Novel Therapeutic Hybrid Systems Using Hydrogels and Nanotechnology: A Focus on Nanoemulgels for the Treatment of Skin Diseases
Abstract
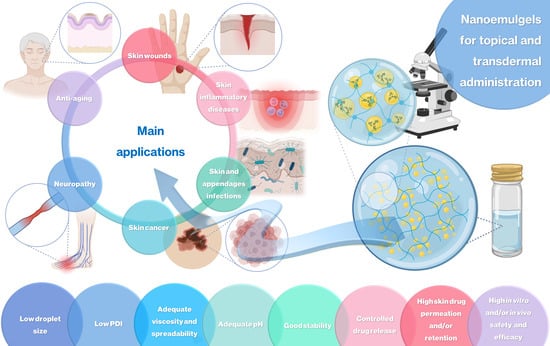
:1. Introduction
1.1. The Skin: Properties and Advantages and Limitations as a Drug Delivery Route
1.2. Nanotechnology for Efficient Skin Drug Delivery: A Special Focus on Nanoemulsions and Nanoemulgels
2. Topical and Transdermal Nanoemulgels for the Treatment of Skin Diseases and Other Applications
2.1. Skin Wound Healing
2.2. Skin and Skin Appendage Infections
2.3. Skin Cancer
2.4. Skin Inflammatory Diseases
2.5. Other Applications: Neuropathy and Antiaging Effects
3. Novel Nanoemulgels for Skin Application: The Future of Topical and Transdermal Drug Delivery?
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Arda, O.; Göksügür, N.; Tüzün, Y. Basic Histological Structure and Functions of Facial Skin. Clin. Dermatol. 2014, 32, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Boer, M.; Duchnik, E.; Maleszka, R.; Marchlewicz, M. Structural and Biophysical Characteristics of Human Skin in Maintaining Proper Epidermal Barrier Function. Adv. Dermatol. Allergol. 2016, 1, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Abdo, J.M.; Sopko, N.A.; Milner, S.M. The Applied Anatomy of Human Skin: A Model for Regeneration. Wound Med. 2020, 28, 100179. [Google Scholar] [CrossRef]
- Baroni, A.; Buommino, E.; De Gregorio, V.; Ruocco, E.; Ruocco, V.; Wolf, R. Structure and Function of the Epidermis Related to Barrier Properties. Clin. Dermatol. 2012, 30, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Hao, T.; Li, C.; Wang, X.; Yu, X.; Liu, L. Automatic Evaluation of Stratum Basale and Dermal Papillae Using Ultrahigh Resolution Optical Coherence Tomography. Biomed. Signal Process Control 2019, 53, 101527. [Google Scholar] [CrossRef]
- Maynard, R.L.; Downes, N. The Skin or the Integument. In Anatomy and Histology of the Laboratory Rat in Toxicology and Biomedical Research; Elsevier: Amsterdam, The Netherlands, 2019; pp. 303–315. [Google Scholar]
- Barbieri, J.S.; Wanat, K.; Seykora, J. Skin: Basic Structure and Function. In Pathobiology of Human Disease; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1134–1144. [Google Scholar]
- McBain, A.J.; O’Neill, C.A.; Oates, A. Skin Microbiology. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Proksch, E.; Brandner, J.M.; Jensen, J. The Skin: An Indispensable Barrier. Exp. Dermatol. 2008, 17, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Nishifuji, K.; Yoon, J.S. The Stratum Corneum: The Rampart of the Mammalian Body. Vet. Dermatol. 2013, 24, 60. [Google Scholar] [CrossRef]
- Tagami, H. Location-related Differences in Structure and Function of the Stratum Corneum with Special Emphasis on Those of the Facial Skin. Int. J. Cosmet. Sci. 2008, 30, 413–434. [Google Scholar] [CrossRef]
- Rippa, A.L.; Kalabusheva, E.P.; Vorotelyak, E.A. Regeneration of Dermis: Scarring and Cells Involved. Cells 2019, 8, 607. [Google Scholar] [CrossRef]
- Carroll, R.G. The Integument. In Elsevier’s Integrated Physiology; Elsevier: Amsterdam, The Netherlands, 2007; pp. 11–17. [Google Scholar]
- Carlson, B.M. Integumentary, Skeletal, and Muscular Systems. In Human Embryology and Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 156–192. [Google Scholar]
- Wong, R.; Geyer, S.; Weninger, W.; Guimberteau, J.; Wong, J.K. The Dynamic Anatomy and Patterning of Skin. Exp. Dermatol. 2016, 25, 92–98. [Google Scholar] [CrossRef]
- Woo, W. Skin Structure and Biology. In Imaging Technologies and Transdermal Delivery in Skin Disorders; Wiley: Hoboken, NJ, USA, 2019; pp. 1–14. [Google Scholar]
- Roberts, W. Air Pollution and Skin Disorders. Int. J. Womens Dermatol. 2021, 7, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Ju, Q.; Zouboulis, C.C. Endocrine-Disrupting Chemicals and Skin Manifestations. Rev. Endocr. Metab. Disord. 2016, 17, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Gromkowska-Kępka, K.J.; Puścion-Jakubik, A.; Markiewicz-Żukowska, R.; Socha, K. The Impact of Ultraviolet Radiation on Skin Photoaging—Review of in Vitro Studies. J. Cosmet. Dermatol. 2021, 20, 3427–3431. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Xiao, Z.; Wu, Y.; Ge, C. Diet and Skin Aging—From the Perspective of Food Nutrition. Nutrients 2020, 12, 870. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, A.; Grando, S.A. Smoking and the Skin. Int. J. Dermatol. 2012, 51, 250–262. [Google Scholar] [CrossRef]
- Liu, S.W.; Lien, M.H.; Fenske, N.A. The Effects of Alcohol and Drug Abuse on the Skin. Clin. Dermatol. 2010, 28, 391–399. [Google Scholar] [CrossRef]
- Lyu, F.; Wu, T.; Bian, Y.; Zhu, K.; Xu, J.; Li, F. Stress and Its Impairment of Skin Barrier Function. Int. J. Dermatol. 2023, 62, 621–630. [Google Scholar] [CrossRef]
- Dąbrowska, A.K.; Spano, F.; Derler, S.; Adlhart, C.; Spencer, N.D.; Rossi, R.M. The Relationship between Skin Function, Barrier Properties, and Body-dependent Factors. Ski. Res. Technol. 2018, 24, 165–174. [Google Scholar] [CrossRef]
- Ita, K.; Silva, M.; Bassey, R. Mechanical Properties of the Skin: What Do We Know? Curr. Cosmet. Sci. 2022, 1, e070122200109. [Google Scholar] [CrossRef]
- Mortazavi, S.M.; Moghimi, H.R. Skin Permeability, a Dismissed Necessity for Anti-wrinkle Peptide Performance. Int. J. Cosmet. Sci. 2022, 44, 232–248. [Google Scholar] [CrossRef]
- Parhi, R.; Mandru, A. Enhancement of Skin Permeability with Thermal Ablation Techniques: Concept to Commercial Products. Drug Deliv. Transl. Res. 2021, 11, 817–841. [Google Scholar] [CrossRef] [PubMed]
- Lundborg, M.; Wennberg, C.L.; Narangifard, A.; Lindahl, E.; Norlén, L. Predicting Drug Permeability through Skin Using Molecular Dynamics Simulation. J. Control. Release 2018, 283, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Alkilani, A.; McCrudden, M.T.; Donnelly, R. Transdermal Drug Delivery: Innovative Pharmaceutical Developments Based on Disruption of the Barrier Properties of the Stratum Corneum. Pharmaceutics 2015, 7, 438–470. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.-Q.; Yang, X.; Wu, X.-F.; Fan, Y.-B. Enhancing Permeation of Drug Molecules Across the Skin via Delivery in Nanocarriers: Novel Strategies for Effective Transdermal Applications. Front. Bioeng. Biotechnol. 2021, 9, 646554. [Google Scholar] [CrossRef] [PubMed]
- Narasimha Murthy, S.; Shivakumar, H.N. Topical and Transdermal Drug Delivery. In Handbook of Non-Invasive Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2010; pp. 1–36. [Google Scholar]
- Kathe, K.; Kathpalia, H. Film Forming Systems for Topical and Transdermal Drug Delivery. Asian J. Pharm. Sci. 2017, 12, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Leppert, W.; Malec–Milewska, M.; Zajaczkowska, R.; Wordliczek, J. Transdermal and Topical Drug Administration in the Treatment of Pain. Molecules 2018, 23, 681. [Google Scholar] [CrossRef] [PubMed]
- Prausnitz, M.R.; Langer, R. Transdermal Drug Delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef]
- Majdi, A.; Sadigh-Eteghad, S.; Gjedde, A. Effects of Transdermal Nicotine Delivery on Cognitive Outcomes: A Meta-analysis. Acta Neurol. Scand. 2021, 144, 179–191. [Google Scholar] [CrossRef]
- Buster, J.E. Transdermal Menopausal Hormone Therapy: Delivery through Skin Changes the Rules. Expert Opin. Pharmacother. 2010, 11, 1489–1499. [Google Scholar] [CrossRef]
- Sittl, R. Transdermal Buprenorphine in the Treatment of Chronic Pain. Expert Rev. Neurother. 2005, 5, 315–323. [Google Scholar] [CrossRef]
- Rehman, K.; Zulfakar, M.H. Recent Advances in Gel Technologies for Topical and Transdermal Drug Delivery. Drug Dev. Ind. Pharm. 2014, 40, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Waghule, T.; Singhvi, G.; Dubey, S.K.; Pandey, M.M.; Gupta, G.; Singh, M.; Dua, K. Microneedles: A Smart Approach and Increasing Potential for Transdermal Drug Delivery System. Biomed. Pharmacother. 2019, 109, 1249–1258. [Google Scholar] [CrossRef] [PubMed]
- Sawarkar, S.; Ashtekar, A. Transdermal Vitamin D Supplementation—A Potential Vitamin D Deficiency Treatment. J. Cosmet. Dermatol. 2020, 19, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Houck, C.S.; Sethna, N.F. Transdermal Analgesia with Local Anesthetics in Children: Review, Update and Future Directions. Expert Rev. Neurother. 2005, 5, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Touitou, E.; Natsheh, H. Topical Administration of Drugs Incorporated in Carriers Containing Phospholipid Soft Vesicles for the Treatment of Skin Medical Conditions. Pharmaceutics 2021, 13, 2129. [Google Scholar] [CrossRef] [PubMed]
