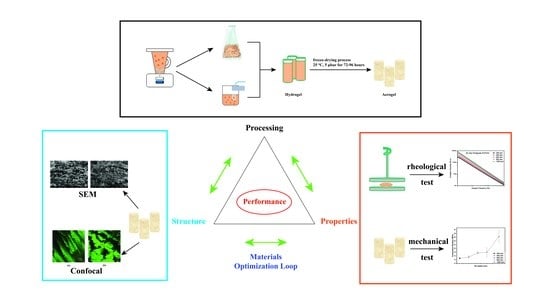
The Relation between the Rheological Properties of Gels and the Mechanical Properties of Their Corresponding Aerogels
Abstract
:1. Introduction
2. Results and Discussion
2.1. Processing Rate
2.2. Standing Times
2.3. Polymer Molecular Weights
3. Conclusions
4. Experimental
4.1. Materials
4.2. Hydrogel Preparation
4.3. Viscosity Testing
4.4. Aerogel Formation
4.5. Mechanical Testing
4.6. SEM and Confocal Microscopy
Author Contributions
Conflicts of Interest
Abbreviations
| G’ | storage modulus |
| G’’ | loss modulus |
| G* | complex modulus |
| η* | complex viscosity |
| PVOH | poly(vinyl alcohol) |
| Mw | molecular weight |
References
- Olson, G.B. Computational design of hierarchically structured materials. Science 1997, 277, 1237–1242. [Google Scholar] [CrossRef]
- Callister, W.D., Jr.; Rethwisch, D.G. Fundamentals of Materials Science and Engineering: An Integrated Approach; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Aliya, D. The Failure Analysis Process: An Overview; ASM International: Materials Park, OH, USA, 2002; pp. 315–323. [Google Scholar]
- Al-Biloushi, M.I.; Milliman, H.; Gawryla, M.D.; Schiraldi, D.A. Oil absorption performance of polymer/clay aerogel materials. J. Appl. Polym. Sci. 2018, 135. [Google Scholar] [CrossRef]
- Shang, K.; Yang, J.C.; Cao, Z.J.; Liao, W.; Wang, Y.-Z.; Schiraldi, D.A. Novel Polymer Aerogel toward High Dimensional Stability, Mechanical Property, and Fire Safety. ACS Appl. Mater. Interfaces 2017, 9, 22985–22993. [Google Scholar] [CrossRef] [PubMed]
- Griebel, J.J.; Gawryla, M.D.; Milliman, H.W.; Schiraldi, D.A. Clay Aerogel Supported Palladium Nanoparticles as Catalysts. Gels 2016, 2, 15. [Google Scholar] [CrossRef]
- Shang, K.; Liao, W.; Wang, J.; Wang, Y.-T.; Wang, Y.-Z.; Schiraldi, D.A. Nonflammable alginate nanocomposite aerogels prepared by a simple freeze-drying and post-cross-linking method. ACS Appl. Mater. Interfaces 2015, 8, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Pojanavaraphan, T.; Magaraphan, R.; Chiou, B.S.; Schiraldi, D.A. Development of biodegradable foamlike materials based on casein and sodium montmorillonite clay. Biomacromolecules 2010, 11, 2640–2646. [Google Scholar] [CrossRef] [PubMed]
- Schiraldi, D.A. Green Polymer Aerogels Green Polymer Chemistry: Biobased Materials and Biocatalysis; American Chemical Society: Washington, DC, USA, 2015; pp. 471–482. [Google Scholar]
- Chen, H.B.; Hollinger, E.; Wang, Y.Z.; Schiraldi, D.A. Facile fabrication of poly (vinyl alcohol) gels and derivative aerogels. Polymer 2014, 55, 380–384. [Google Scholar] [CrossRef]
- Somlai, L.S.; Bandi, S.A.; Schiraldi, D.A.; Mathias, L.J. Facile processing of clays into organically-modified aerogels. AIChE J. 2006, 52, 1162–1168. [Google Scholar] [CrossRef]
- Gawryla, M.D. Low Density Materials through Freeze-Drying: Clay Aerogels and Beyond. Ph.D. Thesis, Case Western Reserve University, Cleveland, OH, USA, 2009. [Google Scholar]
- Sun, M.; Sun, H.; Hostler, S.; Schiraldi, D.A. Effects of feather-fiber reinforcement on poly (vinyl alcohol)/clay aerogels: Structure, property and applications. Polymer 2018, 137, 201–208. [Google Scholar] [CrossRef]
