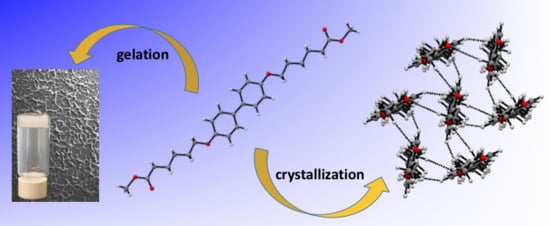
Non-Steroidal Biphenyl Gelators: Correlation of Xerogel Structure with Solid-State Structure and Circular Dichroism Spectroscopy
Abstract
:1. Introduction
2. Results and Discussion
2.1. Gelation Studies
2.2. Phase Transition Temperature (Tg)
2.3. Spectroscopic Studies
2.4. Circular Dichroism
2.5. Scanning Electron Microscopy Studies
2.6. X-ray and XRD Diffraction
3. Conclusions
4. Materials and Methods
4.1. Materials and Instrumentation
4.2. Gel Preparation and Characterization
4.3. Synthetic Details
4.3.1. Synthesis of Acid Precursors
4,4′-biphenoxydioctanoic Acid (BBO8A)
4,4′-biphenoxydihexanoic Acid (BBO6A)
4,4′-biphenoxydidecanoic Acid (BBO10A)
4.3.2. Synthesis of Esters
4,4′-bis-(7-methyloxycarbonyl heptyloxy) Biphenyl (BBO8-Me)
4,4′-bis-(5-methyloxycarbonylpentyloxy) Biphenyl (BBO6-Me)
4,4′-bis-(5-ethyloxycarbonylpentyloxy) Biphenyl (BBO6Et)
4,4′-bis-(7-ethyloxycarbonyl heptyloxy) Biphenyl (BBO8Et)
4,4′-bis-(9-methyloxycarbonylnonyloxy) Biphenyl (BBO10Me)
4,4′-bis-(9-ethyloxycarbonylnonyloxy) Biphenyl (BBO10Et)
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Weiss, R.G. The Past, Present, and Future of Molecular Gels. What Is the Status of the Field, and Where Is It Going? J. Am. Chem. Soc. 2014, 136, 7519–7530. [Google Scholar] [CrossRef] [PubMed]
- Babu, S.S.; Praveen, V.K.; Ajayaghosh, A. Functional π-Gelators and Their Applications. Chem. Rev. 2014, 114, 1973–2129. [Google Scholar] [CrossRef] [PubMed]
- Terech, P.; Weiss, R.G. Low Molecular Mass Gelators of Organic Liquids and the Properties of Their Gels. Chem. Rev. 1997, 97, 3133–3159. [Google Scholar] [CrossRef] [PubMed]
- Ostuni, E.; Kamaras, P.; Weiss, R.G. Novel X-ray Method for in Situ Determination of Gelator Strand Structure: Polymorphism of Cholesteryl Antraquinone-2-carboxylate. Angew. Chem. Int. Ed. 1996, 35, 1324–1326. [Google Scholar] [CrossRef]
- Wang, R.; Geiger, H.C.; Chen, L.; Swanson, B.; Whitten, D.G. Direct Observation of Sol-Gel Conversion: The Role of the Solvent in Organogel Formation. J. Am. Chem. Soc. 2000, 122, 2399–2400. [Google Scholar] [CrossRef]
- Geiger, H.C.; Stanescu, M.; Chen, L.; Whitten, D.G. Organogels Resulting from Competing Self-Assembly Units in the Gelator: Structure, Dynamics, and Photophysical Behavior of Gels Formed from Cholesterol-Stilbene and Cholesterol-Squarine Gelators. Langmuir 1999, 15, 2241–2245. [Google Scholar] [CrossRef]
- Abdallah, D.J.; Bachman, R.E.; Perlstein, J.; Weiss, R.G. Crystal Structures of Symmetrical Tetra-n-Alkyl Ammonium and Phosphonium Halides. Dissection of Competing Interactions Leading to “Biradial” and “Tetraradial” Shapes. J. Phys. Chem. B 1999, 103, 9269–9278. [Google Scholar] [CrossRef]
- Perlstein, J.; Steppe, K.; Vaday, S.; Ndip, E.M.N. Molecular Self-Assemblies. 5. Analysis of the Vector Properties of Hydrogen Bonding in Crystal Engineering. J. Am. Chem. Soc. 1996, 118, 8433–8443. [Google Scholar] [CrossRef]
