Biological Synthesis of Low Cytotoxicity Silver Nanoparticles (AgNPs) by the Fungus Chaetomium thermophilum—Sustainable Nanotechnology
Abstract
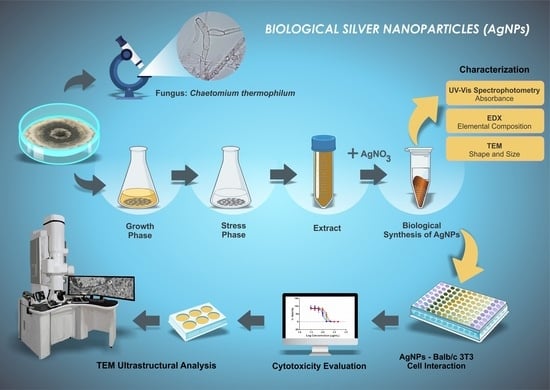
:1. Introduction
2. Materials and Methods
2.1. Production of Chaetomium thermophilum Cell-Free Extract
2.2. Biological Synthesis and Characterisation of AgNPs
2.2.1. AgNP Synthesis
2.2.2. AgNP Characterization
2.3. Mammalian Cell Culture
2.4. Cytotoxicity Evaluation Profile in Mammalian Cell
2.5. Evaluation of Balb/c 3T3 NIH Cells and AgNP Interaction by TEM
2.6. Statistical Analysis
3. Results
3.1. Production of Chaetomium thermophilum Cell-Free Extract
3.2. AgNP Characterisation
3.3. NRU Cytotoxicity Evaluation
3.4. AgNPs-Balb/c 3T3 NIH Cells Interaction
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Mukherjee, D.; Singh, S.; Kumar, M.; Kumar, V.; Datta, S.; Dhanjal, D.S. Fungal Biotechnology: Role and Aspects. In Fungi and Their Role in Sustainable Development: Current Perspectives; Gehlot, P., Singh, J., Eds.; Springer: Singapore, 2018; pp. 91–103. [Google Scholar] [CrossRef]
- United Nations. Transforming Our World, the 2030 Agenda for Sustainable Development, D.o.E.a.S. Affairs, Editor. 2020. Available online: https://sustainabledevelopment.un.org/content/documents/21252030%20Agenda%20for%20Sustainable%20Development%20web.pdf (accessed on 22 May 2022).
- Alves, M.F.; Murray, P.G. Biological Synthesis of Monodisperse Uniform-Size Silver Nanoparticles (AgNPs) by Fungal Cell-Free Extracts at Elevated Temperature and pH. J. Fungi 2022, 8, 439. [Google Scholar] [CrossRef] [PubMed]
- Alghuthaymi, M.A.; Almoammar, H.; Rai, M.; Said-Galiev, E.; Abd-Elsalam, K.A. Myconanoparticles: Synthesis and their role in phytopathogens management. Biotechnol. Biotechnol. Equip. 2015, 29, 221–236. [Google Scholar] [CrossRef] [PubMed]
- Kaabipour, S.; Hemmati, S. A review on the green and sustainable synthesis of silver nanoparticles and one-dimensional silver nanostructures. Beilstein J. Nanotechnol. 2021, 12, 102–136. [Google Scholar] [CrossRef] [PubMed]
- Otari, S.V.; Pawar, S.H.; Patel, S.K.S.; Singh, R.K.; Kim, S.Y.; Lee, J.H.; Zhang, L.; Lee, J.K. Canna edulis Leaf Extract-Mediated Preparation of Stabilized Silver Nanoparticles: Characterization, Antimicrobial Activity, and Toxicity Studies. J. Microbiol. Biotechnol. 2017, 27, 731–738. [Google Scholar] [CrossRef] [Green Version]
- Brown, A.N.; Smith, K.; Samuels, T.A.; Obare, S.O.; Scott, M.E. Nanoparticles functionalized with ampicillin destroy multiple-antibiotic-resistant isolates of Pseudomonas aeruginosa and Enterobacter aerogenes and methicillin-resistant Staphylococcus aureus. Appl. Environ. Microbiol. 2012, 78, 2768–2774. [Google Scholar] [CrossRef] [Green Version]
- Husseiny, S.M.; Salah, T.A.; Anter, H.A. Biosynthesis of size controlled silver nanoparticles by Fusarium oxysporum, their an-tibacterial and antitumor activities. Beni-Suef Univ. J. Basic Appl. Sci. 2015, 4, 225–231. [Google Scholar] [CrossRef] [Green Version]
