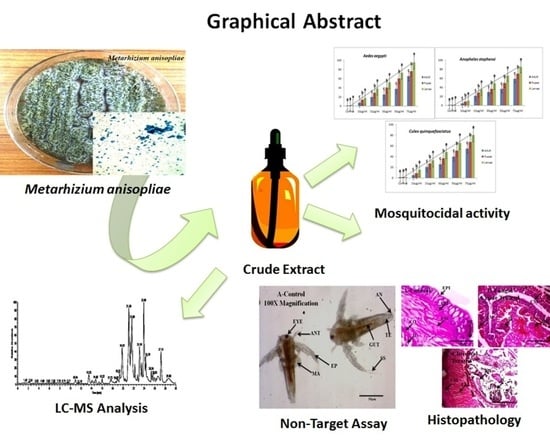
Insecticidal Efficacy of Metarhizium anisopliae Derived Chemical Constituents against Disease-Vector Mosquitoes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Cultures
2.2. Mass Culturing of M. anisopliae
2.3. Extraction of Secondary Metabolites
2.4. Larval Collection and Maintenance
2.5. Non-Target Organisms
2.6. Mosquitocidal Bioassays
2.7. Non-Target Bioassays
2.8. Fourier Transformed Infrared Spectroscopy Analysis
2.9. Liquid Chromatography-Mass Spectrophotometer Analysis
2.10. Statistical Analysis
3. Results
3.1. M. anisopliae metabolites against Ae. aegypti, An. stephensi, Cx. quinquefasciatus Mosquitoes
3.2. Non-Target Organisms
3.3. LC-MS and FT-IR Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Ethical Statement
References
- Huang, W.; Wang, S.; Jacobs-Lorena, M. Use of microbiota to fight mosquito-borne disease. Front. Genet. 2020, 11, 196. [Google Scholar] [CrossRef] [PubMed]
- Chareonviriyaphap, T.; Bangs, M.J.; Suwonkerd, W.; Kongmee, M.; Corbel, V.; Ngoen-Klan, R. Review of insecticide resistance and behavioral avoidance of vectors of human diseases in Thailand. Parasites Vectors 2013, 6, 280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, A.; Chowdhury, N.; Chandra, G. Plant extracts as potential mosquito larvicides. Indian J. Med. Res. 2012, 135, 581–598. [Google Scholar] [PubMed]
- Busvine, J.R. Recommended Methods for Measurement of Pest Resistance to Pesticides; FAO: Rome, Italy, 1980. [Google Scholar]
- Vivekanandhan, P.; Thendralmanikandan, A.; Kweka, E.J.; Mahande, A.M. Resistance to temephos in Anopheles stephensi larvae is associated with increased cytochrome P450 and α-esterase genes overexpression. Int. J. Trop. Insect Sci. 2021, 41, 2543–2548. [Google Scholar] [CrossRef]
- Vatandoost, H.; Hanafi-Bojd, A.A. Indication of pyrethroid resistance in the main malaria vector, Anopheles stephensi from Iran. Asian Pac. J. Trop. Med. 2012, 5, 722–726. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.Q.; Loganath, A.; Chong, Y.S.; Tan, J.; Obbard, J.P. Persistent organic pollutants and adverse health effects in humans. J. Toxicol. Environ. Health Part A 2006, 69, 1987–2005. [Google Scholar]
- Sarwar, M. Biopesticides: An effective and environmental friendly insect-pests inhibitor line of action. Int. J. Eng. Adv. Res. Technol. 2015, 1, 10–15. [Google Scholar]
- Morales-Rodriguez, A.; Peck, D.C. Synergies between biological and neonicotinoid insecticides for the curative control of the white grubs Amphimallon majale and Popillia japonica. Biol. Control 2009, 51, 169–180. [Google Scholar] [CrossRef]
- Ruiu, L.; Satta, A.; Floris, I. Emerging entomopathogenic bacteria for insect pest management. Bull. Insectol. 2013, 66, 181–186. [Google Scholar]
- Bojke, A.; Tkaczuk, C.; Stepnowski, P.; Gołębiowski, M. Comparison of volatile compounds released by entomopathogenic fungi. Microbiol. Res. 2018, 214, 129–136. [Google Scholar] [CrossRef]
- Rai, D.; Updhyay, V.; Mehra, P.; Rana, M.; Pandey, A.K. Potential of entomopathogenic fungi as biopesticides. Indian J. Sci. Res. Technol. 2014, 2, 7–13. [Google Scholar]