- Singh Malik, D.; Mital, N.; Kaur, G. Topical Drug Delivery Systems: A Patent Review. Expert Opin. Ther. Pat. 2016, 26, 213–228. [Google Scholar] [CrossRef]
- Roberts, M.S.; Cheruvu, H.S.; Mangion, S.E.; Alinaghi, A.; Benson, H.A.E.; Mohammed, Y.; Holmes, A.; van der Hoek, J.; Pastore, M.; Grice, J.E. Topical Drug Delivery: History, Percutaneous Absorption, and Product Development. Adv. Drug Deliv. Rev. 2021, 177, 113929. [Google Scholar] [CrossRef]
- Bonamonte, D.; De Marco, A.; Giuffrida, R.; Conforti, C.; Barlusconi, C.; Foti, C.; Romita, P. Topical Antibiotics in the Dermatological Clinical Practice: Indications, Efficacy, and Adverse Effects. Dermatol. Ther. 2020, 33, e13824. [Google Scholar] [CrossRef]
- Lé, A.M.; Torres, T. New Topical Therapies for Psoriasis. Am. J. Clin. Dermatol. 2022, 23, 13–24. [Google Scholar] [CrossRef]
- Lax, S.J.; Harvey, J.; Axon, E.; Howells, L.; Santer, M.; Ridd, M.J.; Lawton, S.; Langan, S.; Roberts, A.; Ahmed, A.; et al. Strategies for Using Topical Corticosteroids in Children and Adults with Eczema. Cochrane Database Syst. Rev. 2022, 2022, CD013356. [Google Scholar] [CrossRef]
- Piraccini, B.M.; Blume-Peytavi, U.; Scarci, F.; Jansat, J.M.; Falqués, M.; Otero, R.; Tamarit, M.L.; Galván, J.; Tebbs, V.; Massana, E. Efficacy and Safety of Topical Finasteride Spray Solution for Male Androgenetic Alopecia: A Phase III, Randomized, Controlled Clinical Trial. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Ivens, U.I.; Steinkjer, B.; Serup, J.; Tetens, V. Ointment Is Evenly Spread on the Skin, in Contrast to Creams and Solutions. Br. J. Dermatol. 2001, 145, 264–267. [Google Scholar] [CrossRef] [PubMed]
- Ridd, M.J.; Santer, M.; MacNeill, S.J.; Sanderson, E.; Wells, S.; Webb, D.; Banks, J.; Sutton, E.; Roberts, A.; Liddiard, L.; et al. Effectiveness and Safety of Lotion, Cream, Gel, and Ointment Emollients for Childhood Eczema: A Pragmatic, Randomised, Phase 4, Superiority Trial. Lancet Child. Adolesc. Health 2022, 6, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Grice, J.; Wang, G.; Roberts, M. Cutaneous Metabolism in Transdermal Drug Delivery. Curr. Drug Metab. 2009, 10, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Svensson, C.K. Biotransformation of Drugs in Human Skin. Drug Metab. Dispos. 2009, 37, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Mugglestone, C.J.; Mariz, S.; Lane, M.E. The Development and Registration of Topical Pharmaceuticals. Int. J. Pharm. 2012, 435, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano Based Drug Delivery Systems: Recent Developments and Future Prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Sultana, A.; Zare, M.; Thomas, V.; Kumar, T.S.S.; Ramakrishna, S. Nano-Based Drug Delivery Systems: Conventional Drug Delivery Routes, Recent Developments and Future Prospects. Med. Drug Discov. 2022, 15, 100134. [Google Scholar] [CrossRef]
- Demetzos, C.; Pippa, N. Advanced Drug Delivery Nanosystems (ADDnSs): A Mini-Review. Drug Deliv. 2014, 21, 250–257. [Google Scholar] [CrossRef]
- Pires, P.C.; Santos, A.O. Nanosystems in Nose-to-Brain Drug Delivery: A Review of Non-Clinical Brain Targeting Studies. J. Control. Release 2018, 270, 89–100. [Google Scholar] [CrossRef]
- Zhang, L.; Gu, F.; Chan, J.; Wang, A.; Langer, R.; Farokhzad, O. Nanoparticles in Medicine: Therapeutic Applications and Developments. Clin. Pharmacol. Ther. 2008, 83, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Rezić, I. Nanoparticles for Biomedical Application and Their Synthesis. Polymers 2022, 14, 4961. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering Precision Nanoparticles for Drug Delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Afzal, O.; Altamimi, A.S.A.; Nadeem, M.S.; Alzarea, S.I.; Almalki, W.H.; Tariq, A.; Mubeen, B.; Murtaza, B.N.; Iftikhar, S.; Riaz, N.; et al. Nanoparticles in Drug Delivery: From History to Therapeutic Applications. Nanomaterials 2022, 12, 4494. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mageed, H.M.; AbuelEzz, N.Z.; Radwan, R.A.; Mohamed, S.A. Nanoparticles in Nanomedicine: A Comprehensive Updated Review on Current Status, Challenges and Emerging Opportunities. J. Microencapsul. 2021, 38, 414–436. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.D.; Duarte, J.; Veiga, F.; Paiva-Santos, A.C.; Pires, P.C. Nanosystems for Brain Targeting of Antipsychotic Drugs: An Update on the Most Promising Nanocarriers for Increased Bioavailability and Therapeutic Efficacy. Pharmaceutics 2023, 15, 678. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, A.; Almotairy, A.R.Z.; Henidi, H.; Alshehri, O.Y.; Aldughaim, M.S. Nanoparticles as Drug Delivery Systems: A Review of the Implication of Nanoparticles’ Physicochemical Properties on Responses in Biological Systems. Polymers 2023, 15, 1596. [Google Scholar] [CrossRef]
- Sechi, M.; Sanna, V.; Pala, N. Targeted Therapy Using Nanotechnology: Focus on Cancer. Int. J. Nanomed. 2014, 9, 467–483. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, Applications and Toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Christian, P.; Von der Kammer, F.; Baalousha, M.; Hofmann, T. Nanoparticles: Structure, Properties, Preparation and Behaviour in Environmental Media. Ecotoxicology 2008, 17, 326–343. [Google Scholar] [CrossRef]
- Hore, M.J.A. Polymers on Nanoparticles: Structure & Dynamics. Soft Matter 2019, 15, 1120–1134. [Google Scholar] [CrossRef] [PubMed]
- Moradifar, N.; Kiani, A.A.; Veiskaramian, A.; Karami, K. Role of Organic and Inorganic Nanoparticles in the Drug Delivery System for Hypertension Treatment: A Systematic Review. Curr. Cardiol. Rev. 2022, 18, e110621194025. [Google Scholar] [CrossRef] [PubMed]
- Khalid, K.; Tan, X.; Mohd Zaid, H.F.; Tao, Y.; Lye Chew, C.; Chu, D.-T.; Lam, M.K.; Ho, Y.-C.; Lim, J.W.; Chin Wei, L. Advanced in Developmental Organic and Inorganic Nanomaterial: A Review. Bioengineered 2020, 11, 328–355. [Google Scholar] [CrossRef] [PubMed]
- Alshammari, B.H.; Lashin, M.M.A.; Mahmood, M.A.; Al-Mubaddel, F.S.; Ilyas, N.; Rahman, N.; Sohail, M.; Khan, A.; Abdullaev, S.S.; Khan, R. Organic and Inorganic Nanomaterials: Fabrication, Properties and Applications. RSC Adv. 2023, 13, 13735–13785. [Google Scholar] [CrossRef] [PubMed]
- Jafari, S.; Derakhshankhah, H.; Alaei, L.; Fattahi, A.; Varnamkhasti, B.S.; Saboury, A.A. Mesoporous Silica Nanoparticles for Therapeutic/Diagnostic Applications. Biomed. Pharmacother. 2019, 109, 1100–1111. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Feng, N. Mesoporous Silica Nanoparticles: Synthesis, Classification, Drug Loading, Pharmacokinetics, Biocompatibility, and Application in Drug Delivery. Expert Opin. Drug Deliv. 2019, 16, 219–237. [Google Scholar] [CrossRef]
- Jain, P.; Hassan, N.; Iqbal, Z.; Dilnawaz, F. Mesoporous Silica Nanoparticles: A Versatile Platform for Biomedical Applications. Recent. Pat. Drug Deliv. Formul. 2019, 12, 228–237. [Google Scholar] [CrossRef]
- Porrang, S.; Davaran, S.; Rahemi, N.; Allahyari, S.; Mostafavi, E. How Advancing Are Mesoporous Silica Nanoparticles? A Comprehensive Review of the Literature. Int. J. Nanomed. 2022, 17, 1803–1827. [Google Scholar] [CrossRef]
- Huang, R.; Shen, Y.-W.; Guan, Y.-Y.; Jiang, Y.-X.; Wu, Y.; Rahman, K.; Zhang, L.-J.; Liu, H.-J.; Luan, X. Mesoporous Silica Nanoparticles: Facile Surface Functionalization and Versatile Biomedical Applications in Oncology. Acta Biomater. 2020, 116, 1–15. [Google Scholar] [CrossRef]
- He, H.; Pham-Huy, L.A.; Dramou, P.; Xiao, D.; Zuo, P.; Pham-Huy, C. Carbon Nanotubes: Applications in Pharmacy and Medicine. Biomed. Res. Int. 2013, 2013, 578290. [Google Scholar] [CrossRef]
- Rahamathulla, M.; Bhosale, R.R.; Osmani, R.A.M.; Mahima, K.C.; Johnson, A.P.; Hani, U.; Ghazwani, M.; Begum, M.Y.; Alshehri, S.; Ghoneim, M.M.; et al. Carbon Nanotubes: Current Perspectives on Diverse Applications in Targeted Drug Delivery and Therapies. Materials 2021, 14, 6707. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wu, L.; de Perrot, M.; Zhao, X. Carbon Nanotubes: A Summary of Beneficial and Dangerous Aspects of an Increasingly Popular Group of Nanomaterials. Front. Oncol. 2021, 11, 693814. [Google Scholar] [CrossRef] [PubMed]