- Rajeshkumar, S.; Malarkodi, C.; Vanaja, M.; Annadurai, G. Anticancer and enhanced antimicrobial activity of biosynthesizd silver nanoparticles against clinical pathogens. J. Mol. Struct. 2016, 1116, 165–173. [Google Scholar] [CrossRef]
- Ameen, F.; Al-Homaidan, A.A.; Al-Sabri, A.; Almansob, A.; AlNadhari, S. Anti-oxidant, anti-fungal and cytotoxic effects of silver nanoparticles synthesized using marine fungus Cladosporium halotolerans. Appl. Nanosci. 2021. [CrossRef]
- Firdhouse, M.J.; Lalitha, P. Biosynthesis of silver nanoparticles and its applications. J. Nanotechnol. 2015, 2015, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.F.; Liu, Z.G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Elamawi, R.M.; Al-Harbi, R.E.; Hendi, A.A. Biosynthesis and characterization of silver nanoparticles using Trichoderma longibrachiatum and their effect on phytopathogenic fungi. Egypt. J. Biol. Pest. Control. 2018, 28, 28. [Google Scholar] [CrossRef] [Green Version]
- Nayak, B.K.; Nanda, A.; Prabhakar, V. Biogenic synthesis of silver nanoparticle from wasp nest soil fungus, Penicillium italicum and its analysis against multi drug resistance pathogens. Biocatal. Agric. Biotechnol. 2018, 16, 412–418. [Google Scholar] [CrossRef]
- Shahzad, A.; Saeed, H.; Iqtedar, M.; Hussain, S.Z.; Kaleem, A.; Abdullah, R.; Sharif, S.; Naz, S.; Saleem, F.; Aihetasham, A.; et al. Size Controlled Production of Silver Nanoparticles by Aspergillus fumigatus BTCB10: Likely Anti-bacterial and Cytotoxic Effects. J. Nanomater. 2019, 2019, 1–14. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Hwang, H.M. Nanotechnology in food science: Functionality, applicability, and safety assessment. J. Food Drug Anal. 2016, 24, 671–681. [Google Scholar] [CrossRef] [Green Version]
- Krug, H. Nanosafety Research-Are We on the Right Track? Angew. Chem. Int. Ed. Engl. 2014, 53, 12304–12319. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Zhang, B.; Chang, X.; Gan, J.; Li, W.; Niu, S.; Wu, T.; Zhang, T.; Tang, M.; Xue, Y. Silver nanoparticles modulate mitochondrial dynamics and biogenesis in HepG2 cells. Environ. Pollut. 2020, 256, 113430. [Google Scholar] [CrossRef]
- ICCVAM. Guidance Document on Using In Vitro Data to Estimate In Vivo Starting Doses for Acute Toxicity, H.a.H. Services, Editor. US Public Health Service. 2001. Available online: https://ntp.niehs.nih.gov/iccvam/docs/acutetox_docs/guidance0801/iv_guide.pdf (accessed on 22 May 2022).
- Organisation for Economic Co-Operation and Development. Guidance Document on Using Cytotoxicity Tests to Estimate Starting Doses for Acute Oral Systemic Toxicity Tests. 2010. Available online: https://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=env/jm/mono%282010%2920&doclanguage=en (accessed on 22 May 2022).
