Ovarian Cell Encapsulation in an Enzymatically Crosslinked Silk-Based Hydrogel with Tunable Mechanical Properties
Abstract
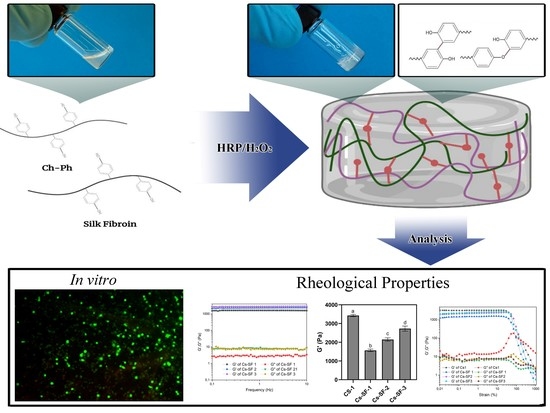
:1. Introduction
2. Results and Discussion
2.1. Cs-Ph Synthesis
2.2. Hydrogel Formation and Gelation Time
2.3. Rheological Properties
2.4. Microstructure, Water Uptake, and Degradation
2.5. Ovarian Cell Encapsulation and Viability
3. Conclusions
4. Materials and Methods
4.1. Materials and Reagents
4.2. Silk Fibroin Extraction
4.3. Chitosan–Phenol Conjugation
4.4. Hydrogel Formation
4.5. Physiochemical Characterization
4.6. Rheological Properties
4.7. Cell Isolation and Culture
4.8. 3D Cell Encapsulation
4.9. Mitochondrial Activity
4.10. Cell Viability (LIVE/DEAD)
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Donnez, J.; Martinez-Madrid, B.; Jadoul, P.; Van Langendonckt, A.; Demylle, D.; Dolmans, M.-M. Ovarian tissue cryopreservation and transplantation: A review. Hum. Reprod. Update 2006, 12, 519–535. [Google Scholar] [CrossRef]
- Amorim, C.A.; Shikanov, A. The artificial ovary: Current status and future perspectives. Future Oncol. 2016, 12, 2323–2332. [Google Scholar] [CrossRef]
- Amorim, C.A. Artificial ovary. In Gonadal Tissue Cryopreservation in Fertility Preservation; Springer: Berlin/Heidelberg, Germany, 2016; pp. 175–192. [Google Scholar]
- Abdi, S.; Salehnia, M.; Hosseinkhani, S. Quality of oocytes derived from vitrified ovarian follicles cultured in two-and three-dimensional culture system in the presence and absence of kit ligand. Biopreserv. Biobank. 2016, 14, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Chiti, M.C.; Dolmans, M.-M.; Donnez, J.; Amorim, C. Fibrin in reproductive tissue engineering: A review on its application as a biomaterial for fertility preservation. Ann. Biomed. Eng. 2017, 45, 1650–1663. [Google Scholar] [CrossRef]
- Wood, C.D.; Vijayvergia, M.; Miller, F.H.; Carroll, T.; Fasanati, C.; Shea, L.D.; Brinson, L.C.; Woodruff, T.K. Multi-modal magnetic resonance elastography for noninvasive assessment of ovarian tissue rigidity in vivo. Acta Biomater. 2015, 13, 295–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, R.M.; Woodruff, T.K.; Shea, L.D. Designing follicle–environment interactions with biomaterials. Oncofertility 2010, 156, 11–24. [Google Scholar]
- Biswal, T. Biopolymers for tissue engineering applications: A review. Mater. Today Proc. 2020, 41, 397–402. [Google Scholar] [CrossRef]
- Jafari, H.; Bernaerts, K.V.; Dodi, G.; Shavandi, A. Chitooligosaccharides for wound healing biomaterials engineering. Mater. Sci. Eng. C 2020, 117, 111266. [Google Scholar] [CrossRef]
- Cui, C.; Shao, C.; Meng, L.; Yang, J. High-strength, self-adhesive, and strain-sensitive chitosan/poly (acrylic acid) double-network nanocomposite hydrogels fabricated by salt-soaking strategy for flexible sensors. ACS Appl. Mater. Interfaces 2019, 11, 39228–39237. [Google Scholar] [CrossRef]
- Thi, T.T.H.; Lee, Y.; Le Thi, P.; Park, K.D. Engineered horseradish peroxidase-catalyzed hydrogels with high tissue adhesiveness for biomedical applications. J. Ind. Eng. Chem. 2019, 78, 34–52. [Google Scholar]
- Li, Y.; Sun, S.; Gao, P.; Zhang, M.; Fan, C.; Lua, Q.; Lia, C.; Chen, C.; Lin, B.; Jiang, Y. A tough chitosan-alginate porous hydrogel prepared by simple foaming method. J. Solid State Chem. 2021, 294, 121797. [Google Scholar] [CrossRef]