- Zare, H.; Ahmadi, S.; Ghasemi, A.; Ghanbari, M.; Rabiee, N.; Bagherzadeh, M.; Karimi, M.; Webster, T.J.; Hamblin, M.R.; Mostafavi, E. Carbon Nanotubes: Smart Drug/Gene Delivery Carriers. Int. J. Nanomed. 2021, 16, 1681–1706. [Google Scholar] [CrossRef] [PubMed]
- Pu, Z.; Wei, Y.; Sun, Y.; Wang, Y.; Zhu, S. Carbon Nanotubes as Carriers in Drug Delivery for Non-Small Cell Lung Cancer, Mechanistic Analysis of Their Carcinogenic Potential, Safety Profiling and Identification of Biomarkers. Int. J. Nanomed. 2022, 17, 6157–6180. [Google Scholar] [CrossRef] [PubMed]
- Din, F.U.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective Use of Nanocarriers as Drug Delivery Systems for the Treatment of Selected Tumors. Int. J. Nanomed. 2017, 12, 7291–7309. [Google Scholar] [CrossRef]
- Francis, A.P.; Devasena, T. Toxicity of Carbon Nanotubes: A Review. Toxicol. Ind. Health 2018, 34, 200–210. [Google Scholar] [CrossRef]
- Kobayashi, N.; Izumi, H.; Morimoto, Y. Review of Toxicity Studies of Carbon Nanotubes. J. Occup. Health 2017, 59, 394–407. [Google Scholar] [CrossRef]
- López-Dávila, V.; Seifalian, A.M.; Loizidou, M. Organic Nanocarriers for Cancer Drug Delivery. Curr. Opin. Pharmacol. 2012, 12, 414–419. [Google Scholar] [CrossRef]
- Palazzolo, S.; Bayda, S.; Hadla, M.; Caligiuri, I.; Corona, G.; Toffoli, G.; Rizzolio, F. The Clinical Translation of Organic Nanomaterials for Cancer Therapy: A Focus on Polymeric Nanoparticles, Micelles, Liposomes and Exosomes. Curr. Med. Chem. 2018, 25, 4224–4268. [Google Scholar] [CrossRef]
- Calzoni, E.; Cesaretti, A.; Polchi, A.; Di Michele, A.; Tancini, B.; Emiliani, C. Biocompatible Polymer Nanoparticles for Drug Delivery Applications in Cancer and Neurodegenerative Disorder Therapies. J. Funct. Biomater. 2019, 10, 4. [Google Scholar] [CrossRef]
- Hadinoto, K.; Sundaresan, A.; Cheow, W.S. Lipid–Polymer Hybrid Nanoparticles as a New Generation Therapeutic Delivery Platform: A Review. Eur. J. Pharm. Biopharm. 2013, 85, 427–443. [Google Scholar] [CrossRef]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, Composition, Types, and Clinical Applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Chen, G.; Zhang, J. A Review of Liposomes as a Drug Delivery System: Current Status of Approved Products, Regulatory Environments, and Future Perspectives. Molecules 2022, 27, 1372. [Google Scholar] [CrossRef] [PubMed]
- Pires, P.C.; Paiva-Santos, A.C.; Veiga, F. Liposome-Derived Nanosystems for the Treatment of Behavioral and Neurodegenerative Diseases: The Promise of Niosomes, Transfersomes, and Ethosomes for Increased Brain Drug Bioavailability. Pharmaceuticals 2023, 16, 1424. [Google Scholar] [CrossRef] [PubMed]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef] [PubMed]
- Taher, M.; Susanti, D.; Haris, M.S.; Rushdan, A.A.; Widodo, R.T.; Syukri, Y.; Khotib, J. PEGylated Liposomes Enhance the Effect of Cytotoxic Drug: A Review. Heliyon 2023, 9, e13823. [Google Scholar] [CrossRef]
- Luo, W.-C.; Lu, X. Solid Lipid Nanoparticles for Drug Delivery. Methods Mol. Biol. 2023, 2622, 139–146. [Google Scholar] [CrossRef]
- Paliwal, R.; Paliwal, S.R.; Kenwat, R.; Das Kurmi, B.; Sahu, M.K. Solid Lipid Nanoparticles: A Review on Recent Perspectives and Patents. Expert Opin. Ther. Pat. 2020, 30, 179–194. [Google Scholar] [CrossRef]
- zur Mühlen, A.; Schwarz, C.; Mehnert, W. Solid Lipid Nanoparticles (SLN) for Controlled Drug Delivery—Drug Release and Release Mechanism. Eur. J. Pharm. Biopharm. 1998, 45, 149–155. [Google Scholar] [CrossRef]
- Corzo, C.; Meindl, C.; Lochmann, D.; Reyer, S.; Salar-Behzadi, S. Novel Approach for Overcoming the Stability Challenges of Lipid-Based Excipients. Part 3: Application of Polyglycerol Esters of Fatty Acids for the next Generation of Solid Lipid Nanoparticles. Eur. J. Pharm. Biopharm. 2020, 152, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Bhardwaj, K.; Bansal, M. Polymeric Micelles as Drug Delivery System: Recent Advances, Approaches, Applications and Patents. Curr. Drug Saf. 2024, 19, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.; Ramsey, J.D.; Kabanov, A.V. Polymeric Micelles for the Delivery of Poorly Soluble Drugs: From Nanoformulation to Clinical Approval. Adv. Drug Deliv. Rev. 2020, 156, 80–118. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, B.; Biswas, S. Polymeric Micelles in Cancer Therapy: State of the Art. J. Control. Release 2021, 332, 127–147. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef]
- Begines, B.; Ortiz, T.; Pérez-Aranda, M.; Martínez, G.; Merinero, M.; Argüelles-Arias, F.; Alcudia, A. Polymeric Nanoparticles for Drug Delivery: Recent Developments and Future Prospects. Nanomaterials 2020, 10, 1403. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-Co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Sharma, S.; Parveen, R.; Chatterji, B.P. Toxicology of Nanoparticles in Drug Delivery. Curr. Pathobiol. Rep. 2021, 9, 133–144. [Google Scholar] [CrossRef]
- Perumal, S.; Atchudan, R.; Lee, W. A Review of Polymeric Micelles and Their Applications. Polymers 2022, 14, 2510. [Google Scholar] [CrossRef]
- Desai, N. Challenges in Development of Nanoparticle-Based Therapeutics. AAPS J. 2012, 14, 282–295. [Google Scholar] [CrossRef]
- Herdiana, Y.; Wathoni, N.; Shamsuddin, S.; Muchtaridi, M. Scale-up Polymeric-Based Nanoparticles Drug Delivery Systems: Development and Challenges. OpenNano 2022, 7, 100048. [Google Scholar] [CrossRef]
- Muthu, M.S.; Wilson, B. Challenges Posed by the Scale-up of Nanomedicines. Nanomedicine 2012, 7, 307–309. [Google Scholar] [CrossRef] [PubMed]
- Yukuyama, M.N.; Kato, E.T.M.; Lobenberg, R.; Bou-Chacra, N.A. Challenges and Future Prospects of Nanoemulsion as a Drug Delivery System. Curr. Pharm. Des. 2017, 23, 495–508. [Google Scholar] [CrossRef] [PubMed]
- Singh, Y.; Meher, J.G.; Raval, K.; Khan, F.A.; Chaurasia, M.; Jain, N.K.; Chourasia, M.K. Nanoemulsion: Concepts, Development and Applications in Drug Delivery. J. Control. Release 2017, 252, 28–49. [Google Scholar] [CrossRef] [PubMed]
- Pires, P.C.; Paiva-Santos, A.C.; Veiga, F. Nano and Microemulsions for the Treatment of Depressive and Anxiety Disorders: An Efficient Approach to Improve Solubility, Brain Bioavailability and Therapeutic Efficacy. Pharmaceutics 2022, 14, 2825. [Google Scholar] [CrossRef] [PubMed]
- Mariyate, J.; Bera, A. A Critical Review on Selection of Microemulsions or Nanoemulsions for Enhanced Oil Recovery. J. Mol. Liq. 2022, 353, 118791. [Google Scholar] [CrossRef]
- Azeem, A.; Rizwan, M.; Ahmad, F.J.; Iqbal, Z.; Khar, R.K.; Aqil, M.; Talegaonkar, S. Nanoemulsion Components Screening and Selection: A Technical Note. AAPS PharmSciTech 2009, 10, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Almeida, F.; Corrêa, M.; Zaera, A.M.; Garrigues, T.; Isaac, V. Influence of Different Surfactants on Development of Nanoemulsion Containing Fixed Oil from an Amazon Palm Species. Colloids Surf. A Physicochem. Eng. Asp. 2022, 643, 128721. [Google Scholar] [CrossRef]
- Koroleva, M.; Nagovitsina, T.; Yurtov, E. Nanoemulsions Stabilized by Non-Ionic Surfactants: Stability and Degradation Mechanisms. Phys. Chem. Chem. Phys. 2018, 20, 10369–10377. [Google Scholar] [CrossRef]
- Mushtaq, A.; Mohd Wani, S.; Malik, A.R.; Gull, A.; Ramniwas, S.; Ahmad Nayik, G.; Ercisli, S.; Alina Marc, R.; Ullah, R.; Bari, A. Recent Insights into Nanoemulsions: Their Preparation, Properties and Applications. Food Chem. X 2023, 18, 100684. [Google Scholar] [CrossRef]
- Ashaolu, T.J. Nanoemulsions for Health, Food, and Cosmetics: A Review. Environ. Chem. Lett. 2021, 19, 3381–3395. [Google Scholar] [CrossRef]
- Kotta, S.; Khan, A.W.; Ansari, S.H.; Sharma, R.K.; Ali, J. Formulation of Nanoemulsion: A Comparison between Phase Inversion Composition Method and High-Pressure Homogenization Method. Drug Deliv. 2015, 22, 455–466. [Google Scholar] [CrossRef]
- Espitia, P.J.P.; Fuenmayor, C.A.; Otoni, C.G. Nanoemulsions: Synthesis, Characterization, and Application in Bio-Based Active Food Packaging. Compr. Rev. Food Sci. Food Saf. 2019, 18, 264–285. [Google Scholar] [CrossRef]
- Yukuyama, M.N.; Ghisleni, D.D.M.; Pinto, T.J.A.; Bou-Chacra, N.A. Nanoemulsion: Process Selection and Application in Cosmetics—A Review. Int. J. Cosmet. Sci. 2016, 38, 13–24. [Google Scholar] [CrossRef]