- Dou, C.D.; Li, D.; Gao, H.Z.; Wang, C.Y.; Zhang, H.Y.; Wang, Y. Sonication-Induced Molecular Gels Based on Mono-Cholesterol Substituted Quinacridone Derivatives. Langmuir 2010, 26, 2113–2118. [Google Scholar] [CrossRef] [PubMed]
- Vintiloiu, A.; Leroux, J.-C. Organogels and their Use in Drug Delivery—A Review. J. Control. Release 2008, 125, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Kumar, N.; Bhattacharya, C.; Sagiri, S.S.; Jain, K.; Pal, K.; Ray, S.S.; Nayak, B. Organogels: Properties and Applications in Drug Delivery. Des. Monomers Polym. 2011, 14, 95–108. [Google Scholar] [CrossRef]
- Culver, H.R.; Clegg, J.R.; Peppas, N.A. Analyte-Responsive Hydrogels: Intelligent Materials for Biosensing and Drug Delivery. Acc. Chem. Res. 2017, 50, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Ng, C.H.B.; Wu, H.; Chan, K.; Shen, J.; Teh, C.; Ying, J.Y.; Zeng, H. Instant Room-Temperature Gelation of Crude Oil by Chiral Organogelators. Chem. Mater. 2016, 28, 4001–4008. [Google Scholar] [CrossRef]
- Shen, X.; Jiao, T.; Zhang, Q.; Guo, H.; Lv, Y.; Zhou, J.; Gao, F. Nanostructures and Self-Assembly of Organogels via Benzimidazole/Benzothiazole Imide Derivatives with Different Alkyl Substituent Chains. J. Nanomater. 2013, 2013, 409087. [Google Scholar] [CrossRef]
- Thool, G.S.; Narayanaswamy, K.; Venkateswararao, A.; Naqvi, S.; Gupta, V.; Chand, S.; Vivekananthan, V.; Koner, R.R.; Krishnan, V.; Singh, S.R. Highly Directional 1D Supramolecular Assembly of New Diketopyrrolopyrrole-Based Gel for Organic Solar Cell Applications. Langmuir 2016, 32, 4346–4351. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xu, M.; Yi, T.; Xiao, S.; Zhou, Z.; Li, F.; Huang, C. Morphology-Tunable and Photoresponsive Properties in a Self-Assembled Two-Component gel System. Langmuir 2007, 23, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Feng, Y.; Deng, G.; He, Y.; Fan, Q. From Weakness to Strength: C−H/π Interaction-Guided Self-Assembly and Gelation of Poly(benzyl ether) Dendrimers. Langmuir 2016, 32, 9313–9320. [Google Scholar] [CrossRef] [PubMed]
- Geiger, H.C.; Lamson, M.; Galka, D.J. Synthesis and Spectroscopic Characterization of Chiral Biphenyl-Cholesterol Gels. Langmuir 2014, 30, 13979–13986. [Google Scholar] [CrossRef] [PubMed]
- Zinic, M.; Vogtle, F.; Fages, F. Cholesterol-Based Gelators. In Topics in Current Chemistry; Springer: Berlin/Heidelberg, Germany, 2005; pp. 39–76. [Google Scholar]
- Ghosh, S.; Mahapatra, R.D.; Dey, J. Thermoreversible as Well as Thermoirreversible Organogel Formation by l-Cysteine-Based Amphiphiles with Poly(ethylene glycol) Tail. Langmuir 2014, 30, 1677–1685. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Terech, P.; Raghavan, S.R.; Weiss, R.G. Kinetics of 5α-Cholestan-3β-yl N-(2-Naphthyl)carbamate/n-Alkane Organogel Formation and Its Influence on the Fibrillar Networks. J. Am. Chem. Soc. 2005, 127, 4336–4344. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.M.; Hong, P.D. Nucleation, Growth, Fractal Aggregation, and Late-Stage Coarsening on Structural Development of Polymer Physical Gels. Macromolecules 2004, 37, 5596–5606. [Google Scholar] [CrossRef]