- Arash, M.E.; Goodarzi, A.; Khanmohammadi, M.; Shokati, A.; Mohandesnezhad, S.; Ataollahi, M.R.; Najafipour, S.; Farahani, M.S.; Ai, J. Chitosan/gelatin hydrogel and endometrial stem cells with subsequent atorvastatin injection impact in regenerating spinal cord tissue. J. Drug Deliv. Sci. Technol. 2020, 58, 101831. [Google Scholar]
- Hasturk, O.; Jordan, K.E.; Choi, J.; Kaplan, D.L. Enzymatically crosslinked silk and silk-gelatin hydrogels with tunable gelation kinetics, mechanical properties and bioactivity for cell culture and encapsulation. Biomaterials 2020, 232, 119720. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, O.; Cooper-White, J.; Janmey, P.A.; Mooney, D.J.; Shenoy, V.B. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 2020, 584, 535–546. [Google Scholar] [CrossRef]
- Jin, R.; Teixeira, L.M.; Dijkstra, P.J.; Karperien, M.; Van Blitterswijk, C.; Zhong, Z.; Feijen, J. Injectable chitosan-based hydrogels for cartilage tissue engineering. Biomaterials 2009, 30, 2544–2551. [Google Scholar] [CrossRef]
- Jin, R.; Lin, C.; Cao, A. Enzyme-mediated fast injectable hydrogels based on chitosan–glycolic acid/tyrosine: Preparation, characterization, and chondrocyte culture. Polym. Chem. 2014, 5, 391–398. [Google Scholar] [CrossRef]
- Gohil, S.V.; Brittain, S.B.; Kan, H.-M.; Drissi, H.; Rowe, D.W.; Nair, L.S. Evaluation of enzymatically crosslinked injectable glycol chitosan hydrogel. J. Mater. Chem. B 2015, 3, 5511–5522. [Google Scholar] [CrossRef]
- Sakai, S.; Nakahata, M. Horseradish peroxidase catalyzed hydrogelation for biomedical, biopharmaceutical, and biofabrication applications. Chem. An. Asian J. 2017, 12, 3098–3109. [Google Scholar] [CrossRef]
- Hu, J.; Quan, Y.; Lai, Y.; Zheng, Z.; Hu, Z.; Wang, X.; Dai, T.; Zhang, Q.; Cheng, Y. A smart aminoglycoside hydrogel with tunable gel degradation, on-demand drug release, and high antibacterial activity. J. Control. Release 2017, 247, 145–152. [Google Scholar] [CrossRef]
- Kuang, L.; Damayanti, N.P.; Jiang, C.; Fei, X.; Liu, W.; Narayanan, N.; Irudayaraj, J.; Campanella, O.; Deng, M. Bioinspired glycosaminoglycan hydrogels via click chemistry for 3D dynamic cell encapsulation. J. Appl. Polym. Sci. 2019, 136, 47212. [Google Scholar] [CrossRef]
- Raia, N.R.; Partlow, B.P.; McGill, M.; Kimmerling, E.P.; Ghezzi, C.E.; Kaplan, D.L. Enzymatically crosslinked silk-hyaluronic acid hydrogels. Biomaterials 2017, 131, 58–67. [Google Scholar] [CrossRef]
- Liu, J.; Yang, B.; Li, M.; Li, J.; Wan, Y. Enhanced dual network hydrogels consisting of thiolated chitosan and silk fibroin for cartilage tissue engineering. Carbohydr. Polym. 2020, 227, 115335. [Google Scholar] [CrossRef]
- Bi, B.; Liu, H.; Kang, W.; Zhuo, R.; Jiang, X. An injectable enzymatically crosslinked tyramine-modified carboxymethyl chitin hydrogel for biomedical applications. Colloids Surf. B Biointerfaces 2019, 175, 614–624. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.-H.; Yu, Q.-Z.; Mo, X.-M. Fabrication and intermolecular interactions of silk fibroin/hydroxybutyl chitosan blended nanofibers. Int. J. Mol. Sci. 2011, 12, 2187–2199. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Tang, E.; Zhao, H. An environmentally sensitive silk fibroin/chitosan hydrogel and its drug release behaviors. Polymers 2019, 11, 1980. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Yu, S.; Liu, B.; Ni, Y.; Yu, C.; Su, Y.; Zhu, X.; Yu, X.; Zhou, Y.; Yan, D. An injectable enzymatically crosslinked carboxymethylated pullulan/chondroitin sulfate hydrogel for cartilage tissue engineering. Sci. Rep. 2016, 6, 20014. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xie, J.; Gao, H.; Cao, Y.; Chen, M.; Liu, Y.; Zeng, B.; Chang, F.-C.; Dai, L. Interpenetration enhancing of Chitosan-PEGLM double network (DN) hydrogel and its properties. Macromol. Res. 2015, 23, 2–12. [Google Scholar] [CrossRef]