- Anton, N.; Vandamme, T.F. The Universality of Low-Energy Nano-Emulsification. Int. J. Pharm. 2009, 377, 142–147. [Google Scholar] [CrossRef]
- Sadurní, N.; Solans, C.; Azemar, N.; García-Celma, M.J. Studies on the Formation of O/W Nano-Emulsions, by Low-Energy Emulsification Methods, Suitable for Pharmaceutical Applications. Eur. J. Pharm. Sci. 2005, 26, 438–445. [Google Scholar] [CrossRef]
- Rao, J.; McClements, D.J. Stabilization of Phase Inversion Temperature Nanoemulsions by Surfactant Displacement. J. Agric. Food Chem. 2010, 58, 7059–7066. [Google Scholar] [CrossRef]
- Ren, G.; Sun, Z.; Wang, Z.; Zheng, X.; Xu, Z.; Sun, D. Nanoemulsion Formation by the Phase Inversion Temperature Method Using Polyoxypropylene Surfactants. J. Colloid. Interface Sci. 2019, 540, 177–184. [Google Scholar] [CrossRef]
- Chuesiang, P.; Siripatrawan, U.; Sanguandeekul, R.; McLandsborough, L.; Julian McClements, D. Optimization of Cinnamon Oil Nanoemulsions Using Phase Inversion Temperature Method: Impact of Oil Phase Composition and Surfactant Concentration. J. Colloid. Interface Sci. 2018, 514, 208–216. [Google Scholar] [CrossRef]
- Bouchemal, K.; Briançon, S.; Perrier, E.; Fessi, H. Nano-Emulsion Formulation Using Spontaneous Emulsification: Solvent, Oil and Surfactant Optimisation. Int. J. Pharm. 2004, 280, 241–251. [Google Scholar] [CrossRef]
- Akram, S.; Anton, N.; Omran, Z.; Vandamme, T. Water-in-Oil Nano-Emulsions Prepared by Spontaneous Emulsification: New Insights on the Formulation Process. Pharmaceutics 2021, 13, 1030. [Google Scholar] [CrossRef]
- Lefebvre, G.; Riou, J.; Bastiat, G.; Roger, E.; Frombach, K.; Gimel, J.-C.; Saulnier, P.; Calvignac, B. Spontaneous Nano-Emulsification: Process Optimization and Modeling for the Prediction of the Nanoemulsion’s Size and Polydispersity. Int. J. Pharm. 2017, 534, 220–228. [Google Scholar] [CrossRef]
- Gupta, A. Nanoemulsions. In Nanoparticles for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 371–384. [Google Scholar]
- Mehanna, M.M.; Mneimneh, A.T. Formulation and Applications of Lipid-Based Nanovehicles: Spotlight on Self-Emulsifying Systems. Adv. Pharm. Bull. 2020, 11, 56–67. [Google Scholar] [CrossRef]
- Preeti; Sambhakar, S.; Malik, R.; Bhatia, S.; Al Harrasi, A.; Rani, C.; Saharan, R.; Kumar, S.; Geeta; Sehrawat, R. Nanoemulsion: An Emerging Novel Technology for Improving the Bioavailability of Drugs. Scientifica 2023, 2023, 6640103. [Google Scholar] [CrossRef]
- Gupta, A.; Eral, H.B.; Hatton, T.A.; Doyle, P.S. Nanoemulsions: Formation, Properties and Applications. Soft Matter 2016, 12, 2826–2841. [Google Scholar] [CrossRef]
- Jaiswal, M.; Dudhe, R.; Sharma, P.K. Nanoemulsion: An Advanced Mode of Drug Delivery System. 3 Biotech 2015, 5, 123–127. [Google Scholar] [CrossRef]
- Sabjan, K.B.; Munawar, S.M.; Rajendiran, D.; Vinoji, S.K.; Kasinathan, K. Nanoemulsion as Oral Drug Delivery—A Review. Curr. Drug Res. Rev. 2020, 12, 4–15. [Google Scholar] [CrossRef]
- Aithal, G.C.; Narayan, R.; Nayak, U.Y. Nanoemulgel: A Promising Phase in Drug Delivery. Curr. Pharm. Des. 2020, 26, 279–291. [Google Scholar] [CrossRef]
- Choudhury, H.; Gorain, B.; Pandey, M.; Chatterjee, L.A.; Sengupta, P.; Das, A.; Molugulu, N.; Kesharwani, P. Recent Update on Nanoemulgel as Topical Drug Delivery System. J. Pharm. Sci. 2017, 106, 1736–1751. [Google Scholar] [CrossRef]
- Anand, K.; Ray, S.; Rahman, M.; Shaharyar, A.; Bhowmik, R.; Bera, R.; Karmakar, S. Nano-Emulgel: Emerging as a Smarter Topical Lipidic Emulsion-Based Nanocarrier for Skin Healthcare Applications. Recent. Pat. Antiinfect. Drug Discov. 2019, 14, 16–35. [Google Scholar] [CrossRef]
- Donthi, M.R.; Munnangi, S.R.; Krishna, K.V.; Saha, R.N.; Singhvi, G.; Dubey, S.K. Nanoemulgel: A Novel Nano Carrier as a Tool for Topical Drug Delivery. Pharmaceutics 2023, 15, 164. [Google Scholar] [CrossRef]
- Sengupta, P.; Chatterjee, B. Potential and Future Scope of Nanoemulgel Formulation for Topical Delivery of Lipophilic Drugs. Int. J. Pharm. 2017, 526, 353–365. [Google Scholar] [CrossRef]
- Salem, H.F.; Kharshoum, R.M.; Abou-Taleb, H.A.; Naguib, D.M. Nanosized Nasal Emulgel of Resveratrol: Preparation, Optimization, in Vitro Evaluation and in Vivo Pharmacokinetic Study. Drug Dev. Ind. Pharm. 2019, 45, 1624–1634. [Google Scholar] [CrossRef]
- Nagaraja, S.; Basavarajappa, G.M.; Attimarad, M.; Pund, S. Topical Nanoemulgel for the Treatment of Skin Cancer: Proof-of-Technology. Pharmaceutics 2021, 13, 902. [Google Scholar] [CrossRef]
- Vichare, R.; Crelli, C.; Liu, L.; Das, A.C.; McCallin, R.; Zor, F.; Kulahci, Y.; Gorantla, V.S.; Janjic, J.M. A Reversibly Thermoresponsive, Theranostic Nanoemulgel for Tacrolimus Delivery to Activated Macrophages: Formulation and In Vitro Validation. Pharmaceutics 2023, 15, 2372. [Google Scholar] [CrossRef]
- Ansari, M.N.; Soliman, G.A.; Rehman, N.U.; Anwer, M.K. Crisaborole Loaded Nanoemulsion Based Chitosan Gel: Formulation, Physicochemical Characterization and Wound Healing Studies. Gels 2022, 8, 318. [Google Scholar] [CrossRef]
- Jeengar, M.K.; Rompicharla, S.V.K.; Shrivastava, S.; Chella, N.; Shastri, N.R.; Naidu, V.G.M.; Sistla, R. Emu Oil Based Nano-Emulgel for Topical Delivery of Curcumin. Int. J. Pharm. 2016, 506, 222–236. [Google Scholar] [CrossRef]
- Pund, S.; Pawar, S.; Gangurde, S.; Divate, D. Transcutaneous Delivery of Leflunomide Nanoemulgel: Mechanistic Investigation into Physicomechanical Characteristics, in Vitro Anti-Psoriatic and Anti-Melanoma Activity. Int. J. Pharm. 2015, 487, 148–156. [Google Scholar] [CrossRef]
- Aggarwal, G.; Dhawan, B.; Harikumar, S. Enhanced Transdermal Permeability of Piroxicam through Novel Nanoemulgel Formulation. Int. J. Pharm. Investig. 2014, 4, 65. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, S.H.; Hwangbo, S.A.; Lee, T.G. A Comparison of Gelling Agents for Stable, Surfactant-Free Oil-in-Water Emulsions. Materials 2022, 15, 6462. [Google Scholar] [CrossRef]
- Morsy, M.A.; Abdel-Latif, R.G.; Nair, A.B.; Venugopala, K.N.; Ahmed, A.F.; Elsewedy, H.S.; Shehata, T.M. Preparation and Evaluation of Atorvastatin-Loaded Nanoemulgel on Wound-Healing Efficacy. Pharmaceutics 2019, 11, 609. [Google Scholar] [CrossRef]
- Rehman, A.; Iqbal, M.; Khan, B.A.; Khan, M.K.; Huwaimel, B.; Alshehri, S.; Alamri, A.H.; Alzhrani, R.M.; Bukhary, D.M.; Safhi, A.Y.; et al. Fabrication, In Vitro, and In Vivo Assessment of Eucalyptol-Loaded Nanoemulgel as a Novel Paradigm for Wound Healing. Pharmaceutics 2022, 14, 1971. [Google Scholar] [CrossRef]
- Yeo, E.; Yew Chieng, C.J.; Choudhury, H.; Pandey, M.; Gorain, B. Tocotrienols-Rich Naringenin Nanoemulgel for the Management of Diabetic Wound: Fabrication, Characterization and Comparative in Vitro Evaluations. Curr. Res. Pharmacol. Drug Discov. 2021, 2, 100019. [Google Scholar] [CrossRef]
- Algahtani, M.S.; Ahmad, M.Z.; Shaikh, I.A.; Abdel-Wahab, B.A.; Nourein, I.H.; Ahmad, J. Thymoquinone Loaded Topical Nanoemulgel for Wound Healing: Formulation Design and In-Vivo Evaluation. Molecules 2021, 26, 3863. [Google Scholar] [CrossRef]
- Algahtani, M.S.; Ahmad, M.Z.; Nourein, I.H.; Albarqi, H.A.; Alyami, H.S.; Alyami, M.H.; Alqahtani, A.A.; Alasiri, A.; Algahtani, T.S.; Mohammed, A.A.; et al. Preparation and Characterization of Curcumin Nanoemulgel Utilizing Ultrasonication Technique for Wound Healing: In Vitro, Ex Vivo, and In Vivo Evaluation. Gels 2021, 7, 213. [Google Scholar] [CrossRef]
- Tayah, D.Y.; Eid, A.M. Development of Miconazole Nitrate Nanoparticles Loaded in Nanoemulgel to Improve Its Antifungal Activity. Saudi Pharm. J. 2023, 31, 526–534. [Google Scholar] [CrossRef]
- Ullah, I.; Alhodaib, A.; Naz, I.; Ahmad, W.; Ullah, H.; Amin, A.; Nawaz, A. Fabrication of Novel Omeprazole-Based Chitosan Coated Nanoemulgel Formulation for Potential Anti-Microbia; In Vitro and Ex Vivo Characterizations. Polymers 2023, 15, 1298. [Google Scholar] [CrossRef]
- Vartak, R.; Menon, S.; Patki, M.; Billack, B.; Patel, K. Ebselen Nanoemulgel for the Treatment of Topical Fungal Infection. Eur. J. Pharm. Sci. 2020, 148, 105323. [Google Scholar] [CrossRef]
- Mahtab, A.; Anwar, M.; Mallick, N.; Naz, Z.; Jain, G.K.; Ahmad, F.J. Transungual Delivery of Ketoconazole Nanoemulgel for the Effective Management of Onychomycosis. AAPS PharmSciTech 2016, 17, 1477–1490. [Google Scholar] [CrossRef]