- Mannerström, M.; Zou, J.; Toimela, T.; Pyykkö, I.; Heinonen, T. The applicability of conventional cytotoxicity assays to predict safety/toxicity of mesoporous silica nanoparticles, silver and gold nanoparticles and multi-walled carbon nanotubes. Toxicol. Vitr. 2016, 37, 113–120. [Google Scholar] [CrossRef]
- Zou, J.; Feng, H.; Mannerström, M.; Heinonen, T.; Pyykkö, I. Toxicity of silver nanoparticle in rat ear and BALB/c 3T3 cell line. J. Nanobiotechnol. 2014, 12, 52. [Google Scholar] [CrossRef] [Green Version]
- McShan, D.; Ray, P.C.; Yu, H. Molecular toxicity mechanism of nanosilver. J. Food Drug Anal. 2014, 22, 116–127. [Google Scholar] [CrossRef] [Green Version]
- Skalska, J.; Dąbrowska-Bouta, B.; Frontczak-Baniewicz, M. Sulkowski, G.; Strużyńska, L. A low dose of nanoparticulate silver induces mitochondrial dysfunction and autophagy in adult rat brain. Neurotox. Res. 2020, 38, 650–664. [Google Scholar] [CrossRef]
- Teodoro, J.S.; Simões, A.M.; Duarte, F.V.; Rolo, A.P.; Murdoch, R.C.; Hussain, S.M.; Palmeira, C.M. Assessment of the toxicity of silver nanoparticles in vitro: A mitochondrial perspective. Toxicol. Vitr. 2011, 25, 664–670. [Google Scholar] [CrossRef] [PubMed]
- AbdelRahim, K.; Mahmoud, S.Y.; Ali, A.M.; Almaary, K.S.; Mustafa, A.E.-Z.M.A.; Husseiny, S.M. Extracellular biosynthesis of silver nanoparticles using Rhizopus stolonifer. Saudi J. Biol. Sci. 2017, 24, 208–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamedi, S.; Ghaseminezhad, M.; Shokrollahzadeh, S.; Shojaosadati, S.A. Controlled biosynthesis of silver nanoparticles using nitrate reductase enzyme induction of filamentous fungus and their antibacterial evaluation. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1588–1596. [Google Scholar] [CrossRef] [PubMed]
- Katapodis, P.; Christakopoulou, V.; Kekos, D.; Christakopoulos, P. Optimization of xylanase production by Chaetomium thermophilum in wheat straw using response surface methodology. Biochem. Eng. J. 2007, 35, 136–141. [Google Scholar] [CrossRef]
- ICCVAM. Recommended Test Method Protocol Normal Human Keratinocyte NRU Cytotoxicity Test Method. 2006. Available online: https://ntp.niehs.nih.gov/iccvam/docs/protocols/ivcyto-nhk.pdf (accessed on 22 May 2022).
- Reus, T.L.; Machado, T.N.; Bezerra, A.G., Jr.; Marcon, B.H.; Paschoal, A.C.C.; Kuligovski, C.; Aguiar, A.M.; Dallagiovanna, B. Dose-dependent cytotoxicity of bismuth nanoparticles produced by LASiS in a reference mammalian cell line BALB/c 3T3. Toxicol. In Vitro 2018, 53, 99–106. [Google Scholar] [CrossRef]
- ICCVAM. In Vitro Cytotoxicity Test Methods for Estimating Acute Oral Systemic Toxicity—Background Review Document. 2006. Available online: https://ntp.niehs.nih.gov/iccvam/docs/acutetox_docs/brd_tmer/at-tmer-complete.pdf (accessed on 22 May 2022).
- Abud, A.P.; Zych, J.; Reus, T.L.; Kuligovski Moraes, E.; Dallagiovanna, B.; Aguiar, A.M. The use of human adipose-derived stem cells based cytotoxicity assay for acute toxicity test. Regul. Toxicol. Pharmacol. 2015, 73, 992–998. [Google Scholar] [CrossRef]
- Reus, T.L.; Machado, T.N.; Marcon, B.H.; Paschoal, A.C.C.; Ribeiro, I.R.S.; Cardoso, M.B.; Dallagiovanna, B.; Aguiar, A.M. Dose-dependent cell necrosis induced by silica nanoparticles. Toxicol. In Vitro 2020, 63, 104723. [Google Scholar] [CrossRef]
- United Nations. Global Harmonized System of Classification and Labelling of Chemicals (GHS). 2019. Available online: https://unece.org/fileadmin/DAM/trans/danger/publi/ghs/ghs_rev08/ST-SG-AC10-30-Rev8e.pdf (accessed on 22 May 2022).
- ICCVAM. In Vitro Cytotoxicity Test Methods for Estimating Starting Doses for Acute Oral Systemic Toxicity Testing. 2006. Available online: https://ntp.niehs.nih.gov/iccvam/docs/acutetox_docs/brd_tmer/brdvol2_nov2006.pdf (accessed on 22 May 2022).
