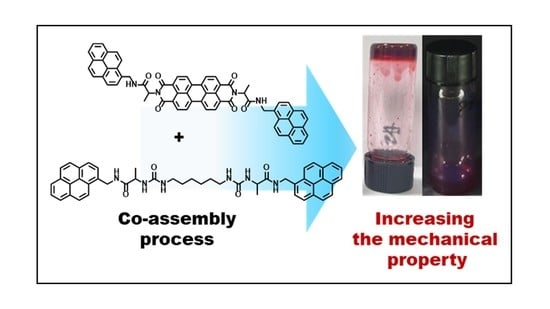
Pyrene-Based Co-Assembled Supramolecular Gel; Morphology Changes and Macroscale Mechanical Property
Abstract
:1. Introduction
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Reagents and Instruments
4.2. FE-SEM Observation
4.3. Rheological Properties
4.4. Preparation of Co-Assembled Supramolecular Gel
4.5. Synthesis of Compound 4
4.6. Synthesis of Compound 3
4.7. Synthesis of Compound 1
4.8. Synthesis of Compound 2
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Duan, P.; Yanai, N.; Nagatomi, H.; Kimizuka, N. Photon Upconversion in Supramolecular Gel Matrixes: Spontaneous Accumulation of Light-Harvesting Donor–Acceptor Arrays in Nanofibers and Acquired Air Stability. J. Am. Chem. Soc. 2015, 137, 1887–1894. [Google Scholar] [CrossRef] [PubMed]
- Valle, C.A.; Felip-León, C.; Angulo-Pachón, C.A.; Mikhailov, M.; Sokolov, M.N.; Miravet, J.F.; Galindo, F. Photoactive Hexanuclear Molybdenum Nanoclusters Embedded in Molecular Organogels. Inorganic Chemistry 2019, 58, 8900–8905. [Google Scholar] [CrossRef] [PubMed]
- Duan, P.; Asthana, D.; Nakashima, T.; Kawai, T.; Yanai, N.; Kimizuka, N. All-or-none switching of photon upconversion in self-assembled organogel systems. Faraday Discuss. 2017, 196, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Cross, E.R.; Sproules, S.; Schweins, R.; Draper, E.R.; Adams, D.J. Controlled Tuning of the Properties in Optoelectronic Self-Sorted Gels. J. Am. Chem. Soc. 2018, 140, 8667–8670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bairi, P.; Chakraborty, P.; Shit, A.; Mondal, S.; Roy, B.; Nandi, A.K. A Co-assembled Gel of a Pyromellitic Dianhydride Derivative and Polyaniline with Optoelectronic and Photovoltaic Properties. Langmuir 2014, 30, 7547–7555. [Google Scholar] [CrossRef]
- Felip-León, C.; Díaz-Oltra, S.; Galindo, F.; Miravet, J.F. Chameleonic, Light Harvesting Photonic Gels Based on Orthogonal Molecular Fibrillization. Chem. Mater. 2016, 28, 7964–7972. [Google Scholar] [CrossRef]
- Chao, Y.-C.; Yeh, S.-D.; Zan, H.-W.; Chang, G.-F.; Meng, H.-F.; Hung, C.-H.; Meng, T.-C. Integrated semiconductor optoelectronic devices for real-time and indicator-free detection of aqueous nitric oxide. Org. Electron. 2011, 12, 751–755. [Google Scholar] [CrossRef] [Green Version]
- Yao, X.; Chen, L.; Chen, X.; Xie, Z.; Ding, J.; He, C.; Zhang, J.; Chen, X. pH-responsive metallo-supramolecular nanogel for synergistic chemo-photodynamic therapy. Acta Biomater. 2015, 25, 162–171. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.; Zou, Q.; Xing, R.; Jiao, T.; Yan, X. An injectable dipeptide–fullerene supramolecular hydrogel for photodynamic antibacterial therapy. J. Mater. Chem. B 2018, 6, 7335–7342. [Google Scholar] [CrossRef]
- Mondal, S.; Das, S.; Nandi, A.K. A review on recent advances in polymer and peptide hydrogels. Soft Matter 2020, 16, 1404–1454. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, Z.; Xie, H.; Ma, Y.; Liu, C.; Liu, S.; Liu, M. Emissive intelligent supramolecular gel for highly selective sensing of Al3+ and writable soft material. Chem. Commun. 2018, 54, 13674–13677. [Google Scholar] [CrossRef] [PubMed]