- McGill, M.; Coburn, J.M.; Partlow, B.P.; Mu, X.; Kaplan, D.L. Molecular and macro-scale analysis of enzyme-crosslinked silk hydrogels for rational biomaterial design. Acta Biomater. 2017, 63, 76–84. [Google Scholar] [CrossRef]
- Li, T.; Song, X.; Weng, C.; Wang, X.; Gu, L.; Gong, X.; Wei, Q.; Duan, X.; Yang, L.; Chen, C. Silk fibroin/carboxymethyl chitosan hydrogel with tunable biomechanical properties has application potential as cartilage scaffold. Int. J. Biol. Macromol. 2019, 137, 382–391. [Google Scholar] [CrossRef]
- Shao, J.; Ding, Z.; Li, L.; Chen, Y.; Zhu, J.; Qian, Q. Improved accumulation of TGF-β by photopolymerized chitosan/silk protein bio-hydrogel matrix to improve differentiations of mesenchymal stem cells in articular cartilage tissue regeneration. J. Photochem. Photobiol. B Biol. 2020, 203, 111744. [Google Scholar] [CrossRef]
- Symes, A.; Shavandi, A.; Zhang, H.; Ahmed, I.A.M.; Al-Juhaimi, F.Y.; Bekhit, A.E.-D.A. Antioxidant activities and Caffeic acid content in New Zealand Asparagus (Asparagus officinalis) roots extracts. Antioxidants 2018, 7, 52. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Allardyce, B.J.; Rajkhowa, R.; Kalita, S.; Dilley, R.J.; Wang, X.; Liu, X. Silk particles, microfibres and nanofibres: A comparative study of their functions in 3D printing hydrogel scaffolds. Mater. Sci. Eng. C 2019, 103, 109784. [Google Scholar] [CrossRef]
- Raia, N.R.; Jia, D.; Ghezzi, C.E.; Muthukumar, M.; Kaplan, D.L. Characterization of silk-hyaluronic acid composite hydrogels towards vitreous humor substitutes. Biomaterials 2020, 233, 119729. [Google Scholar] [CrossRef]
- Gasik, M.; Gantar, A.; Novak, S. Viscoelastic behaviour of hydrogel-based composites for tissue engineering under mechanical load. Biomed. Mater. 2017, 12, 025004. [Google Scholar]
- Jafari, H.; Shahrousvand, M.; Kaffashi, B. Preparation and characterization of reinforced poly (ε-caprolactone) nanocomposites by cellulose nanowhiskers. Polym. Compos. 2020, 41, 624–632. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Liu, Y.; Li, D.; Zhang, L.; Wang, Q.; Xiao, Y.; Zhang, X. Influence of hydrogel network microstructures on mesenchymal stem cell chondrogenesis in vitro and in vivo. Acta Biomater. 2019, 91, 159–172. [Google Scholar] [CrossRef]
- Lee, B.-H.; Li, B.; Guelcher, S.A. Gel microstructure regulates proliferation and differentiation of MC3T3-E1 cells encapsulated in alginate beads. Acta Biomater. 2012, 8, 1693–1702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chi, C.; Li, X.; Zhang, Y.; Miao, S.; Chen, L.; Li, L.; Liang, Y. Understanding the effect of freeze-drying on microstructures of starch hydrogels. Food Hydrocoll. 2020, 101, 105509. [Google Scholar] [CrossRef]
- Yan, J.; Jia, X.; Yan, W.; Yin, L. Double-Network Hydrogels of Corn Fiber Gum and Soy Protein Isolate: Effect of Biopolymer Constituents and pH Values on Textural Properties and Microstructures. Foods 2021, 10, 356. [Google Scholar] [CrossRef]
