures and trends of green new deals. Sustainability 2021, 13, 12529. [Google Scholar] [CrossRef]
- Yildiz, O.; Can, Z.; Laghari, A.Q.; Şahin, H.; Malkoç, M. Wild edible mushrooms as a natural source of phenolics and antioxidants. J. Food Biochem. 2015, 39, 148–154. [Google Scholar] [CrossRef]
- Dos Santos, R.G.; Bouso, J.C.; Rocha, J.M.; Rossi, G.N.; Hallak, J.E. The use of classic hallucinogens/psychedelics in a therapeutic context: Healthcare policy opportunities and challenges. Risk Manag. Healthc. Policy 2021, 14, 901. [Google Scholar] [CrossRef]
- Whelan, A.; Johnson, M.I. Lysergic acid diethylamide and psilocybin for the management of patients with persistent pain: A potential role? Pain Manag. 2018, 8, 217–229. [Google Scholar] [CrossRef] [Green Version]
- Olson, D.E. Psychoplastogens: A Promising Class of Plasticity-Promoting Neurotherapeutics. J. Exp. Neurosci. 2018, 12, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Gill, H.; Gill, B.; Chen-Li, D.; El-Halabi, S.; Rodrigues, N.B.; Cha, D.S.; Lipsitz, O.; Lee, Y.; Rosenblat, J.D.; Majeed, A.; et al. The emerging role of psilocybin and MDMA in the treatment of mental illness. Expert Rev. Neurother. 2020, 20, 1263–1273. [Google Scholar] [CrossRef]
- Pretorius, L.; Smith, C. The trace aminergic system: A gender-sensitive therapeutic target for IBS? J. Biomed. Sci. 2020, 27, 95. [Google Scholar] [CrossRef]
- Mayet, S. A review of common psychedelic drugs. S. Afr. J. Anaesth. Analg. 2020, 26, S113–S117. [Google Scholar] [CrossRef]
- Uthaug, M.V.; Davis, A.K.; Haas, T.F.; Dawis, D.; Dolan, S.B.; Lancelotta, R.; Timmermann, C.; Ramaekers, J.G. The epidemiology of mescaline use: Pattern of use, motivations for consumption, and perceived consequences, benefits, and acute and enduring subjective effects. J. Psychopharmacol. 2021, 36, 309–320. [Google Scholar] [CrossRef]
- Daniel, J.; Haberman, M. Clinical potential of psilocybin as a treatment for mental health conditions. Ment. Health Clin. 2017, 7, 24–28. [Google Scholar] [CrossRef]
- Passie, T.; Halpern, J.H.; Stichtenoth, D.O.; Emrich, H.M.; Hintzen, A. The pharmacology of lysergic acid diethylamide: A review. CNS Neurosci. Ther. 2008, 14, 295–314. [Google Scholar] [CrossRef]
- Lowe, H.; Toyang, N.; Steele, B.; Valentine, H.; Grant, J.; Ali, A.; Ngwa, W.; Gordon, L. The Therapeutic Potential of Psilocybin. Molecules 2021, 26, 2948. [Google Scholar] [CrossRef]
- Halberstadt, A.L.; Geyer, M.A. Multiple receptors contribute to the behavioral effects of indoleamine hallucinogens. Neuropharmacology 2011, 61, 364–381. [Google Scholar] [CrossRef] [Green Version]
- Cumming, P.; Scheidegger, M.; Dornbierer, D.; Palner, M.; Quednow, B.B.; Martin-Soelch, C. Molecular and functional imaging studies of psychedelic drug action in animals and humans. Molecules 2021, 26, 2451. [Google Scholar] [CrossRef]
- Agin-Liebes, G.; Haas, T.F.; Lancelotta, R.; Uthaug, M.V.; Ramaekers, J.G.; Davis, A.K. Naturalistic use of mescaline is associated with self-reported psychiatric improvements and enduring positive life changes. ACS Pharmacol. Transl. Sci. 2021, 4, 543–552. [Google Scholar] [CrossRef]
- Holze, F.; Vizeli, P.; Ley, L.; Müller, F.; Dolder, P.; Stocker, M.; Duthaler, U.; Varghese, N.; Eckert, A.; Borgwardt, S.; et al. Acute dose-dependent effects of lysergic acid diethylamide in a double-blind placebo-controlled study in healthy subjects. Neuropsychopharmacology 2021, 46, 537–544. [Google Scholar] [CrossRef]
- Dinis-Oliveira, R.J. Metabolism of psilocybin and psilocin: Clinical and forensic toxicological relevance. Drug Metab. Rev. 2017, 49, 84–91. [Google Scholar] [CrossRef]