- Christoff-Tempesta, T.; Lew, A.J.; Ortony, J.H. Beyond Covalent Crosslinks: Applications of Supramolecular Gels. Gels 2018, 4, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Draper, E.R.; Adams, D.J. Low-Molecular-Weight Gels: The State of the Art. Chemistry 2017, 3, 390–410. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Kim, K.Y.; Kim, C.; Lee, J.H.; Kim, J.H.; Lee, S.S.; Choi, Y.; Jung, J.H. A crown-ether-based moldable supramolecular gel with unusual mechanical properties and controllable electrical conductivity prepared by cation-mediated cross-linking. Polym. Chem. 2018, 9, 3900–3907. [Google Scholar] [CrossRef]
- Kim, K.Y.; Kim, J.; Moon, C.J.; Liu, J.; Lee, S.S.; Choi, M.Y.; Feng, C.; Jung, J.H. Co-Assembled Supramolecular Nanostructure of Platinum(II) Complex through Helical Ribbon to Helical Tubes with Helical Inversion. Angew. Chem. Int. Ed. 2019, 58, 11709–11714. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Y.; Kim, C.; Choi, Y.; Jung, S.H.; Kim, J.H.; Jung, J.H. Helicity Control of Triphenylamine-Based Supramolecular Polymers: Correlation between Solvent Properties and Helicity in Supramolecular Gels. Chem. Eur. J. 2018, 24, 11763–11770. [Google Scholar] [CrossRef]
- Banerjee, S.; Das, R.K.; Maitra, U. Supramolecular gels “in action”. J. Mater. Chem. 2009, 19, 6649–6687. [Google Scholar] [CrossRef]
- Sangeetha, N.M.; Maitra, U. Supramolecular gels: Functions and uses. Chem. Soc. Rev. 2005, 34, 821–836. [Google Scholar] [CrossRef] [Green Version]
- Jung, J.H.; Lee, J.H.; Silverman, J.R.; John, G. Coordination polymer gels with important environmental and biological applications. Chem. Soc. Rev. 2013, 42, 924–936. [Google Scholar] [CrossRef]
- Chakraborty, P.; Tang, Y.; Yamamoto, T.; Yao, Y.; Guterman, T.; Zilberzwige-Tal, S.; Adadi, N.; Ji, W.; Dvir, T.; Ramamoorthy, A.; et al. Unusual Two-Step Assembly of a Minimalistic Dipeptide-Based Functional Hypergelator. Adv. Mater. 2020, 32, 1906043. [Google Scholar] [CrossRef]
- Chakraborty, P.; Ghosh, M.; Schnaider, L.; Adadi, N.; Ji, W.; Bychenko, D.; Dvir, T.; Adler-Abramovich, L.; Gazit, E. Composite of Peptide-Supramolecular Polymer and Covalent Polymer Comprises a New Multifunctional, Bio-Inspired Soft Material. Macromol. Rapid Commun. 2019, 40, 1900175. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, P.; Guterman, T.; Adadi, N.; Yadid, M.; Brosh, T.; Adler-Abramovich, L.; Dvir, T.; Gazit, E. A Self-Healing, All-Organic, Conducting, Composite Peptide Hydrogel as Pressure Sensor and Electrogenic Cell Soft Substrate. ACS Nano 2019, 13, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Lin, J.; Zhang, L. Supramolecular multicompartment gels formed by ABC graft copolymers: High toughness and recovery properties. Phy. Chem. Chem. Phy. 2018, 20, 15995–16004. [Google Scholar] [CrossRef] [PubMed]
- Makam, P.; Gazit, E. Minimalistic peptide supramolecular co-assembly: Expanding the conformational space for nanotechnology. Chem. Soc. Rev. 2018, 47, 3406–3420. [Google Scholar] [CrossRef]
- Li, Y.; Young, D.J.; Loh, X.J. Fluorescent gels: A review of synthesis, properties, applications and challenges. Mater. Chem. Front. 2019, 3, 1489–1502. [Google Scholar] [CrossRef]