- Almostafa, M.M.; Elsewedy, H.S.; Shehata, T.M.; Soliman, W.E. Novel Formulation of Fusidic Acid Incorporated into a Myrrh-Oil-Based Nanoemulgel for the Enhancement of Skin Bacterial Infection Treatment. Gels 2022, 8, 245. [Google Scholar] [CrossRef] [PubMed]
- Algahtani, M.S.; Ahmad, M.Z.; Nourein, I.H.; Ahmad, J. Co-Delivery of Imiquimod and Curcumin by Nanoemugel for Improved Topical Delivery and Reduced Psoriasis-Like Skin Lesions. Biomolecules 2020, 10, 968. [Google Scholar] [CrossRef] [PubMed]
- Shehata, T.M.; Elnahas, H.M.; Elsewedy, H.S. Development, Characterization and Optimization of the Anti-Inflammatory Influence of Meloxicam Loaded into a Eucalyptus Oil-Based Nanoemulgel. Gels 2022, 8, 262. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.H.; Abu Lila, A.S.; Unissa, R.; Elsewedy, H.S.; Elghamry, H.A.; Soliman, M.S. Preparation, Characterization and Evaluation of Anti-Inflammatory and Anti-Nociceptive Effects of Brucine-Loaded Nanoemulgel. Colloids Surf. B Biointerfaces 2021, 205, 111868. [Google Scholar] [CrossRef] [PubMed]
- Saab, M.; Raafat, K.; El-Maradny, H. Transdermal Delivery of Capsaicin Nanoemulgel: Optimization, Skin Permeation and in Vivo Activity Against Diabetic Neuropathy. Adv. Pharm. Bull. 2021, 12, 780. [Google Scholar] [CrossRef] [PubMed]
- Algahtani, M.S.; Ahmad, M.Z.; Ahmad, J. Nanoemulgel for Improved Topical Delivery of Retinyl Palmitate: Formulation Design and Stability Evaluation. Nanomaterials 2020, 10, 848. [Google Scholar] [CrossRef]
- Childs, D.R.; Murthy, A.S. Overview of Wound Healing and Management. Surg. Clin. N. Am. 2017, 97, 189–207. [Google Scholar] [CrossRef]
- Cañedo-Dorantes, L.; Cañedo-Ayala, M. Skin Acute Wound Healing: A Comprehensive Review. Int. J. Inflam. 2019, 2019, 3706315. [Google Scholar] [CrossRef]
- Wang, P.-H.; Huang, B.-S.; Horng, H.-C.; Yeh, C.-C.; Chen, Y.-J. Wound Healing. J. Chin. Med. Assoc. 2018, 81, 94–101. [Google Scholar] [CrossRef]
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin Wound Healing Process and New Emerging Technologies for Skin Wound Care and Regeneration. Pharmaceutics 2020, 12, 735. [Google Scholar] [CrossRef]
- Velnar, T.; Bailey, T.; Smrkolj, V. The Wound Healing Process: An Overview of the Cellular and Molecular Mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef]
- Almadani, Y.H.; Vorstenbosch, J.; Davison, P.G.; Murphy, A.M. Wound Healing: A Comprehensive Review. Semin. Plast. Surg. 2021, 35, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Gordts, S.; Muthuramu, I.; Amin, R.; Jacobs, F.; De Geest, B. The Impact of Lipoproteins on Wound Healing: Topical HDL Therapy Corrects Delayed Wound Healing in Apolipoprotein E Deficient Mice. Pharmaceuticals 2014, 7, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Bogachkov, Y.Y.; Chen, L.; Le Master, E.; Fancher, I.S.; Zhao, Y.; Aguilar, V.; Oh, M.-J.; Wary, K.K.; DiPietro, L.A.; Levitan, I. LDL Induces Cholesterol Loading and Inhibits Endothelial Proliferation and Angiogenesis in Matrigels: Correlation with Impaired Angiogenesis during Wound Healing. Am. J. Physiol. Cell Physiol. 2020, 318, C762–C776. [Google Scholar] [CrossRef] [PubMed]
- Hata, Y.; Iida, O.; Okamoto, S.; Ishihara, T.; Nanto, K.; Tsujimura, T.; Higashino, N.; Toyoshima, T.; Nakao, S.; Fukunaga, M.; et al. Clinical Outcomes of Patients with Cholesterol Crystal Embolism Accompanied by Lower Extremity Wound. Angiology 2023, 00033197231195671. [Google Scholar] [CrossRef] [PubMed]
- Farsaei, S.; Khalili, H.; Farboud, E.S. Potential Role of Statins on Wound Healing: Review of the Literature. Int. Wound J. 2012, 9, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Fitzmaurice, G.J.; McWilliams, B.; Nölke, L.; Redmond, J.M.; McGuinness, J.G.; O’Donnell, M.E. Do Statins Have a Role in the Promotion of Postoperative Wound Healing in Cardiac Surgical Patients? Ann. Thorac. Surg. 2014, 98, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Toker, S.; Gulcan, E.; Çaycı, M.K.; Olgun, E.G.; Erbilen, E.; Özay, Y. Topical Atorvastatin in the Treatment of Diabetic Wounds. Am. J. Med. Sci. 2009, 338, 201–204. [Google Scholar] [CrossRef]
- Falagas, M.E.; Makris, G.C.; Matthaiou, D.K.; Rafailidis, P.I. Statins for Infection and Sepsis: A Systematic Review of the Clinical Evidence. J. Antimicrob. Chemother. 2008, 61, 774–785. [Google Scholar] [CrossRef]
- Suzuki-Banhesse, V.F.; Azevedo, F.F.; Araujo, E.P.; do Amaral, M.E.C.; Caricilli, A.M.; Saad, M.J.A.; Lima, M.H.M. Effect of Atorvastatin on Wound Healing in Rats. Biol. Res. Nurs. 2015, 17, 159–168. [Google Scholar] [CrossRef]
- Raziyeva, K.; Kim, Y.; Zharkinbekov, Z.; Kassymbek, K.; Jimi, S.; Saparov, A. Immunology of Acute and Chronic Wound Healing. Biomolecules 2021, 11, 700. [Google Scholar] [CrossRef]
- Hurlow, J.; Bowler, P.G. Acute and Chronic Wound Infections: Microbiological, Immunological, Clinical and Therapeutic Distinctions. J. Wound Care 2022, 31, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Mulyaningsih, S.; Sporer, F.; Reichling, J.; Wink, M. Antibacterial Activity of Essential Oils from Eucalyptus and of Selected Components against Multidrug-Resistant Bacterial Pathogens. Pharm. Biol. 2011, 49, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, K.; Manigandan, V.; Jeyapragash, D.; Bharathidasan, V.; Anandharaj, B.; Sathya, M. Eucalyptol Inhibits Biofilm Formation of Streptococcus Pyogenes and Its Mediated Virulence Factors. J. Med. Microbiol. 2020, 69, 1308–1318. [Google Scholar] [CrossRef] [PubMed]
- Baltzis, D.; Eleftheriadou, I.; Veves, A. Pathogenesis and Treatment of Impaired Wound Healing in Diabetes Mellitus: New Insights. Adv. Ther. 2014, 31, 817–836. [Google Scholar] [CrossRef] [PubMed]
- Okur, M.E.; Bülbül, E.Ö.; Mutlu, G.; Eleftherıadou, K.; Karantas, I.D.; Okur, N.Ü.; Siafaka, P.I. An Updated Review for the Diabetic Wound Healing Systems. Curr. Drug Targets 2022, 23, 393–419. [Google Scholar] [CrossRef] [PubMed]
- Jais, S. Various Types of Wounds That Diabetic Patients Can Develop: A Narrative Review. Clin. Pathol. 2023, 16, 2632010X231205366. [Google Scholar] [CrossRef]
- Ahsan, H.; Ahad, A.; Iqbal, J.; Siddiqui, W.A. Pharmacological Potential of Tocotrienols: A Review. Nutr. Metab. 2014, 11, 52. [Google Scholar] [CrossRef]
- Zainal, Z.; Khaza’ai, H.; Kutty Radhakrishnan, A.; Chang, S.K. Therapeutic Potential of Palm Oil Vitamin E-Derived Tocotrienols in Inflammation and Chronic Diseases: Evidence from Preclinical and Clinical Studies. Food Res. Int. 2022, 156, 111175. [Google Scholar] [CrossRef]
- Kandhare, A.D.; Alam, J.; Patil, M.V.K.; Sinha, A.; Bodhankar, S.L. Wound Healing Potential of Naringin Ointment Formulation via Regulating the Expression of Inflammatory, Apoptotic and Growth Mediators in Experimental Rats. Pharm. Biol. 2016, 54, 419–432. [Google Scholar] [CrossRef]
- Kandhare, A.D.; Ghosh, P.; Bodhankar, S.L. Naringin, a Flavanone Glycoside, Promotes Angiogenesis and Inhibits Endothelial Apoptosis through Modulation of Inflammatory and Growth Factor Expression in Diabetic Foot Ulcer in Rats. Chem. Biol. Interact. 2014, 219, 101–112. [Google Scholar] [CrossRef]
- Kmail, A.; Said, O.; Saad, B. How Thymoquinone from Nigella sativa Accelerates Wound Healing through Multiple Mechanisms and Targets. Curr. Issues Mol. Biol. 2023, 45, 9039–9059. [Google Scholar] [CrossRef] [PubMed]
- Sallehuddin, N.; Nordin, A.; Bt Hj Idrus, R.; Fauzi, M.B. Nigella Sativa and Its Active Compound, Thymoquinone, Accelerate Wound Healing in an In Vivo Animal Model: A Comprehensive Review. Int. J. Environ. Res. Public. Health 2020, 17, 4160. [Google Scholar] [CrossRef] [PubMed]
- Rajabian, A.; Hosseinzadeh, H. Dermatological Effects of Nigella Sativa and Its Constituent, Thymoquinone. In Nuts and Seeds in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2020; pp. 329–355. [Google Scholar]
- Kumari, A.; Raina, N.; Wahi, A.; Goh, K.W.; Sharma, P.; Nagpal, R.; Jain, A.; Ming, L.C.; Gupta, M. Wound-Healing Effects of Curcumin and Its Nanoformulations: A Comprehensive Review. Pharmaceutics 2022, 14, 2288. [Google Scholar] [CrossRef] [PubMed]
- Tejada, S.; Manayi, A.; Daglia, M.; Nabavi, S.F.; Sureda, A.; Hajheydari, Z.; Gortzi, O.; Pazoki-Toroudi, H.; Nabavi, S.M. Wound Healing Effects of Curcumin: A Short Review. Curr. Pharm. Biotechnol. 2016, 17, 1002–1007. [Google Scholar] [CrossRef] [PubMed]