- Libânio Osório Marta, R.F. Metabolism of lysergic acid diethylamide (LSD): An update. Drug Metab. Rev. 2019, 51, 378–387. [Google Scholar] [CrossRef]
- Dinis-Oliveira, R.J.; Pereira, C.L.; da Silva, D.D. Pharmacokinetic and Pharmacodynamic Aspects of Peyote and Mescaline: Clinical and Forensic Repercussions. Curr. Mol. Pharmacol. 2019, 12, 184–194. [Google Scholar] [CrossRef]
- Passie, T.; Seifert, J.; Schneider, U.; Emrich, H.M. The pharmacology of psilocybin. Addict. Biol. 2002, 7, 357–364. [Google Scholar] [CrossRef]
- Carhart-Harris, R.L.; Bolstridge, M.; Rucker, J.; Day, C.M.J.; Erritzoe, D.; Kaelen, M.; Bloomfield, M.; Rickard, J.A.; Forbs, B.; Feilding, A.; et al. Psilocybin with psychological support for treatment-resistant depression: An open-label feasibility study. Lancet Psychiatry 2016, 3, 619–627. [Google Scholar] [CrossRef] [Green Version]
- Fuentes, J.J.; Fonseca, F.; Elices, M.; Farré, M.; Torrens, M. Therapeutic use of LSD in psychiatry: A systematic review of randomized-controlled clinical trials. Front. Psychiatry 2020, 10, 943. [Google Scholar] [CrossRef] [Green Version]
- Nichols, D.E. Psychedelics. Pharmacol. Rev. 2016, 68, 264–355. [Google Scholar] [CrossRef] [Green Version]
- Sherwood, A.M.; Prisinzano, T.E. Novel psychotherapeutics—A cautiously optimistic focus on hallucinogens. Expert Rev. Clin. Pharmacol. 2018, 11, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Carlini, E.A.; Maia, L.O. Plant and fungal hallucinogens as toxic and therapeutic agents. In Plant Toxins Toxinology; Gopalakrishnakone, P., Carlini, C., Ligabue-Braun, R., Eds.; Springer: Berlin, Germany, 2017; pp. 37–80. [Google Scholar]
- Olson, D.E. The Promise of Psychedelic Science. ACS Pharmacol. Transl. Sci. 2021, 4, 413–415. [Google Scholar] [CrossRef]
- Ross, S.; Bossis, A.; Guss, J.; Agin-Liebes, G.; Malone, T.; Cohen, B.; Mennenga, S.E.; Belser, A.; Kalliontzi, K.; Babb, J.; et al. Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: A randomized controlled trial. J. Psychopharmacol. 2016, 30, 1165–1180. [Google Scholar] [CrossRef] [Green Version]
- Griffiths, R.R.; Johnson, M.W.; Carducci, M.A.; Umbrich, A.; Richards, W.A.; Richards, B.D.; Cosimano, M.P.; Klinedinst, M.A. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: A randomized double-blind trial. J. Psychopharmacol. 2016, 30, 1181–1197. [Google Scholar] [CrossRef]
- Johnson, M.W.; Garcia-Romeu, A.; Griffiths, R.R. Long-term follow-up of psilocybin-facilitated smoking cessation. Am. J. Drug Alcohol Abus. 2017, 43, 55–60. [Google Scholar] [CrossRef] [Green Version]
- Moreno, F.A.; Wiegand, C.B.; Taitano, E.K.; Delgado, P.L. Safety, tolerability, and efficacy of psilocybin in 9 patients with obsessive-compulsive disorder. J. Clin. Psychiatry 2006, 67, 1735–1740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Negård, M.; Uhlig, S.; Kauserud, H.; Andersen, T.; Høiland, K.; Vrålstad, T. Links between genetic groups, indole alkaloid profiles and ecology within the grass-parasitic Claviceps purpurea species complex. Toxins 2015, 7, 1431–1456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez Arce, J.M.; Winkelman, M.J. Psychedelics, sociality, and human evolution. Front. Psychol. 2021, 12, 729425. [Google Scholar] [CrossRef] [PubMed]
- Halberstadt, A.L. Hallucinogenic drugs: A new study answers old questions about LSD. Curr. Biol. 2017, 27, R156–R158. [Google Scholar] [CrossRef] [Green Version]
- De Gregorio, D.; Comai, S.; Posa, L.; Gobbi, G. d-Lysergic acid diethylamide (LSD) as a model of psychosis: Mechanism of action and pharmacology. Int. J. Mol. Sci. 2016, 17, 1953. [Google Scholar] [CrossRef] [Green Version]