- La-Touche, C.J. On a thermophile species of Chaetomium. Trans. Br. Mycol. Soc. 1950, 33, 94–104. [Google Scholar] [CrossRef]
- Dar, J.; Soytong, K. Construction and characterization of copolymer nanomaterials loaded with bioactive compounds from Chaetomium species. J. Agric. Technol. 2014, 10, 823–831. Available online: https://www.thaiscience.info/journals/Article/IJAT/10934631.pdf (accessed on 22 May 2022).
- Kaul, R.K.; Kumar, P.; Burman, U.; Joshi, P.; Agrawal, A.; Raliya, R.; Tarafdar, J.C. Magnesium and iron nanoparticles production using microorganisms and various salts. Mater. Sci.-Pol. 2012, 30, 254–258. [Google Scholar] [CrossRef]
- Amlacher, S.; Sarges, P.; Flemming, D.; van Noort, V.; Kunze, R.; Devos Damien, P.; Arumugam, M.; Bork, P.; Hurt, E. Insight into Structure and Assembly of the Nuclear Pore Complex by Utilizing the Genome of a Eukaryotic Thermophile. Cell 2011, 146, 277–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, J.; Li, Q.; Li, D. Novel Proteome and N-Glycoproteome of the Thermophilic Fungus Chaetomium thermophilum in Response to High Temperature. Front. Microbiol. 2021, 12, 1200. [Google Scholar] [CrossRef] [PubMed]
- Darwish, A.M.G.; Abdel-Azeem, A.M. Chaetomium Enzymes and Their Applications. Recent Developments on Genus Chaetomium; Springer International Publishing: Cham, Switzerland, 2020; pp. 241–249. [Google Scholar] [CrossRef]
- Fard, J.K.; Jafari, S.; Eghbal, M.A. A Review of Molecular Mechanisms Involved in Toxicity of Nanoparticles. Adv. Pharm. Bull. 2015, 5, 447–454. [Google Scholar] [CrossRef] [PubMed]
- MERCK. Sodium Dodecyl Sulfate—Safety Data Sheet. 2021. Available online: https://www.sigmaaldrich.com/IE/en/sds/mm/8.17034 (accessed on 22 May 2022).
- Salomoni, R.; Leo, P.; Montemor, A.F.; Rinaldi, B.G.; Rodrigues, M.F.A. Antibacterial effect of silver nanoparticles in Pseudomonas aeruginosa. Nanotechnol. Sci. Appl. 2017, 10, 115. [Google Scholar] [CrossRef] [Green Version]
- Halkai, K.; Mudda, J.A.; Shivanna, V.; Patil, V.; Rathod, V.; Halkai, R. Cytotoxicity evaluation of fungal-derived silver nanoparticles on human gingival fibroblast cell line: An In Vitro study. J. Conserv. Dent. 2019, 22, 160–163. [Google Scholar] [CrossRef]
- Rahimi, M.; Noruzi, E.B.; Sheykhsaran, E.; Ebadi, B.; Kariminezhad, Z.; Molaparast, M.; Mehrabani, M.G.; Mehramouz, B.; Yousefi, M.; Ahmadi, R.; et al. Carbohydrate polymer-based silver nanocomposites: Recent progress in the antimicrobial wound dressings. Carbohydr. Polym. 2020, 231, 115696. [Google Scholar] [CrossRef]
- Zawadzka, K.; Felczak, A.; Nowak, M.; Kowalczyk, A.; Piwonski, I.; Lisowska, K. Antimicrobial activity and toxicological risk assessment of silver nanoparticles synthesized using an eco-friendly method with Gloeophyllum striatum. J. Hazard. Mater. 2021, 418, 126316. [Google Scholar] [CrossRef]
- Hasanzadeh, M.; Feyziazar, M.; Solhi, E.; Mokhtarzadeh, A.; Soleymani, J.; Shadjou, N.; Jouyban, A.; Mahboob, S. Ultrasensitive immunoassay of breast cancer type 1 susceptibility protein (BRCA1) using poly (dopamine-beta cyclodextrine-Cetyl trimethylammonium bromide) doped with silver nanoparticles: A new platform in early stage diagnosis of breast cancer and efficient management. Microchem. J. 2019, 145, 778–783. [Google Scholar] [CrossRef]