- Khatun, M.; Nur, M.A.; Biswas, S.; Khan, M.; Amin, M.Z. Assessment of the Anti-Oxidant, Anti-Inflammatory and Anti-Bacterial Activities of Different Types of Turmeric (Curcuma Longa) Powder in Bangladesh. J. Agric. Food Res. 2021, 6, 100201. [Google Scholar] [CrossRef]
- Vitiello, A.; Ferrara, F.; Boccellino, M.; Ponzo, A.; Cimmino, C.; Comberiati, E.; Zovi, A.; Clemente, S.; Sabbatucci, M. Antifungal Drug Resistance: An Emergent Health Threat. Biomedicines 2023, 11, 1063. [Google Scholar] [CrossRef] [PubMed]
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef]
- Fothergill, A.W. Miconazole: A Historical Perspective. Expert Rev. Anti Infect. Ther. 2006, 4, 171–175. [Google Scholar] [CrossRef]
- Quatresooz, P.; Vroome, V.; Borgers, M.; Cauwenbergh, G.; Piérard, G.E. Novelties in the Multifaceted Miconazole Effects on Skin Disorders. Expert Opin. Pharmacother. 2008, 9, 1927–1934. [Google Scholar] [CrossRef]
- Gatta, L. Antimicrobial Activity of Esomeprazole versus Omeprazole against Helicobacter Pylori. J. Antimicrob. Chemother. 2003, 51, 439–442. [Google Scholar] [CrossRef]
- Anagnostopoulos, G.K.; Tsiakos, S.; Margantinis, G.; Kostopoulos, P.; Arvanitidis, D. Esomeprazole versus Omeprazole for the Eradication of Helicobacter Pylori Infection. J. Clin. Gastroenterol. 2004, 38, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Kumar, L.; Verma, S.; Bhardwaj, A.; Vaidya, S.; Vaidya, B. Eradication of Superficial Fungal Infections by Conventional and Novel Approaches: A Comprehensive Review. Artif. Cells Nanomed. Biotechnol. 2014, 42, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Hu, S.; Wu, C.; Gu, F.; Yang, Y. Probiotics: Potential Novel Therapeutics Against Fungal Infections. Front. Cell Infect. Microbiol. 2022, 11, 793419. [Google Scholar] [CrossRef] [PubMed]
- Rauseo, A.M.; Coler-Reilly, A.; Larson, L.; Spec, A. Hope on the Horizon: Novel Fungal Treatments in Development. Open Forum Infect. Dis. 2020, 7, ofaa016. [Google Scholar] [CrossRef] [PubMed]
- Garland, M.; Hryckowian, A.J.; Tholen, M.; Oresic Bender, K.; Van Treuren, W.W.; Loscher, S.; Sonnenburg, J.L.; Bogyo, M. The Clinical Drug Ebselen Attenuates Inflammation and Promotes Microbiome Recovery in Mice after Antibiotic Treatment for CDI. Cell Rep. Med. 2020, 1, 100005. [Google Scholar] [CrossRef] [PubMed]
- Sarma, B.K.; Mugesh, G. Antioxidant Activity of the Anti-Inflammatory Compound Ebselen: A Reversible Cyclization Pathway via Selenenic and Seleninic Acid Intermediates. Chem. Eur. J. 2008, 14, 10603–10614. [Google Scholar] [CrossRef]
- Maślanka, M.; Mucha, A. Antibacterial Activity of Ebselen. Int. J. Mol. Sci. 2023, 24, 1610. [Google Scholar] [CrossRef]
- Leung, A.K.C.; Lam, J.M.; Leong, K.F.; Hon, K.L.; Barankin, B.; Leung, A.A.M.; Wong, A.H.C. Onychomycosis: An Updated Review. Recent. Pat. Inflamm. Allergy Drug Discov. 2020, 14, 32–45. [Google Scholar] [CrossRef]
- Gupta, A.K.; Stec, N.; Summerbell, R.C.; Shear, N.H.; Piguet, V.; Tosti, A.; Piraccini, B.M. Onychomycosis: A Review. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1972–1990. [Google Scholar] [CrossRef]
- Ahmed, I.S.; Elnahas, O.S.; Assar, N.H.; Gad, A.M.; El Hosary, R. Nanocrystals of Fusidic Acid for Dual Enhancement of Dermal Delivery and Antibacterial Activity: In Vitro, Ex Vivo and In Vivo Evaluation. Pharmaceutics 2020, 12, 199. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Castanheira, M.; Sader, H.S.; Jones, R.N. Evaluation of the Activity of Fusidic Acid Tested against Contemporary Gram-Positive Clinical Isolates from the USA and Canada. Int. J. Antimicrob. Agents 2010, 35, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Curbete, M.M.; Salgado, H.R.N. A Critical Review of the Properties of Fusidic Acid and Analytical Methods for Its Determination. Crit. Rev. Anal. Chem. 2016, 46, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Algarin, Y.A.; Jambusaria-Pahlajani, A.; Ruiz, E.; Patel, V.A. Advances in Topical Treatments of Cutaneous Malignancies. Am. J. Clin. Dermatol. 2023, 24, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, L.; Saldías-Fuentes, C.; Carrasco, K.; Halpern, A.C.; Mao, J.J.; Navarrete-Dechent, C. Complementary and Alternative Therapies in Skin Cancer a Literature Review of Biologically Active Compounds. Dermatol. Ther. 2022, 35, e15842. [Google Scholar] [CrossRef] [PubMed]
- Conforti, C.; Corneli, P.; Harwood, C.; Zalaudek, I. Evolving Role of Systemic Therapies in Non-Melanoma Skin Cancer. Clin. Oncol. 2019, 31, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Salari, N.; Faraji, F.; Jafarpour, S.; Faraji, F.; Rasoulpoor, S.; Dokaneheifard, S.; Mohammadi, M. Anti-Cancer Activity of Chrysin in Cancer Therapy: A Systematic Review. Indian J. Surg. Oncol. 2022, 13, 681–690. [Google Scholar] [CrossRef]
- Kasala, E.R.; Bodduluru, L.N.; Madana, R.M.; Athira, K.V.; Gogoi, R.; Barua, C.C. Chemopreventive and Therapeutic Potential of Chrysin in Cancer: Mechanistic Perspectives. Toxicol. Lett. 2015, 233, 214–225. [Google Scholar] [CrossRef]
- Talebi, M.; Talebi, M.; Farkhondeh, T.; Simal-Gandara, J.; Kopustinskiene, D.M.; Bernatoniene, J.; Samarghandian, S. Emerging Cellular and Molecular Mechanisms Underlying Anticancer Indications of Chrysin. Cancer Cell Int. 2021, 21, 214. [Google Scholar] [CrossRef]
- Chang, S.-H.; Wu, C.-Y.; Chuang, K.-C.; Huang, S.-W.; Li, Z.-Y.; Wang, S.-T.; Lai, Z.-L.; Chang, C.-C.; Chen, Y.-J.; Wong, T.-W.; et al. Imiquimod Accelerated Antitumor Response by Targeting Lysosome Adaptation in Skin Cancer Cells. J. Investig. Dermatol. 2021, 141, 2219–2228. [Google Scholar] [CrossRef]
- Bubna, A. Imiquimod—Its Role in the Treatment of Cutaneous Malignancies. Indian J. Pharmacol. 2015, 47, 354. [Google Scholar] [CrossRef]
- Lelli, D.; Pedone, C.; Sahebkar, A. Curcumin and Treatment of Melanoma: The Potential Role of MicroRNAs. Biomed. Pharmacother. 2017, 88, 832–834. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.M.; Clark, C.; Herman-Ferdinandez, L.; Moore-Medlin, T.; Rong, X.; Gill, J.R.; Clifford, J.L.; Abreo, F.; Nathan, C.O. Curcumin Inhibits Skin Squamous Cell Carcinoma Tumor Growth In Vivo. Otolaryngol. Head Neck Surg. 2011, 145, 58–63. [Google Scholar] [CrossRef] [PubMed]
- van der Fits, L.; Mourits, S.; Voerman, J.S.A.; Kant, M.; Boon, L.; Laman, J.D.; Cornelissen, F.; Mus, A.-M.; Florencia, E.; Prens, E.P.; et al. Imiquimod-Induced Psoriasis-Like Skin Inflammation in Mice Is Mediated via the IL-23/IL-17 Axis. J. Immunol. 2009, 182, 5836–5845. [Google Scholar] [CrossRef] [PubMed]
- Carlos, E.C.D.S.; Cristovão, G.A.; Silva, A.A.; de Santos Ribeiro, B.C.; Romana-Souza, B. Imiquimod-induced Ex Vivo Model of Psoriatic Human Skin via Interleukin-17A Signalling of T Cells and Langerhans Cells. Exp. Dermatol. 2022, 31, 1791–1799. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, C.E.M.; Armstrong, A.W.; Gudjonsson, J.E.; Barker, J.N.W.N. Psoriasis. Lancet 2021, 397, 1301–1315. [Google Scholar] [CrossRef] [PubMed]
- Raharja, A.; Mahil, S.K.; Barker, J.N. Psoriasis: A Brief Overview. Clin. Med. 2021, 21, 170–173. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.W.; Read, C. Pathophysiology, Clinical Presentation, and Treatment of Psoriasis. JAMA 2020, 323, 1945. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, M.L.; Schleyerbach, R.; Kirschbaum, B.J. Leflunomide: An Immunomodulatory Drug for the Treatment of Rheumatoid Arthritis and Other Autoimmune Diseases. Immunopharmacology 2000, 47, 273–289. [Google Scholar] [CrossRef]
- Alamri, R.D.; Elmeligy, M.A.; Albalawi, G.A.; Alquayr, S.M.; Alsubhi, S.S.; El-Ghaiesh, S.H. Leflunomide an Immunomodulator with Antineoplastic and Antiviral Potentials but Drug-Induced Liver Injury: A Comprehensive Review. Int. Immunopharmacol. 2021, 93, 107398. [Google Scholar] [CrossRef]
- Boehncke, W.-H. Immunomodulatory Drugs for Psoriasis. BMJ 2003, 327, 634–635. [Google Scholar] [CrossRef]
- Nagai, N.; Ogata, F.; Otake, H.; Kawasaki, N. Oral Administration System Based on Meloxicam Nanocrystals: Decreased Dose Due to High Bioavailability Attenuates Risk of Gastrointestinal Side Effects. Pharmaceutics 2020, 12, 313. [Google Scholar] [CrossRef] [PubMed]