- Rogers, T.J. The molecular basis for neuroimmune receptor signaling. J. Neuroimmune Pharmacol. 2012, 7, 722–724. [Google Scholar] [CrossRef] [Green Version]
- Hutten, N.R.P.W.; Mason, N.L.; Dolder, P.C.; Kuypers, K.P.C. Self-rated effectiveness of microdosing with psychedelics for mental and physical health problems among microdosers. Front. Psychiatry 2019, 10, 672. [Google Scholar] [CrossRef]
- Ramaekers, J.G.; Hutten, N.; Mason, N.L.; Dolder, P.; Theunissen, E.L.; Holze, F.; Liechti, M.E.; Feilding, A.; Kuypers, K.P.C. A low dose of lysergic acid diethylamide decreases pain perception in healthy volunteers. J. Psychopharmacol. 2021, 35, 398–405. [Google Scholar] [CrossRef]
- Tupper, K.W.; Wood, E.; Yensen, R.; Johnson, M.W. Psychedelic medicine: A re-emerging therapeutic paradigm. Can. Med. Assoc. J. 2015, 187, 1054–1059. [Google Scholar] [CrossRef] [Green Version]
- Lünne, F.; Köhler, J.; Stroh, C.; Müller, L.; Daniliuc, C.G.; Mück-Lichtenfeld, C.; Würthwein, E.-U.; Esselen, M.; Humpf, H.-U.; Kalinina, S.A. Insights into Ergochromes of the Plant Pathogen Claviceps purpurea. J. Nat. Prod. 2021, 84, 2630–2643. [Google Scholar] [CrossRef]
- Carboué, Q.; Lopez, M. Amanita muscaria: Ecology, Chemistry, Myths. Encyclopedia 2021, 1, 905–914. [Google Scholar] [CrossRef]
- Michelot, D.; Melendez-Howell, L.M. Amanita muscaria: Chemistry, biology, toxicology, and ethnomycology. Mycol. Res. 2003, 107, 131–146. [Google Scholar] [CrossRef]
- Mikaszewska-Sokolewicz, M.A.; Pankowska, S.; Janiak, M.; Pruszczyk, P.; Łazowski, T.; Jankowski, K. Coma in the course of severe poisoning after consumption of red fly agaric (Amanita muscaria). Acta Biochim. Pol. 2016, 63, 181–182. [Google Scholar] [CrossRef]
- Corbett, R.; Fielding, S.; Cornfeldt, M.; Dunn, R.W. GABAmimetic agents display anxiolytic-like effects in the social interaction and elevated plus maze procedures. Psychopharmacology 1991, 104, 312–316. [Google Scholar] [CrossRef]
- Hosseini, M.; Karami, Z.; Yousefifard, M.; Janzadeh, A.; Zamani, E.; Nasirinezhad, F. Simultaneous intrathecal injection of muscimol and endomorphin-1 alleviates neuropathic pain in rat model of spinal cord injury. Brain Behav. 2020, 10, e01576. [Google Scholar] [CrossRef]
- Mishraki-Berkowitz, T.; Kochelski, E.; Kavanagh, P.; O’Brien, J.; Dunne, C.; Talbot, B.; Ennis, P.; Wolf, U. The Psilocin (4-hydroxy-N, N-dimethyltryptamine) and Bufotenine (5-hydroxy-N, N-dimethyltryptamine) Case: Ensuring the Correct Isomer has Been Identified. J. Forensic Sci. 2020, 65, 1450–1457. [Google Scholar] [CrossRef]
- Blei, F.; Dörner, S.; Fricke, J.; Baldeweg, F.; Trottmann, F.; Komor, A.; Meyer, F.; Hertweck, C.; Hoffmeister, D. Simultaneous production of psilocybin and a cocktail of β--carboline monoamine oxidase inhibitors in “magic” mushrooms. Chem.–A Eur. J. 2020, 26, 729–734. [Google Scholar] [CrossRef] [Green Version]
- Davis, A.K.; Barsuglia, J.P.; Lancelotta, R.; Grant, R.M.; Renn, E. The epidemiology of 5-methoxy- N, N-dimethyltryptamine (5-MeO-DMT) use: Benefits, consequences, patterns of use, subjective effects, and reasons for consumption. J. Psychopharmacol. 2018, 32, 779–792. [Google Scholar] [CrossRef]
- Ma, B.-J.; Shen, J.-W.; Yu, H.-Y.; Ruan, Y.; Wu, T.-T.; Zhao, X. Hericenones and erinacines: Stimulators of nerve growth factor (NGF) biosynthesis in Hericium erinaceus. Mycology 2010, 1, 92–98. [Google Scholar] [CrossRef] [Green Version]
