Injectable Composite Systems Based on Microparticles in Hydrogels for Bioactive Cargo Controlled Delivery
Abstract
:1. Introduction
2. Microparticles in Hydrogel Systems
2.1. Gelation/Cross-Linking of the System
2.1.1. In Situ Gelation
2.1.2. Gelation before Injection
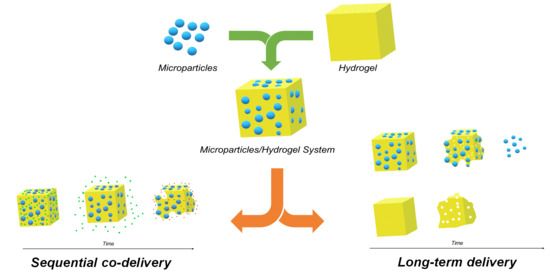
2.2. Microparticle Mixture with the Hydrogel
2.3. Particle Release: Mesh Pore Size and Degradation of the Matrix
2.4. Surface Charge and Hydrophobicity of the Polymers
2.5. Rheological Properties
2.6. Swelling
3. Microparticles in Hydrogel Systems as DDS
3.1. Controllable Drug/Bioactive Agents Release Rate
3.2. In Loco Stability and Drug Release
3.3. Sequential Release and Co Delivery
3.4. Encapsulation of Hydrophobic Drugs
4. Fields of Application
4.1. Cancer Treatment
4.2. Cardiovascular Diseases
4.3. Spinal Diseases
4.4. Cartilage
4.5. Bone
4.6. Skin
4.7. Other
4.8. Tissue Engineering
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Benoit, D.S.W.; Overby, C.T.; Sims, K.R., Jr.; Ackun-Farmmer, M.A. 2.5.12—Drug delivery systems. In Biomaterials Science, 4th ed.; Wagner, W.R., Sakiyama-Elbert, S.E., Zhang, G., Yaszemski, M.J., Eds.; Academic Press: Cambridge, UK, 2020; pp. 1237–1266. [Google Scholar]
- Jain, K.K. An overview of drug delivery systems. In Drug Delivery Systems. Methods in Molecular Biology; Humana: New York, NY, USA, 2021; Volume 2059, pp. 1–51. [Google Scholar] [CrossRef]
- Karlsson, J.; Vaughan, H.J.; Green, J.J. Biodegradable polymeric nanoparticles for therapeutic cancer treatments. Annu. Rev. Chem. Biomol. Eng. 2018, 9, 105–127. [Google Scholar] [CrossRef] [PubMed]
- Packhaeuser, C.; Schnieders, J.; Oster, C.; Kissel, T. In Situ forming parenteral drug delivery systems: An overview. Eur. J. Pharm. Biopharm. 2004, 58, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Dutta, K.; Das, R.; Ling, J.; Monibas, R.M.; Carballo-Jane, E.; Kekec, A.; Feng, D.D.; Lin, S.; Mu, J.; Saklatvala, R.; et al. In Situ forming injectable thermoresponsive hydrogels for controlled delivery of biomacromolecules. ACS Omega 2020, 5, 17531–17542. [Google Scholar] [CrossRef]
- Redaelli, F.; Sorbona, M.; Rossi, F. Synthesis and processing of hydrogels for medical applications. In Bioresorbable Polymers for Biomedical Applications; Perale, G., Hilborn, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 205–228. [Google Scholar]
- Bahram, M.; Mohseni, N.; Moghtader, M. An introduction to hydrogels and some recent applications. In Emerging Concepts in Analysis and Applications of Hydrogels; Majee, S.B., Ed.; IntechOpen: London, UK, 2016; pp. 9–38. [Google Scholar]
- Lengyel, M.; Kállai-Szabó, N.; Antal, V.; Laki, A.J.; Antal, I. Microparticles, microspheres, and microcapsules for advanced drug delivery. Sci. Pharm. 2019, 87, 20. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.K.; Pack, D.W. Microspheres for drug delivery. In BioMEMS and Biomedical Nanotechnology I: Biological and Biomedical Nanotechnology; Ferrari, M., Lee, A.P., Lee, L.J., Eds.; Springer: Boston, MA, USA, 2006; Volume 1, pp. 19–50. [Google Scholar]
- Delgado, B.; Carrêlo, H.; Loureiro, M.V.; Marques, A.C.; Borges, J.P.; Cidade, M.T. Injectable hydrogels with two different rates of drug release based on pluronic/water system filled with poly (ε-caprolactone) microcapsules. J. Mater. Sci. 2021, 56, 13416–13428. [Google Scholar] [CrossRef]
- Cidade, M.; Ramos, D.J.; Santos, J.; Carrelo, H.; Calero, N.; Borges, J.P. Injectable hydrogels based on pluronic/water systems filled with alginate microparticles for biomedical applications. Material 2019, 12, 1083. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Mitragotri, S.; Burke, P.A.; Langer, R. Overcoming the challenges in administering biopharmaceuticals: Formulation and delivery strategies. Nat. Rev. Drug Discov. 2014, 13, 655–672. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Cheng, Y.; Chen, J.; Ding, J.; Li, M.; Li, C.; Wang, J.-C.; Chen, X. Injectable hydrogel–microsphere construct with sequential degradation for locally synergistic chemotherapy. ACS Appl. Mater. Interfaces 2017, 9, 3487–3496. [Google Scholar] [CrossRef]
- Huang, F.; Chen, T.; Chang, J.; Zhang, C.; Liao, F.; Wu, L.; Wang, W.; Yin, Z. A conductive dual-network hydrogel composed of oxidized dextran and hyaluronic-hydrazide as BDNF delivery systems for potential spinal cord injury repair. Int. J. Biol. Macromol. 2021, 167, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Aliaghaie, M.; Mirzadeh, H.; Dashtimoghadam, E.; Taranejoo, S. Investigation of gelation mechanism of an injectable hydrogel based on chitosan by rheological measurements for a drug delivery application. Soft Matter 2012, 8, 7128–7137. [Google Scholar] [CrossRef]