- Rostom, A.; Goldkind, L.; Laine, L. Nonsteroidal Anti-Inflammatory Drugs and Hepatic Toxicity: A Systematic Review of Randomized Controlled Trials in Arthritis Patients. Clin. Gastroenterol. Hepatol. 2005, 3, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Jain, B.; Jain, N.; Jain, S.; Teja, P.K.; Chauthe, S.K.; Jain, A. Exploring Brucine Alkaloid: A Comprehensive Review on Pharmacology, Therapeutic Applications, Toxicity, Extraction and Purification Techniques. Phytomed. Plus 2023, 3, 100490. [Google Scholar] [CrossRef]
- Song, X.; Wang, Y.; Chen, H.; Jin, Y.; Wang, Z.; Lu, Y.; Wang, Y. Dosage-Efficacy Relationship and Pharmacodynamics Validation of Brucine Dissolving Microneedles against Rheumatoid Arthritis. J. Drug Deliv. Sci. Technol. 2021, 63, 102537. [Google Scholar] [CrossRef]
- Lu, L.; Huang, R.; Wu, Y.; Jin, J.-M.; Chen, H.-Z.; Zhang, L.-J.; Luan, X. Brucine: A Review of Phytochemistry, Pharmacology, and Toxicology. Front. Pharmacol. 2020, 11, 377. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Wang, T.-S.; Yin, F.-Z.; Cai, B.-C. Analgesic and Anti-Inflammatory Properties of Brucine and Brucine N-Oxide Extracted from Seeds of Strychnos Nux-Vomica. J. Ethnopharmacol. 2003, 88, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Zhu, W.; Yang, Z.; He, C. Brucine Inhibits TNF-α-induced HFLS-RA Cell Proliferation by Activating the JNK Signaling Pathway. Exp. Ther. Med. 2019, 18, 735–740. [Google Scholar] [CrossRef]
- Elafros, M.A.; Andersen, H.; Bennett, D.L.; Savelieff, M.G.; Viswanathan, V.; Callaghan, B.C.; Feldman, E.L. Towards Prevention of Diabetic Peripheral Neuropathy: Clinical Presentation, Pathogenesis, and New Treatments. Lancet Neurol. 2022, 21, 922–936. [Google Scholar] [CrossRef]
- Jensen, T.S.; Karlsson, P.; Gylfadottir, S.S.; Andersen, S.T.; Bennett, D.L.; Tankisi, H.; Finnerup, N.B.; Terkelsen, A.J.; Khan, K.; Themistocleous, A.C.; et al. Painful and Non-Painful Diabetic Neuropathy, Diagnostic Challenges and Implications for Future Management. Brain 2021, 144, 1632–1645. [Google Scholar] [CrossRef]
- Cernea, S.; Raz, I. Management of Diabetic Neuropathy. Metabolism 2021, 123, 154867. [Google Scholar] [CrossRef]
- Sharma, S.K.; Vij, A.S.; Sharma, M. Mechanisms and Clinical Uses of Capsaicin. Eur. J. Pharmacol. 2013, 720, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Chen, C.; Lan, Y.; Xiao, J.; Li, R.; Huang, J.; Huang, Q.; Cao, Y.; Ho, C.-T. Capsaicin—The Major Bioactive Ingredient of Chili Peppers: Bio-Efficacy and Delivery Systems. Food Funct. 2020, 11, 2848–2860. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, E.S.; Cerqueira, A.R.A.; Soares, A.G.; Costa, S.K.P. Capsaicin and Its Role in Chronic Diseases. In Drug Discovery from Mother Nature; Springer: Berlin/Heidelberg, Germany, 2016; pp. 91–125. [Google Scholar]
- Basith, S.; Cui, M.; Hong, S.; Choi, S. Harnessing the Therapeutic Potential of Capsaicin and Its Analogues in Pain and Other Diseases. Molecules 2016, 21, 966. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Salam, O.M.E.; Mózsik, G. Capsaicin, The Vanilloid Receptor TRPV1 Agonist in Neuroprotection: Mechanisms Involved and Significance. Neurochem. Res. 2023, 48, 3296–3315. [Google Scholar] [CrossRef] [PubMed]
- Fattori, V.; Hohmann, M.; Rossaneis, A.; Pinho-Ribeiro, F.; Verri, W. Capsaicin: Current Understanding of Its Mechanisms and Therapy of Pain and Other Pre-Clinical and Clinical Uses. Molecules 2016, 21, 844. [Google Scholar] [CrossRef] [PubMed]
- Frydas, S.; Varvara, G.; Murmura, G.; Saggini, A.; Caraffa, A.; Antinolfi, P.; Tetè, S.; Tripodi, D.; Conti, F.; Cianchetti, E.; et al. Impact of Capsaicin on Mast Cell Inflammation. Int. J. Immunopathol. Pharmacol. 2013, 26, 597–600. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-S.; Kawada, T.; Kim, B.-S.; Han, I.-S.; Choe, S.-Y.; Kurata, T.; Yu, R. Capsaicin Exhibits Anti-Inflammatory Property by Inhibiting IkB-a Degradation in LPS-Stimulated Peritoneal Macrophages. Cell Signal 2003, 15, 299–306. [Google Scholar] [CrossRef]
- Srinivasan, K. Biological Activities of Red Pepper (Capsicum Annuum) and Its Pungent Principle Capsaicin: A Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1488–1500. [Google Scholar] [CrossRef]
- Rollyson, W.D.; Stover, C.A.; Brown, K.C.; Perry, H.E.; Stevenson, C.D.; McNees, C.A.; Ball, J.G.; Valentovic, M.A.; Dasgupta, P. Bioavailability of Capsaicin and Its Implications for Drug Delivery. J. Control. Release 2014, 196, 96–105. [Google Scholar] [CrossRef]
- Babbar, S.; Marier, J.-F.; Mouksassi, M.-S.; Beliveau, M.; Vanhove, G.F.; Chanda, S.; Bley, K. Pharmacokinetic Analysis of Capsaicin after Topical Administration of a High-Concentration Capsaicin Patch to Patients with Peripheral Neuropathic Pain. Ther. Drug Monit. 2009, 31, 502–510. [Google Scholar] [CrossRef]
- Oliveira, M.B.; do Prado, A.H.; Bernegossi, J.; Sato, C.S.; Lourenço Brunetti, I.; Scarpa, M.V.; Leonardi, G.R.; Friberg, S.E.; Chorilli, M. Topical Application of Retinyl Palmitate-Loaded Nanotechnology-Based Drug Delivery Systems for the Treatment of Skin Aging. Biomed. Res. Int. 2014, 2014, 632570. [Google Scholar] [CrossRef] [PubMed]
- Salem, H.F.; Kharshoum, R.M.; Awad, S.M.; Ahmed Mostafa, M.; Abou-Taleb, H.A. Tailoring of Retinyl Palmitate-Based Ethosomal Hydrogel as a Novel Nanoplatform for Acne Vulgaris Management: Fabrication, Optimization, and Clinical Evaluation Employing a Split-Face Comparative Study. Int. J. Nanomed. 2021, 16, 4251–4276. [Google Scholar] [CrossRef] [PubMed]
- Milosheska, D.; Roškar, R. Use of Retinoids in Topical Antiaging Treatments: A Focused Review of Clinical Evidence for Conventional and Nanoformulations. Adv. Ther. 2022, 39, 5351–5375. [Google Scholar] [CrossRef] [PubMed]
- Nandy, A.; Lee, E.; Mandal, A.; Saremi, R.; Sharma, S. Microencapsulation of Retinyl Palmitate by Melt Dispersion for Cosmetic Application. J. Microencapsul. 2020, 37, 205–219. [Google Scholar] [CrossRef]
- Gholizadeh, M.; Basafa Roodi, P.; Abaj, F.; Shab-Bidar, S.; Saedisomeolia, A.; Asbaghi, O.; Lak, M. Influence of Vitamin A Supplementation on Inflammatory Biomarkers in Adults: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Sci. Rep. 2022, 12, 21384. [Google Scholar] [CrossRef] [PubMed]
- Farooq, U.; Mahmood, T.; Shahzad, Y.; Yousaf, A.M.; Akhtar, N. Comparative Efficacy of Two Anti-aging Products Containing Retinyl Palmitate in Healthy Human Volunteers. J. Cosmet. Dermatol. 2018, 17, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.P.; Cheng, S.-H.; Coop, L.; Xia, Q.; Culp, S.J.; Tolleson, W.H.; Wamer, W.G.; Howard, P.C. Photoreaction, Phototoxicity, and Photocarcinogenicity of Retinoids. J. Environ. Sci. Health Part. C 2003, 21, 165–197. [Google Scholar] [CrossRef]
- Maugard, T.; Rejasse, B.; Legoy, M.D. Synthesis of Water-Soluble Retinol Derivatives by Enzymatic Method. Biotechnol. Prog. 2002, 18, 424–428. [Google Scholar] [CrossRef]
- Suh, D.-C.; Kim, Y.; Kim, H.; Ro, J.; Cho, S.-W.; Yun, G.; Choi, S.-U.; Lee, J. Enhanced In Vitro Skin Deposition Properties of Retinyl Palmitate through Its Stabilization by Pectin. Biomol. Ther. 2014, 22, 73–77. [Google Scholar] [CrossRef]
- Strati, F.; Neubert, R.H.H.; Opálka, L.; Kerth, A.; Brezesinski, G. Non-Ionic Surfactants as Innovative Skin Penetration Enhancers: Insight in the Mechanism of Interaction with Simple 2D Stratum Corneum Model System. Eur. J. Pharm. Sci. 2021, 157, 105620. [Google Scholar] [CrossRef]
- Ren, Q.; Deng, C.; Meng, U.; Chen, Y.; Chen, U.; Sha, X.; Fang, X. In Vitro, Ex Vivo, and In Vivo Evaluation of the Effect of Saturated Fat Acid Chain Length on the Transdermal Behavior of Ibuprofen-Loaded Microemulsions. J. Pharm. Sci. 2014, 103, 1680–1691. [Google Scholar] [CrossRef] [PubMed]
- Erdal, M.S.; Özhan, G.; Mat, C.; Özsoy, Y.; Güngör, S. Colloidal Nanocarriers for the Enhanced Cutaneous Delivery of Naftifine: Characterization Studies and in Vitro and in Vivo Evaluations. Int. J. Nanomed. 2016, 1027, 1027–1037. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Won, M.; Yang; Lee, K.M.; Kim, C.S. In Vitro Permeation Studies of Nanoemulsions Containing Ketoprofen as a Model Drug. Drug Deliv. 2008, 15, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Osborne, D.W.; Musakhanian, J. Skin Penetration and Permeation Properties of Transcutol®—Neat or Diluted Mixtures. AAPS PharmSciTech 2018, 19, 3512–3533. [Google Scholar] [CrossRef] [PubMed]