- Rai, S.N.; Mishra, D.; Singh, P.; Vamanu, E.; Singh, M.P. Therapeutic applications of mushrooms and their biomolecules along with a glimpse of in silico approach in neurodegenerative diseases. Biomed. Pharmacother. 2021, 137, 111377. [Google Scholar] [CrossRef]
- Yao, W.; Zhang, J.-c.; Dong, C.; Zhuang, C.; Hirota, S.; Inanaga, K.; Hashimoto, K. Effects of amycenone on serum levels of tumor necrosis factor-α, interleukin-10, and depression-like behavior in mice after lipopolysaccharide administration. Pharmacol. Biochem. Behav. 2015, 136, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Li, I.; Lee, L.-Y.; Tzeng, T.-T.; Chen, W.-P.; Chen, Y.-P.; Shiao, Y.-J.; Chen, C.-C. Neurohealth properties of Hericium erinaceus mycelia enriched with erinacines. Behav. Neurol. 2018, 2018, 5802634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.O.; Lee, S.W.; Kim, J.S. A comprehensive review of the therapeutic effects of Hericium erinaceus in neurodegenerative disease. J. Mushroom 2014, 12, 77–81. [Google Scholar] [CrossRef] [Green Version]
- Nagano, M.; Shimizu, K.; Kondo, R.; Hayashi, C.; Sato, D.; Kitagawa, K.; Ohnuki, K. Reduction of depression and anxiety by 4 weeks Hericium erinaceus intake. Biomed. Res. 2010, 31, 231–237. [Google Scholar] [CrossRef] [Green Version]
- Chiu, C.-H.; Chyau, C.-C.; Chen, C.-C.; Lee, L.-Y.; Chen, W.-P.; Liu, Y.-L.; Lin, W.-H.; Mong, M.-C. Erinacine A-enriched Hericium erinaceus mycelium produces antidepressant-like effects through modulating BDNF/PI3K/Akt/GSK-3β signaling in mice. Int. J. Mol. Sci. 2018, 19, 341. [Google Scholar] [CrossRef] [Green Version]
- Chong, P.S.; Poon, C.H.; Roy, J.; Tsui, K.C.; Lew, S.Y.; Lok Phang, M.W.; Yuenyinn Tan, R.J.; Cheng, P.G.; Fung, M.-L.; Wong, K.H.; et al. Neurogenesis-dependent antidepressant-like activity of Hericium erinaceus in an animal model of depression. Chin. Med. 2021, 16, 132. [Google Scholar] [CrossRef]
- Cheah, I.K.; Halliwell, B. Ergothioneine, recent developments. Redox Biol. 2021, 42, 101868. [Google Scholar] [CrossRef]
- Nakamichi, N.; Nakayama, K.; Ishimoto, T.; Masuo, Y.; Wakayama, T.; Sekiguchi, H.; Sutoh, K.; Usumi, K.; Iseki, S.; Kato, Y. Food-derived hydrophilic antioxidant ergothioneine is distributed to the brain and exerts antidepressant effect in mice. Brain Behav. 2016, 6, e00477. [Google Scholar] [CrossRef]
- Matsuda, Y.; Ozawa, N.; Shinozaki, T.; Wakabayashi, K.-i.; Suzuki, K.; Kawano, Y.; Ohtsu, I.; Tatebayashi, Y. Ergothioneine, a metabolite of the gut bacterium Lactobacillus reuteri, protects against stress-induced sleep disturbances. Transl. Psychiatry 2020, 10, 170. [Google Scholar] [CrossRef]
- Orrico-Sanchez, A.; Chausset-Boissarie, L.; Alves de Sousa, R.; Coutens, B.; Rezai Amin, S.; Vialou, V.; Louis, F.; Hessani, A.; Dansette, P.M.; Zornoza, T.; et al. Antidepressant efficacy of a selective organic cation transporter blocker in a mouse model of depression. Mol. Psychiatry 2020, 25, 1245–1259. [Google Scholar] [CrossRef]
- Dolder, P.C.; Schmid, Y.; Steuer, A.E.; Kraemer, T.; Rentsch, K.M.; Hammann, F.; Liechti, M.E. Pharmacokinetics and pharmacodynamics of lysergic acid diethylamide in healthy subjects. Clin. Pharmacokinet. 2017, 56, 1219–1230. [Google Scholar] [CrossRef] [Green Version]
- Brown, R.T.; Nicholas, C.R.; Cozzi, N.V.; Gassman, M.C.; Cooper, K.M.; Muller, D.; Thomas, C.D.; Hetzel, S.J.; Henriquez, K.M.; Ribaudo, A.S.; et al. Pharmacokinetics of escalating doses of oral psilocybin in healthy adults. Clin. Pharmacokinet. 2017, 56, 1543–1554. [Google Scholar] [CrossRef]
- Masterson, C.H.; Murphy, E.J.; Gonzalez, H.; Major, I.; McCarthy, S.D.; O’Toole, D.; Laffey, J.G.; Rowan, N.J. Purified β-glucans from the Shiitake mushroom ameliorates antibiotic-resistant Klebsiella pneumoniae-induced pulmonary sepsis. Lett. Appl. Microbiol. 2020, 71, 405–412. [Google Scholar] [CrossRef]