- Zhang, L.; Fasoyin, O.E.; Molnár, I.; Xu, Y. Secondary metabolites from hypocrealean entomopathogenic fungi: Novel bioactive compounds. Nat. Prod. Rep. 2020, 37, 1181–1206. [Google Scholar] [CrossRef]
- Darbro, J.M.; Thomas, M.B. Spore persistence and likelihood of aeroallergenicity of entomopathogenic fungi used for mosquito control. Am. J. Trop. Med. Hyg. 2009, 80, 992–997. [Google Scholar] [CrossRef]
- Islam, W.; Adnan, M.; Shabbir, A.; Naveed, H.; Abubakar, Y.S.; Qasim, M.; Tayyab, M.; Noman, A.; Nisar, M.S.; Khan, K.A.; et al. Insect-fungal-interactions: A detailed review on entomopathogenic fungi pathogenicity to combat insect pests. Microb. Pathog. 2021, 159, 105122. [Google Scholar] [CrossRef]
- Vyas, N.; Dua, K.K.; Prakash, S. Efficacy of Lagenidium giganteum metabolites on mosquito larvae with reference to nontarget organisms. Parasitol. Res. 2007, 101, 385–390. [Google Scholar] [CrossRef]
- Vivekanandhan, P.; Swathy, K.; Thomas, A.; Kweka, E.J.; Rahman, A.; Pittarate, S.; Krutmuang, P. Insecticidal Efficacy of Microbial-Mediated Synthesized Copper Nano-Pesticide against Insect Pests and Non-Target Organisms. Int. J. Environ. Res. Public Health 2021, 18, 10536. [Google Scholar] [CrossRef]
- Vivekanandhan, P.; Arunthirumeni, M.; Vengateswari, G.; Shivakumar, M.S. 5 Bioprospecting of Novel Fungal Secondary Metabolites for Mosquito Control. In Microbial Control of Vector-Borne Diseases; Taylor & Francis: Raton, FL, USA, 2018; pp. 61–89. [Google Scholar]
- Lu, Y.; Yu, J. A Well-Established Method for the Rapid Assessment of Toxicity Using Artemia spp. Model. In Assessment and Management of Radioactive and Electronic Wastes; IntechOpen: London, UK, 2019; pp. 1–15. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Guidelines for Laboratory and Field Testing of Mosquito Larvicides. Communicable Disease Control, Prevention and Eradication; WHO, Pesticide Evaluation Scheme; WHO: Geneva, Switzerland, 2005; pp. 1–219. [Google Scholar]
- World Health Organization. Global programme to eliminate lymphatic filariasis-progress report on mass drug administration in 2016. Wkly. Epidemiol. Rec. 2016, 85, 365–372. [Google Scholar]
- Norris, E.J.; Bloomquist, J.R. Nutritional status significantly affects toxicological endpoints in the CDC bottle bioassay. Pest Manag. Sci. 2022, 78, 743–748. [Google Scholar] [CrossRef]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Thakur, N.; Kaur, S.; Tomar, P.; Thakur, S.; Yadav, A.N. Microbial biopesticides: Current status and advancement for sustainable agriculture and environment. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 243–282. [Google Scholar]
- Soni, N.; Prakash, S. Effect of Chrysosporium keratinophilum metabolites against Culex quinquefasciatus after chromatographic purification. Parasitol. Res. 2010, 107, 1329–1336. [Google Scholar] [CrossRef]
- Soni, N.; Prakash, S. Entomopathogenic fungus generated Nanoparticles for enhancement of efficacy in Culex quinquefasciatus and Anopheles stephensi. Asian Pac. J. Trop. Dis. 2012, 2, S356–S361. [Google Scholar] [CrossRef]
- Vivekanandhan, P.; Karthi, S.; Shivakumar, M.S.; Benelli, G. Synergistic effect of entomopathogenic fungus Fusarium oxysporum extract in combination with temephos against three major mosquito vectors. Pathog. Glob. Health 2018, 112, 37–46. [Google Scholar] [CrossRef]