- Liu, L.; Wu, Q.; Ma, X.; Xiong, D.; Gong, C.; Qian, Z.; Zhao, X.; Wei, Y. Camptothecine encapsulated composite drug delivery system for colorectal peritoneal carcinomatosis therapy: Biodegradable microsphere in thermosensitive hydrogel. Colloids Surf. B Biointerfaces 2013, 106, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.-H.; Lee, S.Y.; Kim, S.; Yang, M.; Jeong, D.I.; Hwang, C.; Kim, M.-H.; Kim, H.-J.; Lee, J.; Lee, K.; et al. Monopotassium phosphate-reinforced in situ forming injectable hyaluronic acid hydrogels for subcutaneous injection. Int. J. Biol. Macromol. 2020, 163, 2134–2144. [Google Scholar] [CrossRef]
- Hori, Y.; Winans, A.M.; Irvine, D.J. Modular injectable matrices based on alginate solution/microsphere mixtures that gel In Situ and co-deliver immunomodulatory factors. Acta Biomater. 2009, 5, 969–982. [Google Scholar] [CrossRef] [Green Version]
- Yan, J.; Miao, Y.; Tan, H.; Zhou, T.; Ling, Z.; Chen, Y.; Xing, X.; Hu, X. Injectable alginate/hydroxyapatite gel scaffold combined with gelatin microspheres for drug delivery and bone tissue engineering. Mater. Sci. Eng. C 2016, 63, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Wu, Y.; Chen, W.; Li, J.; Wang, X.; Chen, Y.; Yu, Y.; Shen, Z.; Zhang, Y. Sustained release of bioactive IGF-1 from a silk fibroin microsphere-based injectable alginate hydrogel for the treatment of myocardial infarction. J. Mater. Chem. B 2020, 8, 308–315. [Google Scholar] [CrossRef]
- Zhao, F.; Wu, D.; Yao, D.; Guo, R.; Wang, W.; Dong, A.; Kong, D.; Zhang, J. An injectable particle-hydrogel hybrid system for glucose-regulatory insulin delivery. Acta Biomater. 2017, 64, 334–345. [Google Scholar] [CrossRef]
- Zhang, X.; Pan, Y.; Li, S.; Xing, L.; Du, S.; Yuan, G.; Li, J.; Zhou, T.; Xiong, D.; Tan, H.; et al. Doubly crosslinked biodegradable hydrogels based on gellan gum and chitosan for drug delivery and wound dressing. Int. J. Biol. Macromol. 2020, 164, 2204–2214. [Google Scholar] [CrossRef] [PubMed]
- Talebian, S.; Mehrali, M.; Taebnia, N.; Pennisi, C.P.; Kadumudi, F.B.; Foroughi, J.; Hasany, M.; Nikkhah, M.; Akbari, M.; Orive, G.; et al. Self-healing hydrogels: The next paradigm shift in tissue engineering? Adv. Sci. 2019, 6, 1801664. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Ning, C.; Xu, W.; Hu, H.; Li, M.; Zhao, G.; Ding, J.; Chen, X. Precision-guided long-acting analgesia by gel-immobilized bupivacaine-loaded microsphere. Theranostics 2018, 8, 3331–3347. [Google Scholar] [CrossRef]
- Zhao, C.; Tian, S.; Liu, Q.; Xiu, K.; Lei, I.; Wang, Z.; Ma, P.X. Biodegradable nanofibrous temperature-responsive gelling microspheres for heart regeneration. Adv. Funct. Mater. 2020, 30, 2000776. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, M.; Wang, M.; Song, X.; Zhang, C.; Dong, Z.; Zhang, J.; Yang, Z. Polymerizable microsphere-induced high mechanical strength of hydrogel composed of acrylamide. Materials 2018, 11, 880. [Google Scholar] [CrossRef] [Green Version]
- Moura, M.; Gil, M.H.; Figueiredo, M. Cisplatin delivery systems based on different drug encapsulation techniques. Eur. Polym. J. 2019, 113, 357–364. [Google Scholar] [CrossRef]
- Karamzadeh, Y.; Asl, A.A.; Rahmani, S. PCL microsphere/PEG-based composite hydrogels for sustained release of methadone hydrochloride. J. Appl. Polym. Sci. 2020, 137, 48967. [Google Scholar] [CrossRef]
- França, C.G.; Plaza, T.; Naveas, N.; Santana, M.H.A.; Manso-Silván, M.; Recio, G.; Hernandez-Montelongo, J. Nanoporous silicon microparticles embedded into oxidized hyaluronic acid/adipic acid dihydrazide hydrogel for enhanced controlled drug delivery. Microporous Mesoporous Mater. 2021, 310, 110634. [Google Scholar] [CrossRef]
- Mohammadi, M.; Patel, K.; Alaie, S.P.; Shmueli, R.B.; Besirli, C.G.; Larson, R.G.; Green, J.J. Injectable drug depot engineered to release multiple ophthalmic therapeutic agents with precise time profiles for postoperative treatment following ocular surgery. Acta Biomater. 2018, 73, 90–102. [Google Scholar] [CrossRef]
- Tsaryk, R.; Gloria, A.; Russo, T.; Anspach, L.; De Santis, R.; Ghanaati, S.; Unger, R.E.; Ambrosio, L.; Kirkpatrick, C.J. Collagen-low molecular weight hyaluronic acid semi-interpenetrating network loaded with gelatin microspheres for cell and growth factor delivery for nucleus pulposus regeneration. Acta Biomater. 2015, 20, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Ma, Y.; Tan, H.; Jia, Y.; Zou, S.; Guo, S.; Zhao, M.; Huang, H.; Ling, Z.; Chen, Y.; et al. Covalent and injectable chitosan-chondroitin sulfate hydrogels embedded with chitosan microspheres for drug delivery and tissue engineering. Mater. Sci. Eng. C 2017, 71, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Herrero, E.; García-García, P.; Morales, J.G.; Llabrés, M.; Delgado, A.; Évora, C. New injectable two-step forming hydrogel for delivery of bioactive substances in tissue regeneration. Regen. Biomater. 2019, 6, 149–162. [Google Scholar] [CrossRef]