- Karuppaiah, A.; Siram, K.; Selvaraj, D.; Ramasamy, M.; Babu, D.; Sankar, V. Synergistic and enhanced anticancer effect of a facile surface modified non-cytotoxic silver nanoparticle conjugated with gemcitabine in metastatic breast cancer cells. Mater. Today Commun. 2020, 23, 100884. [Google Scholar] [CrossRef]
- Khansa, I.; Schoenbrunner, A.R.; Kraft, C.T.; Janis, J.E. Silver in Wound Care—Friend or Foe?: A Comprehensive Review. Plast. Reconstr. Surg.-Glob. Open 2019, 7, e2390. [Google Scholar] [CrossRef]
- Nigam Joshi, P.; Agawane, S. Athalye, M.C.; Jadhav, V.; Sarkar, D.; Prakash, R. Multifunctional inulin tethered silver-graphene quantum dots nanotheranostic module for pancreatic cancer therapy. Mater. Sci. Eng. C 2017, 78, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Pothipor, C.; Wiriyakun, N.; Putnin, T.; Ngamaroonchote, A.; Jakmunee, J.; Ounnunkad, K.; Laocharoensuk, R.; Aroonyadet, N. Highly sensitive biosensor based on graphene–poly (3-aminobenzoic acid) modified electrodes and porous-hollowed-silver-gold nanoparticle labelling for prostate cancer detection. Sens. Actuators B Chem. 2019, 296, 126657. [Google Scholar] [CrossRef]
- Składanowski, M.; Golinska, P.; Rudnicka, K.; Dahm, H.; Rai, M. Evaluation of cytotoxicity, immune compatibility and antibacterial activity of biogenic silver nanoparticles. Med. Microbiol. Immunol. 2016, 205, 603–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wypij, M.; Czarnecka, J.; Swiecimska, M.; Dahm, H.; Rai, M.; Golinska, P. Synthesis, characterization and evaluation of antimicrobial and cytotoxic activities of biogenic silver nanoparticles synthesized from Streptomyces xinghaiensis OF1 strain. World J. Microbiol. Biotechnol. 2018, 34, 23. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.-H.; Cheng, F.-Y.; Chiu, H.-W.; Tsai, J.-C.; Fang, C.-Y.; Chen, C.-W.; Wang, Y.-J. Cytotoxicity, oxidative stress, apoptosis and the autophagic effects of silver nanoparticles in mouse embryonic fibroblasts. Biomaterials 2014, 35, 4706–4715. [Google Scholar] [CrossRef]
- Baluku, J.B.; Nuwagira, E.; Bongomin, F.; Denning, D.W. Pulmonary TB and chronic pulmonary aspergillosis: Clinical differences and similarities. Int. J. Tuberc. Lung Dis. 2021, 25, 537–546. [Google Scholar] [CrossRef]
- Bennett, J.W.; Klinch, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef] [Green Version]
- Phan, C.-W.; David, P.; Naidu, M.; Wong, K.-H.; Sabaratnam, V. Neurite outgrowth stimulatory effects of culinary-medicinal mushrooms and their toxicity assessment using differentiating Neuro-2a and embryonic fibroblast BALB/3T3. BMC Complement. Altern. Med. 2013, 13, 261. [Google Scholar] [CrossRef] [Green Version]
- Ali, H.; Aboud, M.; Alwan, S. Biological Synthesis of Silver Nanoparticles from Saprolegnia parasitica. J. Phys. Conf. Ser. 2019, 1294, 062090. [Google Scholar] [CrossRef] [Green Version]
- Bressan, E.; Ferroni, L.; Gardin, C.; Rigo, C.; Stocchero, M.; Vindigni, V.; Cairns, W.; Zavan, B. Silver Nanoparticles and Mitochondrial Interaction. Int. J. Dent. 2013, 2013, 312747. [Google Scholar] [CrossRef]