- Vivekanandhan, P.; Deepa, S.; Kweka, E.J.; Shivakumar, M.S. Toxicity of Fusarium oxysporum-VKFO-01 Derived Silver Nanoparticles as Potential Inseciticide Against Three Mosquito Vector Species (Diptera: Culicidae). J. Clust. Sci. 2018, 29, 1139–1149. [Google Scholar] [CrossRef]
- Balumahendhiran, K.; Vivekanandhan, P.; Shivakumar, M.S. Mosquito control potential of secondary metabolites isolated from Aspergillus flavus and Aspergillus fumigatus. Biocatal. Agric. Biotechnol. 2019, 21, 101334. [Google Scholar] [CrossRef]
- Manfra, L.; Canepa, S.; Piazza, V.; Faimali, M. Lethal and sublethal endpoints observed for Artemia exposed to two reference toxicants and an ecotoxicological concern organic compound. Ecotoxicol. Environ. Saf. 2016, 123, 60–64. [Google Scholar] [CrossRef]
- Uwizeyimana, H.; Wang, M.; Chen, W.; Khan, K. The eco-toxic effects of pesticide and heavy metal mixtures towards earthworms in soil. Environ. Toxicol. Pharmacol. 2017, 55, 20–29. [Google Scholar] [CrossRef]
- Dubey, N.K.; Shukla, R.; Kumar, A.; Singh, P.; Prakash, B. Global scenario on the application of natural products in integrated pest management programmes. Nat. Prod. Plant Pest. Manag. 2011, 1, 1–20. [Google Scholar]
- Libralato, G.; Prato, E.; Migliore, L.; Cicero, A.M.; Manfra, L. A review of toxicity testing protocols and endpoints with Artemia spp. Ecol. Indic. 2016, 69, 35–49. [Google Scholar] [CrossRef] [Green Version]
- Libralato, G. The case of Artemia spp. in nanoecotoxicology. Mar. Environ. Res. 2014, 101, 38–43. [Google Scholar] [CrossRef]
- Gao, Q.; Jin, K.; Ying, S.H.; Zhang, Y.; Xiao, G.; Shang, Y.; Duan, Z.; Hu, X.; Xie, X.Q.; Zhou, G.; et al. Genome sequencing and comparative transcriptomics of the model entomopathogenic fungi Metarhizium anisopliae and M. acridum. PLoS Genet. 2011, 7, e1001264. [Google Scholar] [CrossRef] [Green Version]
- Vasantha-Srinivasan, P.; Senthil-Nathan, S.; Thanigaivel, A.; Edwin, E.; Ponsankar, A.; Selin-Rani, S.; Pradeepa, V.; Sakthi-Bhagavathy, M.; Kalaivani, K.; Hunter, W.B.; et al. Developmental response of Spodoptera litura Fab. to treatments of crude volatile oil from Piper betle L. and evaluation of toxicity to earthworm, Eudrilus eugeniae Kinb. Chemosphere 2016, 155, 336–347. [Google Scholar] [CrossRef] [PubMed]





| Mosquito | Stage | N = Insect Number | LC50 (LCL-UCL) | LC90 (LCL-UCL) | χ2 (df = 12) |
|---|---|---|---|---|---|
| Ae. aegypti | Larvae | 450 | 29.631 (25.440–36.833) | 80.560 (74.910–87.001) | 5.673 |
| Pupae | 450 | 45.530 (39.920–51.532) | 103.430 (98.571–109.642) | 4.041 | |
| Adult | 450 | 62.589 (57.439–67.991) | 123.775 (115.679–129.002) | 6.090 | |
| An. stephensi | Larvae | 450 | 32.578 (27.871–35.900) | 88.003 (82.717–93.966) | 5.214 |
| Pupae | 450 | 52.491 (46.913–56.331) | 98.110 (95.332–105.88) | 1.287 | |
| Adult | 450 | 70.235 (66.057–75.339) | 150.921 (141.883–157.991) | 3.002 | |
| Cx. quinquefasciatus | Larvae | 450 | 48.003 (41.771–53.994) | 96.883 (93.880–103.439) | 6.454 |
| Pupae | 450 | 69.017 (64.771–74.000) | 158.881 (151.875–164.640) | 0.989 | |