- Pan, Y.; Xiao, C.; Tan, H.; Yuan, G.; Li, J.; Li, S.; Jia, Y.; Xiong, D.; Hu, X.; Niu, X. Covalently injectable chitosan/chondroitin sulfate hydrogel integrated gelatin/heparin microspheres for soft tissue engineering. Int. J. Polym. Mater. 2021, 70, 149–157. [Google Scholar] [CrossRef]
- Liu, W.; Borrell, M.A.; Venerus, D.C.; Mieler, W.F.; Kang-Mieler, J.J. Characterization of biodegradable microsphere-hydrogel ocular drug delivery system for controlled and extended release of ranibizumab. Transl. Vis. Sci. Technol. 2019, 8, 12. [Google Scholar] [CrossRef]
- Yoo, J.; Won, Y.-Y. Phenomenology of the initial burst release of drugs from PLGA microparticles. ACS Biomater. Sci. Eng. 2020, 6, 6053–6062. [Google Scholar] [CrossRef]
- Heo, J.Y.; Noh, J.H.; Park, S.H.; Ji, Y.B.; Ju, H.J.; Kim, D.Y.; Lee, B.; Kim, M.S. An injectable click-crosslinked hydrogel that prolongs dexamethasone release from dexamethasone-loaded microspheres. Pharmaceutics 2019, 11, 438. [Google Scholar] [CrossRef] [Green Version]
- Osswald, C.R.; Kang-Mieler, J.J. Controlled and extended In Vitro release of bioactive anti-vascular endothelial growth factors from a microsphere-hydrogel drug delivery system. Curr. Eye Res. 2016, 41, 1216–1222. [Google Scholar] [CrossRef]
- Lourenço, A.H.; Neves, N.; Machado, C.; Sousa, S.R.; Lamghari, M.; Barrias, C.; Cabral, A.T.; Barbosa, M.; Ribeiro, C.C. Injectable hybrid system for strontium local delivery promotes bone regeneration in a rat critical-sized defect model. Sci. Rep. 2017, 7, 5098. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.; Song, W.; He, Y.; Li, H. Multilayer injectable hydrogel system sequentially delivers bioactive substances for each wound healing stage. ACS Appl. Mater. Interfaces 2020, 12, 29787–29806. [Google Scholar] [CrossRef]
- Wu, Y.; Chang, T.; Chen, W.; Wang, X.; Li, J.; Chen, Y.; Yu, Y.; Shen, Z.; Yu, Q.; Zhang, Y. Release of VEGF and BMP9 from injectable alginate based composite hydrogel for treatment of myocardial infarction. Bioact. Mater. 2021, 6, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Niu, S.; Williams, G.R.; Wu, J.; Chen, X.; Zheng, H.; Zhu, L.-M. Dual-responsive nanoparticles based on chitosan for enhanced breast cancer therapy. Carbohydr. Polym. 2019, 221, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Loepfe, M.; Duss, A.; Zafeiropoulou, K.-A.; Björgvinsdóttir, O.; D’Este, M.; Eglin, D.; Fortunato, G.; Klasen, J.; Ferguson, S.J.; Wuertz-Kozak, K.; et al. Electrospray-based microencapsulation of epigallocatechin 3-gallate for local delivery into the intervertebral disc. Pharmaceutics 2019, 11, 435. [Google Scholar] [CrossRef] [Green Version]
- Chan, P.S.; Xian, J.W.; Li, Q.; Chan, C.W.; Leung, S.S.Y.; To, K.K.W. Biodegradable thermosensitive PLGA-PEG-PLGA polymer for non-irritating and sustained ophthalmic drug delivery. AAPS J. 2019, 21, 59. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Lin, Z.; Zhang, Q.; Zhang, Y.; Liu, Y.; Lyu, Y.; Li, X.; Zhou, C.; Wu, G.; Ao, N.; et al. Injectable and In Situ-formable thiolated chitosan-coated liposomal hydrogels as curcumin carriers for prevention of In Vivo breast cancer recurrence. ACS Appl. Mater. Interfaces 2020, 12, 17936–17948. [Google Scholar] [CrossRef] [PubMed]
- Shaker, D.; Shaker, M.A.; Klingner, A.; Hanafy, M.S. In Situ thermosensitive tamoxifen citrate loaded hydrogels: An effective tool in breast cancer loco-regional therapy. J. Drug Deliv. Sci. Technol. 2016, 35, 155–164. [Google Scholar] [CrossRef]
- Larrañeta, E.; Stewart, S.; Ervine, M.; Al-Kasasbeh, R.; Donnelly, R.F. Hydrogels for hydrophobic drug delivery. Classification, synthesis and applications. J. Funct. Biomater. 2018, 9, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davoodi, P.; Ng, W.C.; Yan, W.C.; Srinivasan, M.P.; Wang, C.-H. Double-walled microparticles-embedded self-cross-linked, injectable, and antibacterial hydrogel for controlled and sustained release of chemotherapeutic agents. ACS Appl. Mater. Interfaces 2016, 8, 22785–22800. [Google Scholar] [CrossRef] [PubMed]
- Arunkumar, P.; Indulekha, S.; Vijayalakshmi, S.; Srivastava, R. Poly (caprolactone) microparticles and chitosan thermogels based injectable formulation of etoricoxib for the potential treatment of osteoarthritis. Mater. Sci. Eng. C 2016, 61, 534–544. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Available online: www.cancer.org (accessed on 17 June 2021).