- Sutar, P.; Suresh, V.M.; Maji, T.K. Tunable emission in lanthanide coordination polymer gels based on a rationally designed blue emissive gelator. Chem. Commun. 2015, 51, 9876–9879. [Google Scholar] [CrossRef]
- Ma, Y.; Cametti, M.; Džolić, Z.; Jiang, S. Responsive aggregation-induced emissive supramolecular gels based on bis-cyanostilbene derivatives. J. Mater. Chem. C 2016, 4, 10786–10790. [Google Scholar] [CrossRef] [Green Version]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Low, Z.W.K.; Li, Z.; Owh, C.; Chee, P.L.; Ye, E.; Kai, D.; Yang, D.P.; Loh, X.J. Using Artificial Skin Devices as Skin Replacements: Insights into Superficial Treatment. Small 2019, 15, 1805453. [Google Scholar] [CrossRef]
- Khan, Q.U.; Tian, G.; Bao, L.; Qi, S.; Wu, D. Highly uniform supramolecular nano-films derived from carbazole-containing perylene diimide via surface-supported self-assembly and their electrically bistable memory behavior. New J. Chem. 2018, 42, 11506–11515. [Google Scholar] [CrossRef]
- Sukul, P.K.; Asthana, D.; Mukhopadhyay, P.; Summa, D.; Muccioli, L.; Zannoni, C.; Beljonne, D.; Rowan, A.E.; Malik, S. Assemblies of perylene diimide derivatives with melamine into luminescent hydrogels. Chem. Commun. 2011, 47, 11858–11860. [Google Scholar] [CrossRef] [PubMed]
- Draper, E.R.; Greeves, B.J.; Barrow, M.; Schweins, R.; Zwijnenburg, M.A.; Adams, D.J. pH-Directed Aggregation to Control Photoconductivity in Self-Assembled Perylene Bisimides. Chem 2017, 3, 390–410. [Google Scholar] [CrossRef] [Green Version]
- Yeh, M.-Y.; Huang, C.-W.; Chang, J.-W.; Huang, Y.-T.; Lin, J.-H.; Hsu, S.-M.; Hung, S.-C.; Lin, H.-C. A novel nanostructured supramolecular hydrogel self-assembled from tetraphenylethylene-capped dipeptides. Soft Matter 2016, 12, 6347–6351. [Google Scholar] [CrossRef] [PubMed]
- Beneduci, A.; Cospito, S.; Deda, M.L.; Chidichimo, G. Highly Fluorescent Thienoviologen-Based Polymer Gels for Single Layer Electrofluorochromic Devices. Adv. Funct. Mater. 2015, 25, 1240–1247. [Google Scholar] [CrossRef]
- Jung, S.H.; Kim, K.Y.; Woo, D.K.; Lee, S.S.; Jung, J.H. Tb3+-triggered luminescence in a supramolecular gel and its use as a fluorescent chemoprobe for proteins containing alanine. Chem. Commun. 2014, 50, 13107–13110. [Google Scholar] [CrossRef]
- Kim, C.; Kim, K.Y.; Lee, J.H.; Ahn, J.; Sakurai, K.; Lee, S.S.; Jung, J.H. Chiral Supramolecular Gels with Lanthanide Ions: Correlation between Luminescence and Helical Pitch. ACS Appl. Mater. Interfaces 2017, 9, 3799–3807. [Google Scholar] [CrossRef]
- Kotova, O.; Bradberry, S.J.; Savyasachi, A.J.; Gunnlaugsson, T. Recent advances in the development of luminescent lanthanide-based supramolecular polymers and soft materials. Dalton Trans. 2018, 47, 16377–16387. [Google Scholar] [CrossRef]
- Cayuela, A.; Soriano, M.L.; Kennedy, S.R.; Steed, J.W.; Valcárcel, M. Fluorescent carbon quantum dot hydrogels for direct determination of silver ions. Talanta 2016, 151, 100–105. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Zhao, X.; Tang, B.; Xu, W.; Li, J.; Hu, J.; Gu, Z. Quantum-Dot-Tagged Bioresponsive Hydrogel Suspension Array for Multiplex Label-Free DNA Detection. Adv. Funct. Mater. 2010, 20, 976–982. [Google Scholar] [CrossRef]