- Pereira, L.C.; Pazin, M.; Franco-Bernardes, M.F.; Martins, A.D.C., Jr.; Barcelos, G.R.M.; Pereira, M.C.; Mesquita, J.P.; Rodrigues, J.L.; Barbosa, F.; Dorta, D.J. A perspective of mitochondrial dysfunction in rats treated with silver and titanium nanoparticles (AgNPs and TiNPs). J. Trace Elem. Med. Biol. 2018, 47, 63–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osellame, L.D.; Blacker, T.S.; Duchen, M.R. Cellular and molecular mechanisms of mitochondrial function. Best Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 711–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farshbaf, M.J.; Ghaedi, K. Huntington’s Disease and Mitochondria. Neurotox. Res. 2017, 32, 518–529. [Google Scholar] [CrossRef] [PubMed]
- Ruhoy, I.S.; Saneto, R.P. The genetics of Leigh syndrome and its implications for clinical practice and risk management. Appl. Clin. Genet. 2014, 7, 221–234. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Chen, M.; Jiang, J. Mitochondrial dysfunction in neurodegenerative diseases and drug targets via apoptotic signaling. Mitochondrion 2019, 49, 35–45. [Google Scholar] [CrossRef] [PubMed]








| IC | Predicted LD50 | GHS | |
|---|---|---|---|
| µg/mL | mg/kg | ||
| IC80 | 144.92 ± 23.33 | 624.31 ± 41.87 | 4 |
| IC50 | 119.69 ± 21.15 | ||
| IC20 | 91.77 ± 24.24 | ||
| AgNP SYNTHESIS | IC50 | CYTOTOXIC TEST | CELL LINEAGE | REF. |
|---|---|---|---|---|
| µg/mL | ||||
| Biological: Fusarium semitectum | 260.00 | MTT | HGF human fibroblast | [45] |
| Biological: Gloeophyllum striatum | 28.76 | MTT | L929 mouse fibroblasts | [47] |
| Biological: Streptomyces sp. | 64.50 | MTT | L929 mouse fibroblasts | [53] |
| Biological: Streptomyces xinghaiensis | 4.0 | MTT | BALB/c 3T3 fibroblasts | [54] |
| Biological: Canna edulis | 18.00 | NRU/MTT | L929 mouse fibroblasts | [6] |
| Chemical: PVP-AgNP | 2.80 | NRU | BALB/c 3T3 fibroblasts | [22] |
| Chemical: PVP-AgNP | 2.80 | NRU | BALB/c 3T3 fibroblasts | [21] |
| Chemical: Na3C6H5O7-AgNP | 10.00 * | MTS | BALB/c 3T3 fibroblasts | [55] |
| Chemical: Na3C6H5O7-AgNP | 7.00 | NRU | NCTC 929 fibroblast | [44] |
| Biological: Chaetomium thermophilum | 119.69 | NRU | Balb/c 3T3 fibroblast | Present study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves, M.F.; Paschoal, A.C.C.; Klimeck, T.D.F.; Kuligovski, C.; Marcon, B.H.; de Aguiar, A.M.; Murray, P.G. Biological Synthesis of Low Cytotoxicity Silver Nanoparticles (AgNPs) by the Fungus Chaetomium thermophilum—Sustainable Nanotechnology. J. Fungi 2022, 8, 605. https://doi.org/10.3390/jof8060605
Alves MF, Paschoal ACC, Klimeck TDF, Kuligovski C, Marcon BH, de Aguiar AM, Murray PG. Biological Synthesis of Low Cytotoxicity Silver Nanoparticles (AgNPs) by the Fungus Chaetomium thermophilum—Sustainable Nanotechnology. Journal of Fungi. 2022; 8(6):605. https://doi.org/10.3390/jof8060605
Chicago/Turabian StyleAlves, Mariana Fuinhas, Ariane Caroline Campos Paschoal, Tabata D’Maiella Freitas Klimeck, Crisciele Kuligovski, Bruna Hilzendeger Marcon, Alessandra Melo de Aguiar, and Patrick G. Murray. 2022. "Biological Synthesis of Low Cytotoxicity Silver Nanoparticles (AgNPs) by the Fungus Chaetomium thermophilum—Sustainable Nanotechnology" Journal of Fungi 8, no. 6: 605. https://doi.org/10.3390/jof8060605
APA StyleAlves, M. F., Paschoal, A. C. C., Klimeck, T. D. F., Kuligovski, C., Marcon, B. H., de Aguiar, A. M., & Murray, P. G. (2022). Biological Synthesis of Low Cytotoxicity Silver Nanoparticles (AgNPs) by the Fungus Chaetomium thermophilum—Sustainable Nanotechnology. Journal of Fungi, 8(6), 605. https://doi.org/10.3390/jof8060605