- Wind, N.S.; Holen, I. Multidrug resistance in breast cancer: From In Vitro models to clinical studies. Int. J. Breast Cancer 2011, 2011, 967419. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Kwon, D.Y.; Kwon, J.S.; Park, J.H.; Park, S.H.; Oh, H.J.; Kim, J.H.; Min, B.H.; Park, K.; Kim, M.S. Synergistic anti-tumor activity through combinational intratumoral injection of an In-Situ injectable drug depot. Biomaterials 2016, 85, 232–245. [Google Scholar] [CrossRef]
- Luo, J.; Wu, Z.; Lu, Y.; Xiong, K.; Wen, Q.; Zhao, L.; Wang, B.; Gui, Y.; Fu, S. Intraperitoneal administration of biocompatible hyaluronic acid hydrogel containing multi-chemotherapeutic agents for treatment of colorectal peritoneal carcinomatosis. Int. J. Biol. Macromol. 2020, 152, 718–726. [Google Scholar] [CrossRef]
- Pawar, V.; Borse, V.; Thakkar, R.; Srivastava, R.; Pawar, V.B.V. Dual-purpose injectable doxorubicin conjugated alginate gel containing polycaprolactone microparticles for anti-cancer and anti-inflammatory therapy. Curr. Drug Deliv. 2018, 15, 716–726. [Google Scholar] [CrossRef]
- Zhu, C.; Ding, Z.; Lu, Q.; Lu, G.; Xiao, L.; Zhang, X.; Dong, X.; Ru, C.; Kaplan, D.L. Injectable silk–vaterite composite hydrogels with tunable sustained drug release capacity. ACS Biomater. Sci. Eng. 2019, 5, 6602–6609. [Google Scholar] [CrossRef] [PubMed]
- Ozeki, T.; Kaneko, D.; Hashizawa, K.; Imai, Y.; Tagami, T.; Okada, H. Improvement of survival in C6 rat glioma model by a sustained drug release from localized PLGA microspheres in a thermoreversible hydrogel. Int. J. Pharm. 2012, 427, 299–304. [Google Scholar] [CrossRef]
- Chen, X.; Fan, M.; Tan, H.; Ren, B.; Yuan, G.; Jia, Y.; Li, J.; Xiong, D.; Xing, X.; Niu, X.; et al. Magnetic and self-healing chitosan-alginate hydrogel encapsulated gelatin microspheres via. covalent cross-linking for drug delivery. Mater. Sci. Eng. C 2019, 101, 619–629. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Available online: www.who.int (accessed on 12 July 2021).