- Usuldin, S.R.A.; Wan-Mohtar, W.A.A.Q.I.; Ilham, Z.; Jamaludin, A.A.; Abdullah, N.R.; Rowan, N. In vivo toxicity of bioreactor-grown biomass and exopolysaccharides from Malaysian tiger milk mushroom mycelium for potential future health applications. Sci. Rep. 2021, 11, 23079. [Google Scholar] [CrossRef]

| Disorder | Treatment | Mode of Action | Efficacy | Side Effects |
|---|---|---|---|---|
| Anxiety disorders * | SSRIs e.g., sertraline, escitalopram | Inhibit the reuptake of 5-HT | First-line treatments for PD, GAD, SAD, and PTSD [12] | GI problems (nausea, diarrhoea, dyspepsia, bleeding), dry mouth, headaches, dizziness, anxiety, insomnia, and sexual dysfunction [13] |
| SNRIs e.g., venlafaxine, duloxetine | Inhibit the reuptake of NE and 5-HT (and/or DA) | |||
| TCAs e.g., amitriptyline, imipramine | Inhibit the reuptake of NE and 5-HT | Equivocal efficacy with SSRIs; however, cause more adverse side effects due to their anticholinergic activity [14] | Nausea, dry mouth, constipation, weight gain, blurred vision, light-headedness, confusion, sedation, urine retention and tachycardia [15] | |
| MAOIs e.g., moclobemide, phenelzine | Inhibit the mitochondrial enzyme monoamine oxidase | Third-line treatment for refractory SAD and PD, i.e., considered for patients who are non-responsive to other treatments [16] | Dry mouth, nausea, diarrhoea, constipation, drowsiness, insomnia, dizziness/or light-headedness, fatigue, urinary problems, sexual dysfunction, hypertensive crisis reaction, and serotonin syndrome [17] | |
| Benzodiazepines e.g., alprazolam, diazepam, clonazepam | Positive allosteric modulators of GABA-A, resulting in increased frequency of chloride channel opening | Effective and fast acting in the treatment of GAD. Recommended as second-line therapy and for short duration use due potential risks of tolerance, dependence, abuse, or misuse [13] | Drowsiness, lethargy, fatigue, and potential for dependence. Higher doses can cause impaired motor coordination, dizziness, vertigo, slurred speech, blurry vision, mood swings, euphoria and hostile or erratic behaviour | |
| Pregabalin | Calcium channel modulator [18] | Effective as a monotherapy for GAD, or as an adjunct to SSRIs/SNRIs in treatment-resistant GAD [19,20] | Sedation, dizziness, somnolence, dry mouth, amblyopia, diarrhoea, weight gain and potential for dependence [12] | |
| Buspirone | High affinity for 5-HT1A receptors [21] | Nausea, headaches, dizziness, and fatigue [13] | ||
| Mood disorders * | Lithium | Multiple mechanisms including modulation of (GABA)-ergic and glutamatergic neurotransmission, and alteration of voltage-gated ion channels or intracellular signalling pathways [22,23] | First-line treatment for prevention of manic and depressive episodes of bipolar disorder (BD) [24] | Cardiac problems, cognitive problems, acne, psoriasis, thyroid problems, nausea, vomiting, weight gain, hyponatremia, sedation, decreased libido, and teratogenic [25] |
| valproic acid | First-line treatment for acute mania and maintenance of BD [26] | Cardiac problems, cognitive problems, hair loss, hypothyroidism, aplastic anaemia, Leukopenia, increased transaminases, hepatitis, SLE-like syndrome, hyponatremia, tremor, decreased libido, infertility and teratogenic [25] | ||