- Voorhees, P.W. The Theory of Ostwald Ripening. J. Stat. Phys. 1985, 38, 231–252. [Google Scholar] [CrossRef]
- Murata, K.; Aoki, M.; Suzuki, T.; Harada, T.; Kawabata, H.; Komri, T.; Ohseto, F.; Ueda, K.; Shinkai, S. Thermal and Light Control of the Sol-Gel Phase Transition in Cholesterol-Based Organic Gels. Novel Helical Aggregation Modes As Detected by Circular Dichroism and Electron Microscopic Observation. J. Am. Chem. Soc. 1994, 116, 6664–6676. [Google Scholar] [CrossRef]
- Peng, J.; Liu, K.; Liu, J.; Zhang, Q.; Feng, X.; Fang, Y. New Dicholesteryl-Based Gelators: Chirality and Spacer Effect. Langmuir 2008, 24, 2992–3000. [Google Scholar] [CrossRef] [PubMed]
- Baddi, S.; Madugula, S.S.; Sarma, D.S.; Soujanya, Y.; Palanisamy, A. Combined Experimental and computational study of the gelation of cyclohexane-based bis (acyl-semicarbazides) and the multi-stimuli-responsive properties of their gels. Langmuir 2016, 32, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Gao, D.; Yan, J.; Ma, Y.; Liu, K.; Fang, Y. Novel Dimeric Cholesteryl Derivatives and Their Smart Thixotropic Gels. Langmuir 2011, 27, 12156–12163. [Google Scholar] [CrossRef] [PubMed]
- Geiger, D.K.; Geiger, H.C.; Moore, S.S.; Williams, R.R. Structural characterization, gelation ability, and energy-framework analysis of two bis (long-chain ester)-substituted 4,4′-biphenyl compounds. Acta Cryst. C 2017, 73, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer17; University of Western Australia: Perth, Australia, 2017; Available online: http://crystalexplorer.scb.uwa.edu.au (accessed on 15 April 2018).
- Turner, M.J.; Grabowsky, S.; Jayatilaka, D.; Spackman, M.A. Accurate and Efficient Model Energies for Exploring Intermolecular Interactions in Molecular Crystals. J. Phys. Chem. Lett. 2014, 5, 4249–4255. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, C.F.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer model energies and energy frameworks: Extension to metal coordination compounds, organic salts, solvates and open-shell systems. IUCrJ 2017, 4, 575–587. [Google Scholar] [CrossRef] [PubMed]










| Solvent | BBO6-Me (1) | BBO-6Et (2) | BBO8-Me (3) | BBO8-Et (4) | BBO10-Me (5) | BBO10-Et (6) |
|---|---|---|---|---|---|---|
| water | I | I | I | I | I | I |
| methanol | I | I | I | I | wG | wG |
| ethanol | wG | P | wG | wG | wG | wG |
| n-butanol | wG | P | sG | sG | sG | wG |
| n-hexanol | wG | P | wG | wG | wG | wG |
| n-octanol | sG | P | sG | sG | sG | sG |
| hexane | I | I | P | P | P | P |
| heptane | I | I | P | P | wG | wG |