- Ciocci, M.; Cacciotti, I.; Seliktar, D.; Melino, S. Injectable silk fibroin hydrogels functionalized with microspheres as adult stem cells-carrier systems. Int. J. Biol. Macromol. 2018, 108, 960–971. [Google Scholar] [CrossRef]
- Nelson, D.M.; Hashizume, R.; Yoshizumi, T.; Blakney, A.K.; Ma, Z.; Wagner, W.R. Intramyocardial injection of a synthetic hydrogel with delivery of bFGF and IGF1 in a rat model of ischemic cardiomyopathy. Biomacromolecules 2014, 15, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyu, Y.; Xie, J.; Liu, Y.; Xiao, M.; Li, Y.; Yang, J.; Yang, J.; Liu, W. Injectable hyaluronic acid hydrogel loaded with functionalized human mesenchymal stem cell aggregates for repairing infarcted myocardium. ACS Biomater. Sci. Eng. 2020, 6, 6926–6937. [Google Scholar] [CrossRef] [PubMed]
- Nazemi, Z.; Nourbakhsh, M.S.; Kiani, S.; Heydari, Y.; Ashtiani, M.K.; Daemi, H.; Baharvand, H. Co-delivery of minocycline and paclitaxel from injectable hydrogel for treatment of spinal cord injury. J. Control. Release 2020, 321, 145–158. [Google Scholar] [CrossRef]
- Khaing, Z.; Agrawal, N.K.; Park, J.H.; Xin, S.; Plumton, G.C.; Lee, K.H.; Huang, Y.-J.; Niemerski, A.L.; Schmidt, C.E.; Grau, J.W. Localized and sustained release of brain-derived neurotrophic factor from injectable hydrogel/microparticle composites fosters spinal learning after spinal cord injury. J. Mater. Chem. B 2016, 4, 7560–7571. [Google Scholar] [CrossRef] [PubMed]
- Segundo, F.S.; Sá, M.J.C.D.; Souza, R.L.D. Cartilage tissue engineering and regeneration. In Cartilage Tissue Engineering and Regeneration Techniques; Nikolopoulos, D.D., Ed.; IntechOpen: London, UK, 2019; pp. 1–14. [Google Scholar]
- Naghi Zadeh, Z.; Karkhaneh, A.; Nokhbatolfoghahaei, H.; Farzad-Mohajeri, S.; Rezai-Rad, M.; Dehghan, M.M.; Aminishakib, P.; Khojasteh, A. Cartilage regeneration with dual-drug-releasing injectable hydrogel/microparticle system: In Vitro and In Vivo study. J. Cell. Physiol. 2021, 236, 2194–2204. [Google Scholar] [CrossRef]
- Bolandi, B.; Imani, R.; Bonakdar, S.; Fakhrzadeh, H. Chondrogenic stimulation in mesenchymal stem cells using scaffold-based sustained release of platelet-rich plasma. J. Appl. Polym. Sci. 2021, 138, 50075. [Google Scholar] [CrossRef]
- Asgari, N.; Bagheri, F.; Eslaminejad, M.B.; Ghanian, M.H.; Sayahpour, F.A.; Ghafari, A.M. Dual functional construct containing kartogenin releasing microtissues and curcumin for cartilage regeneration. Stem Cell Res. Ther. 2020, 11, 289. [Google Scholar] [CrossRef]
- Chen, H.; He, D.; Zhu, Y.; Yu, W.; Ramalingam, M.; Wu, Z. In Situ osteochondral regeneration by controlled release of stromal cell-derived factor-1 chemokine from injectable biomaterials: A preclinical evaluation in animal model. J. Biomater. Tissue Eng. 2019, 9, 958–967. [Google Scholar] [CrossRef]
- Liao, J.; Wang, B.; Huang, Y.; Qu, Y.; Peng, J.; Qian, Z. Injectable alginate hydrogel cross-linked by calcium gluconate-loaded porous microspheres for cartilage tissue engineering. ACS Omega 2017, 2, 443–454. [Google Scholar] [CrossRef]
- Zhao, Y.; Cui, Z.; Liu, B.; Xiang, J.; Qiu, D.; Tian, Y.; Qu, X.; Yang, Z. An injectable strong hydrogel for bone reconstruction. Adv. Health Mater. 2019, 8, 1900709. [Google Scholar] [CrossRef] [PubMed]
- Gaihre, B.; Unagolla, J.M.; Liu, J.; Ebraheim, N.A.; Jayasuriya, A.C. Thermoresponsive injectable microparticle–gel composites with recombinant BMP-9 and VEGF enhance bone formation in rats. ACS Biomater. Sci. Eng. 2019, 5, 4587–4600. [Google Scholar] [CrossRef] [PubMed]
- Dashtimoghadam, E.; Fahimipour, F.; Tongas, N.; Tayebi, L. Microfluidic fabrication of microcarriers with sequential delivery of VEGF and BMP-2 for bone regeneration. Sci. Rep. 2020, 10, 11764. [Google Scholar] [CrossRef]
- Wang, T.; Guo, S.; Zhang, H.; Chen, Y.; Cai, Y. Injectable hydrogel delivering bone morphogenetic protein-2, vascular endothelial growth factor, and adipose-derived stem cells for vascularized bone tissue engineering. J. Drug Deliv. Sci. Technol. 2020, 57, 101637. [Google Scholar] [CrossRef]
- Liao, H.T.; Tsai, M.-J.; Brahmayya, M.; Chen, J.-P. Bone regeneration using adipose-derived stem cells in injectable thermo-gelling hydrogel scaffold containing platelet-rich plasma and biphasic calcium phosphate. Int. J. Mol. Sci. 2018, 19, 2537. [Google Scholar] [CrossRef] [Green Version]
- Min, Q.; Liu, J.; Yu, X.; Zhang, Y.; Wu, J.; Wan, Y. Sequential delivery of dual growth factors from injectable chitosan-based composite hydrogels. Mar. Drugs 2019, 17, 365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-García, P.; Reyes, R.; Segredo-Morales, E.; Pérez-Herrero, E.; Delgado, A.; Evora, C. PLGA-BMP-2 and PLA-17β-estradiol microspheres reinforcing a composite hydrogel for bone regeneration in osteoporosis. Pharmaceutics 2019, 11, 648. [Google Scholar] [CrossRef] [Green Version]
- Segredo-Morales, E.; García-García, P.; Reyes, R.; Perez-Herrero, E.; Delgado, A.; Évora, C. Bone regeneration in osteoporosis by delivery BMP-2 and PRGF from tetronic–alginate composite thermogel. Int. J. Pharm. 2018, 543, 160–168. [Google Scholar] [CrossRef]