| Carbamazepine | Effective as a monotherapy to treat manic symptoms of bipolar or as adjunct to lithium or valproic acid [27] | Cardiac problems, cognitive problems, acne, hair loss, hypothyroidism, PCOS. diarrhoea, nausea, vomiting, pancreatitis, increased transaminases, metabolic syndrome, weight gain, sedation, tremor, decreased sexual function, infertility and teratogenic [25] | ||
| Psychotic disorders | First-generation antipsychotics (FGA) e.g., Chlorpromazine, haloperidol | D2 antagonists: work by inhibiting dopaminergic neurotransmission [28] | Effective in the treatment and maintenance of schizophrenia, acute mania with psychotic symptoms, major depressive order with psychotic features, and delusional disorder [28] | Adverse effects are drug specific and include anticholinergic effects (dry mouth, blurry vision, tachycardia, constipation), sedation, distonias, weight gain, increased lipids, parkinsonism (tremor, rigidity, bradykinesia), akathisia tardive dyskinesia, sialorrhea, orthostatic hypotension, neuroleptic malignant syndrome, sexual disfunction, neutropenia/agranulocytosis, and myocarditis [29] |
| Second-generation antipsychotics (SGA) e.g., quetiapine, aripiprazole | Serotonin-dopamine antagonists: work by blocking D2 dopamine receptors as well as serotonin receptor antagonist action [28] | Same clinical efficacy as FGA, with the exception of clozapine, which has unique efficacy against treatment resistant schizophrenia [30] | ||
| Eating disorder | Olanzapine (SGA) | Block dopaminergic (D1-4 antagonism) and serotonergic (5-HT2A/2C antagonism) receptors [31] | Effective as an adjunctive therapy in treatment of AN, increasing appetite and decreasing anxiety and ruminating thoughts involving body image and food [32] | Dizziness, orthostatic hypotension, hypercholesterolemia, hypertriglyceridemia, hyperglycaemia, weight gain, extra-pyramidal symptoms, dry mouth, hyperprolactinemia, and insomnia [32] |
| Antidepressants(SSRIs, SNRIs, TCAs, MAOIs) | Defined above | Effective as an adjunctive therapy in treatment of BN and BED, reducing the crisis of binge eating, purging phenomena and improving mood and anxiety [33] | Listed above | |
| Mood stabilizers e.g., topiramate | Blocks voltage gated sodium channels, enhances GABA-A receptor activity, reduces membrane depolarization by AMPA/Kainate receptors and is a weak inhibitor of carbonic anhydrase [34] | Shown efficacy in treatment of BN and BED, reducing the crisis of binge eating, purging phenomena and promoting weight loss (in overweight or obese patients) [33] | Paraesthesia, fatigue, cognitive problems, dizziness, somnolence, psychomotor slowing, memory/concentration difficulties, nervousness, confusion, weight loss [34] | |
| Impulse control, addiction, and obsessive-compulsive disorders | Antidepressants e.g., SSRIs and clomipramine (TCA) | Potently inhibit the reuptake of 5-HT | Effective as a monotherapy or as an augmentation agent in the treatment of impulsive (PG, KM, TTM, IED and pyromania), addiction and compulsive disorders [35,36,37,38,39] | Listed above |
| Mood stabilisers e.g., olanzapine, carbamazepine | Defined above | |||
| Naltrexone | Non-specific competitive opioid antagonist with highest affinity for the mu-opioid receptors in the CNS [39] | Nausea, vomiting, abdominal pain, decreased appetite, dizziness, lethargy, headaches and sleep disorders [40] | ||
| Personality disorders | Antidepressants (SSRIs, SNRIs) | Defined above | Shown efficacy in the treatment of BPD [41,42] | Listed above |
| Quetiapine | ||||
| Naltrexone |
| Mescaline | Psilocybin/Psilocin | LSD | |
|---|---|---|---|
| Pharmacodynamics | Naturally occurring substituted phenethylamine extracted from the peyote cactus | Naturally occurring indole-alkylamine (tryptamine) extracted from Psilocybe mushrooms | Semisynthetic indole-alkylamine (ergoline) derived from lysergic acid found in Claviceps purpurea |