- Godwin, D.A.; Kim, N.-H.; Felton, L.A. Influence of Transcutol® CG on the Skin Accumulation and Transdermal Permeation of Ultraviolet Absorbers. Eur. J. Pharm. Biopharm. 2002, 53, 23–27. [Google Scholar] [CrossRef]
- Antunes, F.E.; Gentile, L.; Oliviero Rossi, C.; Tavano, L.; Ranieri, G.A. Gels of Pluronic F127 and Nonionic Surfactants from Rheological Characterization to Controlled Drug Permeation. Colloids Surf. B Biointerfaces 2011, 87, 42–48. [Google Scholar] [CrossRef]
- Zheng, Y.; Ouyang, W.-Q.; Wei, Y.-P.; Syed, S.; Hao, C.-S.; Wang, B.-Z.; Shang, Y.-H. Effects of Carbopol 934 Proportion on Nanoemulsion Gel for Topical and Transdermal Drug Delivery: A Skin Permeation Study. Int. J. Nanomed. 2016, 11, 5971–5987. [Google Scholar] [CrossRef]
- Babu, R.J.; Pandit, J.K. Effect of Penetration Enhancers on the Release and Skin Permeation of Bupranolol from Reservoir-Type Transdermal Delivery Systems. Int. J. Pharm. 2005, 288, 325–334. [Google Scholar] [CrossRef]
- De Jong, W.H.; Geertsma, R.E.; Borchard, G. Regulatory Safety Evaluation of Nanomedical Products: Key Issues to Refine. Drug Deliv. Transl. Res. 2022, 12, 2042–2047. [Google Scholar] [CrossRef]
- Foulkes, R.; Man, E.; Thind, J.; Yeung, S.; Joy, A.; Hoskins, C. The Regulation of Nanomaterials and Nanomedicines for Clinical Application: Current and Future Perspectives. Biomater. Sci. 2020, 8, 4653–4664. [Google Scholar] [CrossRef]
- Liu, L.; Bagia, C.; Janjic, J.M. The First Scale-Up Production of Theranostic Nanoemulsions. Biores Open Access 2015, 4, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Adena, S.K.R.; Herneisey, M.; Pierce, E.; Hartmeier, P.R.; Adlakha, S.; Hosfeld, M.A.I.; Drennen, J.K.; Janjic, J.M. Quality by Design Methodology Applied to Process Optimization and Scale up of Curcumin Nanoemulsions Produced by Catastrophic Phase Inversion. Pharmaceutics 2021, 13, 880. [Google Scholar] [CrossRef] [PubMed]
- Paliwal, R.; Babu, R.J.; Palakurthi, S. Nanomedicine Scale-up Technologies: Feasibilities and Challenges. AAPS PharmSciTech 2014, 15, 1527–1534. [Google Scholar] [CrossRef] [PubMed]














| Disease Intended to Treat | Encapsulated Molecule(s) | Main Formulation Composition | Droplet Size (nm) | PDI | ZP (mV) | pH | Main In Vitro and/or In Vivo Therapeutic Efficacy-Related Results | Reference |
|---|---|---|---|---|---|---|---|---|
| Skin cancer | Chrysin | Capryol® 90, Tween® 80, Transcutol® HP, Pluronic® F127, water | <300 | 0.26 | −15 | NR | Strong antiproliferative effect in human and murine melanoma and human epidermoid carcinoma cell lines | [142] |
| Skin cancer and psoriasis | Leflunomide | Capryol® 90, Cremophor® EL, Transcutol® HP, Pluronic® F-127, water | 98.7 to 280.92 | 0.2 to 0.3 | −7.8 | NR | Significant antimelanoma activity by inducing apoptosis and inhibiting tumor cell proliferation in melanoma cells; potent antipsoriatic activity by inhibiting human keratinocyte proliferation and reducing proinflammatory cytokine levels | [146] |
| Skin wound healing | Atorvastatin | Liquid paraffin, Tween® 80, propylene glycol, carboxymethyl cellulose, water | 100 to 200 | <0.300 | −20 to −30 | 7.6 to 7.8 | Positive effect on wound healing, showing reduced inflammation, increased angiogenesis, and marked improvement in the skin’s histological architecture, after topical application on rat skin for 21 days | [149] |
| Skin wound healing | Eucalyptus oil | Black seed oil, Tween® 80, Span® 60, propylene glycol, Carbopol® 940, water | 139 ± 5.8 | <0.450 | −28.05 | 5 to 6 | Significant improvement in wound healing in a rabbit model, with almost complete wound contraction after a 15-day period | [150] |
| Skin wound healing | Tocotrienols and naringenin | Capryol® 90, Solutol® HS15, Transcutol® P, Carbopol® 934 or Carbopol® 940, water | 145.6 ± 12.5 | 0.452 ± 0.03 | −21.1 ± 3.32 | 4.9 to 5.3 | NR | [151] |
| Skin wound healing | Thymoquinone | Black seed oil, Kolliphor® EL, Transcutol® HP, Carbopol® 940, water | 40.02 to 99.66 | 0.052 to 0.542 | −26.7 to −30.6 | 5.53 ± 0.04 | Accelerated wound closure in an in vivo rat wound model, evidenced by reduced wound size, enhanced re-epithelization, and increased collagen deposition | [152] |
| Skin wound healing | Curcumin | Labrafac™ PG, Tween® 80, PEG 400, Carbopol® 940, water | 49.61 to 84.23 | 0.10 to 0.23 | −15.96 ± 0.55 to −20.26 ± 0.65 | 5.53 ± 0.03 | Significant wound-healing activity in Wistar rats, with almost complete wound healing after 20 days, with reduced inflammatory cells and extensive collagen fiber production | [153] |
| Skin infections | Miconazole nitrate | Olive oil, almond oil, Tween® 80, Span® 80, Carbopol® 940, water | 170 | 0.193 | <−30 | NR | Significant antifungal activity against selected fungal strains (Candida albicans) | [154] |
| Skin infections | Omeprazole | Olive oil, Span® 80, Tween® 80, chitosan, water | 369.7 ± 8.77 | 0.316 | −15.3 ± 6.7 | 6.21 ± 0.21 | Substantial antibacterial effects against both Gram-negative bacteria (Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa) and Gram-positive bacteria (Staphylococcus aureus) | [155] |
| Skin infections | Ebselen | Captex® 300 EP/NF, Kolliphor® ELP, dimethylacetamide, Soluplus®, Aquaphor, water | 54.82 ± 1.26 | NR | −1.69 | NR | Potent antifungal activity against multi-drug-resistant Candida albicans and Candida tropicalis | [156] |
| Skin appendage infections (nails) | Ketoconazole | Labrafac™ Lipophile WL1349, Polysorbate 80, PEG 400, Carbopol® Ultrez 21, glycerin, methylparaben, thioglycolic acid, aminomethyl propanol, water | 77.52 ± 0.92 | 0.128 ± 0.035 | −5.44 ± 0.67 | 6.4 ± 0.24 | Significant antifungal activity against clinical isolates of dermatophytes, namely, Trichophyton rubrum and Candida albicans | [157] |
| Skin infections | Fusidic acid | Myrrh oil, Tween® 80, Transcutol® P, CMC, water | 116 to 226 | NR | NR | 6.61 ± 0.23 | Substantial antibacterial activity, with significant inhibition zones against Staphylococcus Aureus, Bacillus subtilis, Enterococcus faecalis, Candida albicans, Shigella, and Escherichia coli | [158] |
| Skin cancer | Imiquimod and curcumin | Oleic acid, Tween® 20, Transcutol® HP, Carbopol® 934, water | 78.39 | 0.254 | −18.7 | 5.5 | Did not lead to the appearance of psoriasis-like symptoms after topical application to mice | [159] |
| Skin inflammatory diseases | Meloxicam | Eucalyptus oil, Tween® 80, PEG 400, Transcutol® P, distilled water | 139 ± 2.31 to 257 ± 3.61 | NR | NR | 6.58 ± 0.21 | Confirmed anti-inflammatory effects with reduced inflammation percentage in in vivo study | [160] |
| Skin inflammatory diseases | Brucine | Myrrh oil, Tween® 80, PEG 400, ethyl alcohol, carboxymethyl cellulose, water | 151 ± 12 | 0.243 | NR | 6.2 ± 0.2 | Anti-inflammatory effects (significant decrease in inflammation) and antinociceptive effects (reduction in writhing movements) in an animal model, after topical application | [161] |
| Diabetic neuropathy | Capsaicin | Eucalyptus oil, Tween® 80, propylene glycol, ethanol, isopropyl alcohol, Carbopol® 940, water | 28.15 ± 0.24 | 0.27 ± 0.05 | NR | NR | Significant in vivo efficacy in alleviating mechanical allodynia | [162] |
| Skin aging | Retinyl palmitate | Capryol® 90, Captex® 355, Kolliphor® EL, Transcutol® HP, Carbopol® 940, glycerin, water | 16.71 | 0.015 | −20.6 | 5.53 ± 0.06 | NR | [163] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sghier, K.; Mur, M.; Veiga, F.; Paiva-Santos, A.C.; Pires, P.C. Novel Therapeutic Hybrid Systems Using Hydrogels and Nanotechnology: A Focus on Nanoemulgels for the Treatment of Skin Diseases. Gels 2024, 10, 45. https://doi.org/10.3390/gels10010045
Sghier K, Mur M, Veiga F, Paiva-Santos AC, Pires PC. Novel Therapeutic Hybrid Systems Using Hydrogels and Nanotechnology: A Focus on Nanoemulgels for the Treatment of Skin Diseases. Gels. 2024; 10(1):45. https://doi.org/10.3390/gels10010045
Chicago/Turabian StyleSghier, Kamil, Maja Mur, Francisco Veiga, Ana Cláudia Paiva-Santos, and Patrícia C. Pires. 2024. "Novel Therapeutic Hybrid Systems Using Hydrogels and Nanotechnology: A Focus on Nanoemulgels for the Treatment of Skin Diseases" Gels 10, no. 1: 45. https://doi.org/10.3390/gels10010045
APA StyleSghier, K., Mur, M., Veiga, F., Paiva-Santos, A. C., & Pires, P. C. (2024). Novel Therapeutic Hybrid Systems Using Hydrogels and Nanotechnology: A Focus on Nanoemulgels for the Treatment of Skin Diseases. Gels, 10(1), 45. https://doi.org/10.3390/gels10010045