- Gong, Y.; Zhang, Y.; Cao, Z.; Ye, F.; Lin, Z.; Li, Y. Development of CaCO3 microsphere-based composite hydrogel for dual delivery of growth factor and Ca to enhance bone regeneration. Biomater. Sci. 2019, 7, 3614–3626. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.N.; Shah, S.R.; Lu, S.; Tatara, A.M.; Lee, E.J.; Roh, T.T.; Tabata, Y.; Mikos, A.G. Injectable dual-gelling cell-laden composite hydrogels for bone tissue engineering. Biomaterials 2016, 83, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, B.; Chen, X.; Du, S.; Ma, Y.; Chen, H.; Yuan, G.; Li, J.; Xiong, D.; Tan, H.; Ling, Z.; et al. Injectable polysaccharide hydrogel embedded with hydroxyapatite and calcium carbonate for drug delivery and bone tissue engineering. Int. J. Biol. Macromol. 2018, 118, 1257–1266. [Google Scholar] [CrossRef]
- Xing, L.; Sun, J.; Tan, H.; Yuan, G.; Li, J.; Jia, Y.; Xiong, D.; Chen, G.; Lai, J.; Ling, Z.; et al. Covalently polysaccharide-based alginate/chitosan hydrogel embedded alginate microspheres for BSA encapsulation and soft tissue engineering. Int. J. Biol. Macromol. 2019, 127, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Tian, J.; Liu, Y.; Cao, H.; Li, R.; Wang, J.; Wu, J.; Zhang, Q. Dynamic covalent constructed self-healing hydrogel for sequential delivery of antibacterial agent and growth factor in wound healing. Chem. Eng. J. 2019, 373, 413–424. [Google Scholar] [CrossRef]
- Xu, K.; An, N.; Zhang, H.; Zhang, Q.; Zhang, K.; Hu, X.; Wu, Y.; Wu, F.; Xiao, J.; Zhang, H.; et al. Sustained-release of PDGF from PLGA microsphere embedded thermo-sensitive hydrogel promoting wound healing by inhibiting autophagy. J. Drug Deliv. Sci. Technol. 2020, 55, 101405. [Google Scholar] [CrossRef]
- Ghorbani, F.; Zamanian, A.; Behnamghader, A.; Joupari, M.D. Bioactive and biostable hyaluronic acid-pullulan dermal hydrogels incorporated with biomimetic hydroxyapatite spheres. Mater. Sci. Eng. C 2020, 112, 110906. [Google Scholar] [CrossRef]
- Ding, Q.; Zha, R.; He, X.; Sun, A.; Shi, K.; Qu, Y.; Qian, Z. A novel injectable fibroblasts-porous PDLLA microspheres/collagen gel composite for soft-tissue augmentation. Prog. Nat. Sci. 2020, 30, 651–660. [Google Scholar] [CrossRef]
- Osswald, C.R.; Guthrie, M.J.; Avila, A.; Valio, J.A.; Mieler, W.F.; Kang-Mieler, J.J. In Vivo efficacy of an injectable microsphere-hydrogel ocular drug delivery system. Curr. Eye Res. 2017, 42, 1293–1301. [Google Scholar] [CrossRef]
- Wang, B.; Wang, J.; Shao, J.; Kouwer, P.H.; Bronkhorst, E.M.; Jansen, J.A.; Walboomers, X.F.; Yang, F. A tunable and injectable local drug delivery system for personalized periodontal application. J. Control. Release 2020, 324, 134–145. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Ni, S.; Liu, Y.; Sun, H.; Lin, Q. Enhanced osteogenic healing process of rat tooth sockets using a novel simvastatin-loaded injectable microsphere-hydrogel system. J. Cranio-Maxillofac. Surg. 2019, 47, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Mantha, S.; Pillai, S.; Khayambashi, P.; Upadhyay, A.; Zhang, Y.; Tao, O.; Pham, H.M.; Tran, S.D. Smart hydrogels in tissue engineering and regenerative medicine. Materials 2019, 12, 3323. [Google Scholar] [CrossRef] [PubMed] [Green Version]














| Microparticles | Hydrogels | Bioactive Cargo | Observations | Ref. |
|---|---|---|---|---|
| PLGA | PLAF-b- PEG-b- PLA | Combretastatin A-4 and docetaxel | Sequential release of drugs. The synergistic system suppressed the spread of an osteosarcoma in in vivo tests. | [14] |
| PCL–PEG–PCL | PCL–PEG–PCL | Camptothecine | The composite system had a strong anti-tumour effect. The system suppressed growth and metastasis in in vivo tests. | [17] |
| Double walled microparticles with PLGA and PDLLA | Aldehyde functionalized sodium alginate | Cisplatin and paclitaxel | Beyond the use for chemotherapeutics the hydrogel of the system also served as an antibacterial agent, due to the presence of polyethyleneimine within the hydrogel. | [49] |
| PLGA | Pluronic and copolymer of PEG-PCL-PLLA | DOX and 5 FU | Microparticles carried DOX and the hydrogel carried 5 FU. Pluronic rapidly cleared whilst the copolymer endured. The spread of the drug to healthy tissues was not observed in vivo. | [52] |
| PCL–PEG–PCL | Hyaluronic acid | 5 FU, cisplatin and paclitaxel | Hydrogel loaded with 5Fu and cisplatin and microspheres with paclitaxel. Both evaluated separately. Good in vivo performance. | [54] |
| PCL | Alginate | DOX and ibuprofen | Early release of ibuprofen as an anti-inflammatory agent followed by DOX release for cytotoxic effect. | [55] |
| Vaterite microspheres | Silk nanofibers | DOX | Several combinations made. The system significantly decreased viability of MCF-7 cells in in vivo tests. | [56] |
| PLGA | PEG and PNIPAAm conjugates | Camptothecin and vincristine | Drugs loaded only on the microparticles. Vincristine had a better performance than camptothecin, with longer rates of survival. | [57] |