| 5-HT releasing agent, catecholamine-like structure [77] | Close structural analogue of 5-HT | Close structural analogue of 5-HT | |
| Primarily interacts at 5-HT2A/2C and α2-adrenergic receptors [78] | Primarily interacts at 5-HT2A/2C and 5-HT1A, 5-HT2C [79] | Mixed 5-HT2/5-HT1 receptor partial agonist [80] | |
| Low binding affinity at dopaminergic and histaminergic receptors [78] | Indirectly increases DA concentration but has no affinity for D2 receptors [81] | High affinity dopaminergic, adrenergic, and histaminergic receptors [82,83] | |
| Pharmacokinetics | Can be ingested orally, smoked, or insufflated | Can be ingested orally or intravenously | Can be ingested orally, smoked, injected, or snorted |
| Relatively low-potency: active doses in the 200−400 mg range) [84] | 20× more potent than mescaline: active doses in 10–30 mg range [81] | 2000× more potent mescaline: active doses in 25–200 μg range [85] | |
| Rapidly absorbed in GI and distributed to the kidneys and liver | Rapidly absorbed and dephosphorylated to psilocin (bioactive form) [81] | Rapidly absorbed in GI and distributed to tissues and organs | |
| Low lipid solubility, weak ability to penetrate BBB [77] | Lipid soluble, can easily cross BBB [86] | Can easily cross BBB [87] | |
| Detoxification via oxidative deamination | Detoxification via demethylation and oxidative deamination | Detoxification via N-dealkylation and/or oxidation processes | |
| Long-lasting, half-life of 6 hrs | Half-life of 3 hrs | Half-life of 3.6 hrs | |
| Eliminated in urine mainly in the unchanged form (81.4%) and the remaining as the metabolite TMPA [88] | Eliminated in urine mainly as glucuronidated metabolites (80%) as well as unaltered psilocybin (3–10%) [89] | Eliminated in urine mainly as metabolites, only 1% of the dose is excreted unchanged) [87] | |
| Efficacy | Acute experiences of psychological insight during mescaline use are associated with self-reported improvements in anxiety disorders, depression, and substance abuse [78,84] | Therapeutic efficacy in treating mood and anxiety disorders, depression, cluster headaches, chronic pain, intractable phantom pain, obsessive-compulsive disorder, and substance abuse [79,81,90] | Therapeutic efficacy in treating anxiety disorders, depression, cluster headaches, obsessive-compulsive disorder, substance abuse, psychosomatic illnesses, and anxiety in relation to life-threatening diseases [91,92] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meade, E.; Hehir, S.; Rowan, N.; Garvey, M. Mycotherapy: Potential of Fungal Bioactives for the Treatment of Mental Health Disorders and Morbidities of Chronic Pain. J. Fungi 2022, 8, 290. https://doi.org/10.3390/jof8030290
Meade E, Hehir S, Rowan N, Garvey M. Mycotherapy: Potential of Fungal Bioactives for the Treatment of Mental Health Disorders and Morbidities of Chronic Pain. Journal of Fungi. 2022; 8(3):290. https://doi.org/10.3390/jof8030290
Chicago/Turabian StyleMeade, Elaine, Sarah Hehir, Neil Rowan, and Mary Garvey. 2022. "Mycotherapy: Potential of Fungal Bioactives for the Treatment of Mental Health Disorders and Morbidities of Chronic Pain" Journal of Fungi 8, no. 3: 290. https://doi.org/10.3390/jof8030290
APA StyleMeade, E., Hehir, S., Rowan, N., & Garvey, M. (2022). Mycotherapy: Potential of Fungal Bioactives for the Treatment of Mental Health Disorders and Morbidities of Chronic Pain. Journal of Fungi, 8(3), 290. https://doi.org/10.3390/jof8030290