| Gelator | Tg (°C) | ICD |
|---|---|---|
| BBO6-Me | 48–50 | (+) 285, (−) 244 |
| BBO8-Me | 62–64 | (−) 300, (−) 244 |
| BBO8-Et | 46–48 | (−) 300, (−) 237 |
| BBO10-Me | 68–70 | (−) 292, (−) 245 |
| BBO10-Et | 48–50 | (−) 300, (−) 241 |
| BBO8-chol18 | 96–98 |
| Parameter | BBO6-Me | BBO6-Me [28] | BBO6-Et [28] |
|---|---|---|---|
| Molecular formula | C26H34O6 | C26H34O6 | C28H38O6 |
| Space group | P21/c | P21/c | P21/c |
| Temperature, K | 293 K | 200 K | 200 K |
| Cell lengths, Å | a = 25.98(1) | a = 26.022(9) | a = 26.594(7) |
| b = 7.459 (3) | b = 7.410(3) | b = 7.534(2) | |
| c = 6.518(2) | c = 6.531(2) | c = 6.530(1) | |
| Cell angles (α, β, γ), o | 90, 94.37(1), 90 | 90, 94.38(1), 90 | 90, 95.872 (8), 90 |
| Cell volume, Å3 | 1259.3(8) | 1255.7(7) | 1301.6(5) |
| Ratio/Gelator | BBO6-Me | BBO8-Me | BBO8-Et | BBO10-Me | BBO10-Et | |
|---|---|---|---|---|---|---|
| 1:1 | 2θ | 3.51 | 3.01 | 2.61 | 2.44 | 2.32 |
| d (Å) | 25.17 | 29.31 | 33.74 | 36.20 | 37.95 | |
| 1:2 | 2θ | 5.25 | 4.85 | 4.63 | ||
| d (Å) | 16.82 | 18.18 | 19.05 | |||
| 1:3 | 2θ | 7.80 | 7.27 | 6.94 | ||
| d (Å) | 11.32 | 12.4 | 12.71 | |||
| 1:4 | 2θ | 14.01 | 12.06 | 10.41 | 9.71 | 9.28 |
| d (Å) | 6.31 | 7.33 | 8.49 | 9.10 | 9.51 | |
| 1:5 | 2θ | 17.54 | 15.04 | 13.01 | 12.14 | 11.61 |
| d (Å) | 5.05 | 5.88 | 6.80 | 7.28 | 7.61 | |
| 1:6 | 2θ | 21.07 | 18.12 | 15.63 | 14.57 | 13.95 |
| d (Å) | 4.21 | 4.89 | 5.66 | 6.07 | 6.43 | |
| 1:7 | 2θ | 21.09 | 17.02 | 16.31 | ||
| d (Å) | 4.21 | 5.20 | 5.43 | |||
| 1:8 | 2θ | 28.23 | 24.39 | 19.47 | ||
| d (Å) | 3.16 | 3.64 | 4.55 | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geiger, H.C.; Geiger, D.K.; Roberts, W.R.; Morell, D.L.; Huttunen, P.; Schulman, J.L.; Tran, M.; Farthing, D. Non-Steroidal Biphenyl Gelators: Correlation of Xerogel Structure with Solid-State Structure and Circular Dichroism Spectroscopy. Gels 2018, 4, 34. https://doi.org/10.3390/gels4020034
Geiger HC, Geiger DK, Roberts WR, Morell DL, Huttunen P, Schulman JL, Tran M, Farthing D. Non-Steroidal Biphenyl Gelators: Correlation of Xerogel Structure with Solid-State Structure and Circular Dichroism Spectroscopy. Gels. 2018; 4(2):34. https://doi.org/10.3390/gels4020034
Chicago/Turabian StyleGeiger, H. Cristina, David K. Geiger, William R. Roberts, Dominic L. Morell, Paul Huttunen, Jennifer L. Schulman, Melanie Tran, and Dori Farthing. 2018. "Non-Steroidal Biphenyl Gelators: Correlation of Xerogel Structure with Solid-State Structure and Circular Dichroism Spectroscopy" Gels 4, no. 2: 34. https://doi.org/10.3390/gels4020034
APA StyleGeiger, H. C., Geiger, D. K., Roberts, W. R., Morell, D. L., Huttunen, P., Schulman, J. L., Tran, M., & Farthing, D. (2018). Non-Steroidal Biphenyl Gelators: Correlation of Xerogel Structure with Solid-State Structure and Circular Dichroism Spectroscopy. Gels, 4(2), 34. https://doi.org/10.3390/gels4020034