| Gelatine | Alginate and chitosan | 5 FU and iron oxide nanoparticles | Under an external magnetic field, the system released higher quantities of 5 FU for higher times but had no effect in the initial release. | [58] |
| Microparticles | Hydrogels | Bioactive Cargo | Observations | Ref. |
|---|---|---|---|---|
| Silk fibroin | Alginate | IGF-1 | After 28 days microspheres in hydrogel system with IGF-1 had the higher enhancement in cardiac function. | [21] |
| PLLA-PEG-PNIPAAm | PLLA-PEG-PNIPAAm | Stem cells | Copolymer assembled into microspheres. PNIPAAm branch served has physical links that formed a hydrogel in the presence of water. | [26] |
| Silk fibroin | Alginate | VEGF and BMP9 | Sequential release of two bioactive agents. VEGF was loaded to the hydrogel (for rapid release) and BMP9 to the microparticles (for prolonged release). | [42] |
| Albumin | Silk fibroin and PEGDA | Stem cells | In vitro 3D cell culture revealed proteins characteristic of early cardiac muscle cell differentiation. | [60] |
| PLGA | Poly (NIPAAm-co-HEMA-co-MAPLA) copolymer | IGF-1 and bFGF | In vivo presence of growth factors was high but no improvements to cardiac functions were noted. | [61] |
| Protein coated PLGA | Based-hyaluronic acid | Stem cells | Formation of functionalized mesenchymal stem cell aggregates for the delivery of bioactive factors with the protection of hyaluronic acid hydrogel. | [62] |
| Microparticles | Hydrogels | Bioactive Cargo | Observations | Ref. |
|---|---|---|---|---|
| PLGA with tannic acid | Oxidized dextran and hyaluronic acid-hydrazide | BDNF | Tannic acid increased electrical conductivity and mechanical properties. | [15] |
| Gelatine | Collagen-low molecular weight hyaluronic acid | TGF-b3 | The hydrogels supported growth and differentiation of MSC. Microparticles delivered TGF-b3 that promoted cell differentiation. | [32] |
| Gelatine | Hyaluronic acid and N-isopropylacrylamide | Epigallocatechin 3-gallate | Reduction of inflammatory response with efficient drug encapsulation with electrospraying. | [44] |
| PLGA | Alginate | Minocycline hydrochloride and paclitaxel | Co-delivery of drugs. MH regulated the inflammatory process and PTX promoted tissue regeneration and decreased scar tissue. | [63] |
| PLGA | Hyaluronic acid and methylcellulose | Brain-derived neurotrophic factor (BDNF) | The composite system had a more controlled release of BDNF compared to the hydrogel alone. | [64] |
| Microparticles | Hydrogels | Bioactive Cargo | Observations | Ref. |
|---|---|---|---|---|
| Chitosan | Oxidized alginate and carboxymethyl chitosan | Melatonin and methylprednisolone | Melatonin conjugated with chitosan. Tripolyphosphate was used to develop microspheres loaded with methylprednisolone. In vivo tests compared the effect of hydrogel alone and microparticles in hydrogel system. The latter had the best cartilage regeneration. | [66] |
| Alginate sulphate | Alginate and PVA | PRP and Rabbit adipose-derived mesenchymal stem cells | Sustained release of PRP to the hydrogel carrying stem cells had a significant gene expression compared with the hydrogel with free PRP. | [67] |
| PLGA | Gelatine methacryloyl | Kartogenin and curcumin | Microparticles loaded with kartogenin were incorporated within MSC aggregates to promote chondrocyte differentiation and the hydrogel served has a scaffold with curcumin to moderate inflammatory response. | [68] |
| PLGA | Chitosan and gelatine | Stromal cell-derived factor-1 | Injectable hydrogel for recovery of osteochondral tissue regeneration. The microparticles in hydrogel system had a prolonged release profile and promoted the cartilage regeneration. | [69] |
| PCL-PEG-PCL | Alginate and PVA | Calcium gluconate | Calcium gluconate served as an in situ cross-linking solution for the alginate hydrogel. | [70] |
| Microparticles | Hydrogels | Bioactive Cargo | Observations | Ref. |
|---|---|---|---|---|
| Hydroxyapatite | Alginate | Strontium | Strontium presence increased the bone formation. Only local delivery occurred. | [40] |
| Bioglass | PVA | BMP2 and VEGF | Increase in mechanical properties. In vivo tests revealed that the microparticles in hydrogel system is more efficient than bulk hydrogel to treat bone defects. | [71] |
| Chitosan | Methyl cellulose and alginate | BMP2 and VEGF | VEGF was loaded to the hydrogel and BMP within the particles. VEGF had a faster release when compared to BMP2. | [72] |
| PLGA | Alginate | BMP2 and VEGF | Injectable composite system for sequential delivery of bioactive factors for vascularization and bone regeneration. In vivo, the system induced vascularization and ectopic bone formation. | [73] |
| PLGA and hydroxyapatite | Chitosan | BMP2 and VEGF | After 12 weeks, the delivery of BMP2 and the co delivery of both agents using the composite system promoted a complete healing of the bone defect. | [74] |
| Biphasic calcium phosphate | Hyaluronic acid and chitosan | Adipose-derived stem cells and platelet rich plasma | Development of a thermoresponsive microparticles in hydrogel system. Improvement of cell proliferation and mineralization of extracellular matrix. | [75] |