| Adult | 450 | 73.937 (66.383–78.382) | 180.440 (176.003–189.337) | 7.046 |
| Mosquito (na = 450) | Concentration (µg/mL) | % Mortality ± SD | LC50 (LCL-UCL) | LC90 (LCL-UCL) | χ2 (df = 12) |
|---|---|---|---|---|---|
| M. anisopliae | Control | 1.33 ± 0.5 | 620.481 (612.550–635.779) | 6893.990 (6587.612–7432.900) | 1.599 |
| 10 | 5.33 ± 0.5 | ||||
| 15 | 12.66 ± 1.0 | ||||
| 30 | 15.0 ± 0.5 | ||||
| 50 | 13.33 ± 1.0 | ||||
| 75 | 18.33 ± 0.5 |
| Treatment | Concentration (µg/mL) | % Mortality ± SD |
|---|---|---|
| M. anisopliae | Control | 1.33 ± 0.5 a |
| 50 | 4.66 ± 1.0 b | |
| 75 | 14.00 ± 1.1 c | |
| Monocrotophos | Control | 1.33 ± 0.5 a |
| 50 | 50.00 ± 0.5 b | |
| 75 | 87.33 ± 0.5 c |
| Treatments | E. eugeniae | ||
|---|---|---|---|
| Epidermis (µm) ± SD | Intestinal Epithelium (µm) ± SD | Body Wall (µm) ± SD | |
| Control | 37.13 ± 0.0 a | 71.14 ± 0.5 a | 280.12 ± 0.0 a |
| M. anisopliae | 36.51 ± 0.5 b | 70.55 ± 0.5 b | 279.10 ± 0.0 b |
| Monocrotophos | 23.32 ± 0.5 c | 55.15 ± 1.1 c | 210.12 ± 0.5 c |
| S. No | Retention Time | Molecular Formula | Molecular Weight | Compound Name | Compound Structure |
|---|---|---|---|---|---|
| 1 | 19.82 | C37H67NO13 | 733.46124 | (-)-Erythromycin |  |
| 2 | 21.08 | C10H16O | 152.12012 | (-)-Camphor |  |
| 3 | 21.66 | C6H11NO | 113.08406 | Caprolactam |  |
| 4 | 23.10 | C16H30O4 | 286.21441 | 2,2,4-Trimethyl-1,3-pentadienol diisobutyrate |  |
| 5 | 23.90 | C12H14O4 | 222.08921 | Monobutyl phthalate |  |
| 6 | 24.30 | C20H38O2 | 310.28718 | Ethyl oleate |  |
| 7 | 27.18 | C16H22O4 | 278.15181 | Dibutyl phthalate |  |
| S. No | Observed Wavenumber (cm−1) | Functional Group | Bonding Pattern |
|---|---|---|---|
| 1 | 3457.62 | O–H stretch | Phenols |
| 2 | 2854.91 | O–H stretch | Carboxylic acids |
| 3 | 2250.11 | -C C- stretch | Alkynes |
| 4 | 1679.00 | C=O stretch | Aldehydes |
| 5 | 1103.42 | C-H wag | Alkyl halides |
| 6 | 655.10 | C-H bends | Aromatics |
| 7 | 650.25 | C-H bends | Aromatics |
| 8 | 520.20 | C-Br stretch | Alkyl halides |
| 9 | 400.21 | C-Br stretch | Alkyl halides |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vivekanandhan, P.; Swathy, K.; Murugan, A.C.; Krutmuang, P. Insecticidal Efficacy of Metarhizium anisopliae Derived Chemical Constituents against Disease-Vector Mosquitoes. J. Fungi 2022, 8, 300. https://doi.org/10.3390/jof8030300
Vivekanandhan P, Swathy K, Murugan AC, Krutmuang P. Insecticidal Efficacy of Metarhizium anisopliae Derived Chemical Constituents against Disease-Vector Mosquitoes. Journal of Fungi. 2022; 8(3):300. https://doi.org/10.3390/jof8030300
Chicago/Turabian StyleVivekanandhan, Perumal, Kannan Swathy, Amarchand Chordia Murugan, and Patcharin Krutmuang. 2022. "Insecticidal Efficacy of Metarhizium anisopliae Derived Chemical Constituents against Disease-Vector Mosquitoes" Journal of Fungi 8, no. 3: 300. https://doi.org/10.3390/jof8030300
APA StyleVivekanandhan, P., Swathy, K., Murugan, A. C., & Krutmuang, P. (2022). Insecticidal Efficacy of Metarhizium anisopliae Derived Chemical Constituents against Disease-Vector Mosquitoes. Journal of Fungi, 8(3), 300. https://doi.org/10.3390/jof8030300