- Reddy, S.M.M.; Dorishetty, P.; Augustine, G.; Deshpande, A.P.; Ayyadurai, N.; Shanmugam, G. A Low-Molecular-Weight Gelator Composed of Pyrene and Fluorene Moieties for Effective Charge Transfer in Supramolecular Ambidextrous Gel. Langmuir 2017, 33, 13504–13514. [Google Scholar] [CrossRef]
- Hahma, A.; Bhat, S.; Leivo, K.; Linnanto, J.; Lahtinen, M.; Rissanen, K. Pyrene derived functionalized low molecular weight organic gelators and gels. New J. Chem. 2008, 32, 1438–1448. [Google Scholar] [CrossRef]
- Wang, K.; Guo, Z.; Zhang, L.; Sun, K.; Yu, P.; Zhou, S.; Wang, W.; Li, Z. Co-assembly of donor and acceptor towards organogels tuned by charge transfer interaction strength. Soft Matter 2017, 13, 1948–1955. [Google Scholar] [CrossRef] [PubMed]
- Sekhar Ghosh, A.; Gupta, D.; Basu, S. State of polarization of monomer and eximer emission from pyrene. J. Photochem. Photobiol. 1975, 4, 227–228. [Google Scholar] [CrossRef]
- El-Mahdy, A.F.M.; Kuo, S.-W. A pyrene-functionalized polytyrosine exhibiting aggregation-induced emission and capable of dispersing carbon nanotubes and hydrogen bonding with P4VP. Polymer 2018, 156, 10–21. [Google Scholar] [CrossRef]
- Nakamura, M.; Fukuda, M.; Takada, T.; Yamana, K. Highly ordered pyrene π-stacks on an RNA duplex display static excimer fluorescence. Org. Biomol. Chem. 2012, 10, 9620–9626. [Google Scholar] [CrossRef]
- Gershberg, J.; Fennel, F.; Rehm, T.H.; Lochbrunner, S.; Würthner, F. Anti-cooperative supramolecular polymerization: A new K2–K model applied to the self-assembly of perylene bisimide dye proceeding via well-defined hydrogen-bonded dimers. Chem. Sci. 2016, 7, 1729–1737. [Google Scholar] [CrossRef] [Green Version]
- Hendsbee, A.D.; Dayneko, S.V.; Pells, J.A.; Cann, J.R.; Welch, G.C. N-annulated perylene diimide dimers: The effect of thiophene bridges on physical, electronic, optical, and photovoltaic properties. Sustainable Energy Fuels 2017, 1, 1137–1147. [Google Scholar] [CrossRef]




| Solvent | State1 | Solvent | State1 |
|---|---|---|---|
| Toluene | I | Ethanol | I |
| H2O | I | MC | I |
| Acetonitrile | I | CHCl3 | S |
| Methanol | I | THF | I |
| Butanol | I | DMSO | G |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.Y.; Ok, M.; Kim, J.; Jung, S.H.; Seo, M.L.; Jung, J.H. Pyrene-Based Co-Assembled Supramolecular Gel; Morphology Changes and Macroscale Mechanical Property. Gels 2020, 6, 16. https://doi.org/10.3390/gels6020016
Kim KY, Ok M, Kim J, Jung SH, Seo ML, Jung JH. Pyrene-Based Co-Assembled Supramolecular Gel; Morphology Changes and Macroscale Mechanical Property. Gels. 2020; 6(2):16. https://doi.org/10.3390/gels6020016
Chicago/Turabian StyleKim, Ka Young, Mirae Ok, Jaehyeong Kim, Sung Ho Jung, Moo Lyong Seo, and Jong Hwa Jung. 2020. "Pyrene-Based Co-Assembled Supramolecular Gel; Morphology Changes and Macroscale Mechanical Property" Gels 6, no. 2: 16. https://doi.org/10.3390/gels6020016
APA StyleKim, K. Y., Ok, M., Kim, J., Jung, S. H., Seo, M. L., & Jung, J. H. (2020). Pyrene-Based Co-Assembled Supramolecular Gel; Morphology Changes and Macroscale Mechanical Property. Gels, 6(2), 16. https://doi.org/10.3390/gels6020016