- Wang, Y.T.; Zhao, H.B.; Degracia, K.; Han, L.-X.; Sun, H.; Sun, M.; Wang, Y.-Z.; Schiraldi, D.A. Green Approach to Improving the Strength and Flame Retardancy of Poly (vinyl alcohol)/Clay Aerogels: Incorporating Biobased Gelatin. ACS Appl. Mater. Interfaces 2017, 9, 42258–42265. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Pochan, D.J. Rheological properties of peptide-based hydrogels for biomedical and other applications. Chem. Soc. Rev. 2010, 39, 3528–3540. [Google Scholar] [CrossRef] [PubMed]
- Anseth, K.S.; Bowman, C.N.; Brannon-Peppas, L. Mechanical properties of hydrogels and their experimental determination. Biomaterials 1996, 17, 1647–1657. [Google Scholar] [CrossRef]
- Macosko, C.W. Rheology: Principles, Measurements, and Applications; Wiley-VCH: Weinheim, Germany, 1994. [Google Scholar]
- Gleissle, W.; Hochstein, B. Validity of the Cox—Merz rule for concentrated suspensions. J. Rheol. 2003, 47, 897–910. [Google Scholar] [CrossRef]
- Sun, M. Structure, Properties and Applications of Layered Materials: Multilayered Films and Aerogel Composites; Case Western Reserve University: Cleveland, OH, USA, 2018. [Google Scholar]
- Mezger, T.G. The Rheology Handbook: For Users of Rotational and Oscillatory Rheometers; Vincentz Network GmbH & Co. KG: Hannover, Germany, 2006. [Google Scholar]
- Gawryla, M.D.; van den Berg, O.; Weder, C.; Schiraldi, D.A. Clay aerogel/cellulose whisker nanocomposites: A nanoscale wattle and daub. J. Mater. Chem. 2009, 19, 2118–2124. [Google Scholar] [CrossRef]
- Pojanavaraphan, T.; Schiraldi, D.A.; Magaraphan, R. Mechanical, rheological, and swelling behavior of natural rubber/montmorillonite aerogels prepared by freeze-drying. Appl. Clay Sci. 2010, 50, 271–279. [Google Scholar] [CrossRef]
- Gawryla, M.D.; Schiraldi, D.A. Novel absorbent materials created via ice templating. Macromol. Mater. Eng. 2009, 294, 570–574. [Google Scholar] [CrossRef]
- Wang, Y.; Gawryla, M.D.; Schiraldi, D.A. Effects of freezing conditions on the morphology and mechanical properties of clay and polymer/clay aerogels. J. Appl. Polym. Sci. 2013, 129, 1637–1641. [Google Scholar] [CrossRef]
- Lamison, K.; Gawryla, M.D.; Schiraldi, D.A. The effect of molecular weight on poly (vinyl alcohol)/clay aerogel composite properties. Polym. Prepr. 2007, 48, 974–975. [Google Scholar]









© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, M.; Sun, H.; Wang, Y.; Sánchez-Soto, M.; Schiraldi, D.A. The Relation between the Rheological Properties of Gels and the Mechanical Properties of Their Corresponding Aerogels. Gels 2018, 4, 33. https://doi.org/10.3390/gels4020033
Sun M, Sun H, Wang Y, Sánchez-Soto M, Schiraldi DA. The Relation between the Rheological Properties of Gels and the Mechanical Properties of Their Corresponding Aerogels. Gels. 2018; 4(2):33. https://doi.org/10.3390/gels4020033
Chicago/Turabian StyleSun, Mingze, Hua Sun, Yutao Wang, Miguel Sánchez-Soto, and David A. Schiraldi. 2018. "The Relation between the Rheological Properties of Gels and the Mechanical Properties of Their Corresponding Aerogels" Gels 4, no. 2: 33. https://doi.org/10.3390/gels4020033
APA StyleSun, M., Sun, H., Wang, Y., Sánchez-Soto, M., & Schiraldi, D. A. (2018). The Relation between the Rheological Properties of Gels and the Mechanical Properties of Their Corresponding Aerogels. Gels, 4(2), 33. https://doi.org/10.3390/gels4020033