| Alginate and chitosan/PLA/alginate | Chitosan and glycerophosphate | BMP2 and platelet-derived growth factor-BB | Sequential release with encapsulation in different microparticles. Release patterns regulated by the number of microparticles embedded in the hydrogel and the initial drug load in microparticles. | [76] |
| PLGA and PLA | Chitosan and collagen | BMP2 and 17β-estradiol | Presence of hydroxyapatite diminished burst effect on PLGA microspheres with BMP2. Results suggested that 17β-estradiol had no effect on bone regeneration. | [77] |
| PLGA and PLA-S | Poloxamine (T-1307) and alginate | BMP2, platelet rich growth factors and 17β-oestradiol | Alginate increased viscosity and diminished the gelation temperature of poloxamine. Microparticles increased viscosity. Drug release had an early fast release followed by a prolonged slow released to up 6 weeks. | [78] |
| CaCO3 | Fibrin-glue | BMP2 | Within 8 weeks in vivo in tibia bone defects of rabbits, the composite system had nearly healed the defects. | [79] |
| Gelatine | Synthetical polymers | Mesenchymal stem cells | Gelatine microparticles served as porogens for the hydrogel to promote cell attachment and proliferation. | [80] |
| Calcium carbonate microparticles and hydroxyapatite nanoparticles | Chitosan and alginate | Tetracycline hydrochloride | Hydroxyapatite and microparticles ratio affected mechanical properties. Sustained release and antibacterial properties were observed. | [81] |
| Microparticles | Hydrogels | Bioactive Cargo | Observations | Ref. |
|---|---|---|---|---|
| PLGA and alginate | Alginate and bioglass | Condition medium of RAW 246.7 cells and pirfenidone | Sequential release that accompanies the different stages of wound healing, with specific agents for each one. | [41] |
| Alginate | Oxidized alginate and N-succinyl chitosan | Bull Serum Albumin | Higher compressive modulus and slower release profiles compared with the hydrogel and microparticles alone. | [82] |
| PLGA | Aminated gelatine, adipic acid dihydrazyde and oxidized dextran | Chlorhexidine acetate and basic fibroblast growth factor (bFGF) | Self-healing hydrogel with antibacterial properties and sequential delivery. Chlorhexidine acetate would serve as an antibacterial agent for an early release, followed by a latter release of bFGF for cell proliferation. | [83] |
| PLGA | Chitosan | Platelet-derived growth factor receptor | Promotion of granulation formation and collagen deposition with the microparticles in hydrogel system. | [84] |
| Hydroxyapatite | Hyaluronic acid | None | Application for rejuvenation of aged skin. Viscosity and storage modulus increased with hydroxyapatite microparticles presence. | [85] |
| PDLLA | Collagen | Fibroblasts | Porous PDLLA microspheres served as scaffolds for cell adhesion | [86] |
| Treatment Objective | Microparticles | Hydrogels | Bioactive Cargo | Observations | Ref. |
|---|---|---|---|---|---|
| Diabetes | PLGA-based | Dopamine-conjugated hyaluronic acid | Insulin | Glucose-regulated insulin delivery system. With a single administration, stabilized glucose’s level for 2 weeks in vivo. | [22] |
| Post-operative ocular system | PLA | Triblock polymers | Fluoroquinolone moxifloxacin, dexamethasone, and beta-blocker levo-bunolol | Different triblock polymers were studied. Hydrophobicity used to regulate the delivery profile and to delay the initial burst. | [31] |
| Macular degeneration | PLGA | PNIPAAm | Anti-VEGF | Prolonged drug delivery. In vitro release prolonged for about 200 days. | [39] |
| Macular degeneration | PLGA | PNIPAAm | Anti-VEGF | The microparticles in hydrogel system decreased the ocular lesion areas in vivo. | [87] |
| Periodontitis | PLGA | Polyisocyanopeptide | Doxycycline and lipoxin | Ester capped PLGA microparticles had a longer release profile than the acid terminated ones | [88] |
| Periodontitis | PLGA | Gelatine | Simvastatin | Controlled release of simvastatin for tooth extraction. The microparticles in hydrogel system promoted bone formation after 5 weeks. | [89] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrêlo, H.; Soares, P.I.P.; Borges, J.P.; Cidade, M.T. Injectable Composite Systems Based on Microparticles in Hydrogels for Bioactive Cargo Controlled Delivery. Gels 2021, 7, 147. https://doi.org/10.3390/gels7030147
Carrêlo H, Soares PIP, Borges JP, Cidade MT. Injectable Composite Systems Based on Microparticles in Hydrogels for Bioactive Cargo Controlled Delivery. Gels. 2021; 7(3):147. https://doi.org/10.3390/gels7030147
Chicago/Turabian StyleCarrêlo, Henrique, Paula I. P. Soares, João Paulo Borges, and Maria Teresa Cidade. 2021. "Injectable Composite Systems Based on Microparticles in Hydrogels for Bioactive Cargo Controlled Delivery" Gels 7, no. 3: 147. https://doi.org/10.3390/gels7030147
APA StyleCarrêlo, H., Soares, P. I. P., Borges, J. P., & Cidade, M. T. (2021). Injectable Composite Systems Based on Microparticles in Hydrogels for Bioactive Cargo Controlled Delivery. Gels, 7(3), 147. https://doi.org/10.3390/gels7030147