- Ounkaew, A.; Kasemsiri, P.; Jetsrisuparb, K.; Uyama, H.; Hsu, Y.-I.; Boonmars, T.; Artchayasawat, A.; Knijnenburg, J.T.; Chindaprasirt, P. Synthesis of nanocomposite hydrogel based carboxymethyl starch/polyvinyl alcohol/nanosilver for biomedical materials. Carbohydr. Polym. 2020, 248, 116767. [Google Scholar] [CrossRef]
- Ahsan, A.; Farooq, M.A.; Parveen, A. Thermosensitive Chitosan-Based Injectable Hydrogel as an Efficient Anticancer Drug Carrier. ACS Omega 2020, 5, 20450–20460. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Song, H.; Gong, Y.; Mao, Z.; Gao, C.; Shen, J. Covalently crosslinked chitosan hydrogel: Properties of in vitro degradation and chondrocyte encapsulation. Acta Biomater. 2007, 3, 23–31. [Google Scholar] [CrossRef]
- Wang, L.-S.; Chung, J.E.; Chan, P.P.-Y.; Kurisawa, M. Injectable biodegradable hydrogels with tunable mechanical properties for the stimulation of neurogenesic differentiation of human mesenchymal stem cells in 3D culture. Biomaterials 2010, 31, 1148–1157. [Google Scholar] [CrossRef]
- Banerjee, A.; Arha, M.; Choudhary, S.; Ashton, R.S.; Bhatia, S.R.; Schaffer, D.V.; Kane, R.S. The influence of hydrogel modulus on the proliferation and differentiation of encapsulated neural stem cells. Biomaterials 2009, 30, 4695–4699. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, E.H.; Zanotelli, M.R.; Schwartz, M.P.; Murphy, W.L. Differential effects of cell adhesion, modulus and VEGFR-2 inhibition on capillary network formation in synthetic hydrogel arrays. Biomaterials 2014, 35, 2149–2161. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; Wu, B.; Li, S.; Song, J.; Song, B.; Lu, W.F. Shear stress analysis and its effects on cell viability and cell proliferation in drop-on-demand bioprinting. Biomed. Phys. Eng. Express 2018, 4, 045028. [Google Scholar] [CrossRef]
- Zeng, S.; Liu, L.; Shi, Y.; Qiu, J.; Fang, W.; Rong, M.; Guo, Z.; Gao, W. Characterization of silk fibroin/chitosan 3D porous scaffold and in vitro cytology. PLoS ONE 2015, 10, e0128658. [Google Scholar] [CrossRef] [Green Version]
- McGill, M.; Grant, J.M.; Kaplan, D.L. Enzyme-mediated conjugation of peptides to silk fibroin for facile hydrogel functionalization. Ann. Biomed. Eng. 2020, 48, 1905–1915. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Park, J.P.; Koh, M.-Y.; Kim, P.; Lee, J.; Shin, M.; Lee, H. Chitosan-catechol: A writable bioink under serum culture media. Biomater. Sci. 2018, 6, 1040–1047. [Google Scholar] [CrossRef]
- Tran, D.L.; Le Thi, P.; Thi, T.T.H.; Park, K.D. Novel enzymatically crosslinked chitosan hydrogels with free-radical-scavenging property and promoted cellular behaviors under hyperglycemia. Prog. Nat. Sci. Mater. Int. 2020, 30, 661–668. [Google Scholar] [CrossRef]
- Jafari, H.; Shahrousvand, M.; Kaffashi, B. Reinforced poly (ε-caprolactone) bimodal foams via phospho-calcified cellulose nanowhisker for osteogenic differentiation of human mesenchymal stem cells. ACS Biomater. Sci. Eng. 2018, 4, 2484–2493. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, M.; Paulini, F.; Corral, A.; Balcerzyk, M.; Lucci, C.M.; Ambroise, J.; Merola, M.; Fernandez-Maza, L.; Risco, R.; Dolmans, M.-M. Evaluation of a new freezing protocol containing 20% dimethyl sulphoxide concentration to cryopreserve human ovarian tissue. Reprod. Biomed. Online 2018, 37, 653–665. [Google Scholar] [CrossRef] [PubMed]
- Asiabi, P.; Dolmans, M.-M.; Ambroise, J.; Camboni, A.; Amorim, C. In vitro differentiation of theca cells from ovarian cells isolated from postmenopausal women. Hum. Reprod. 2020, 35, 2793–2807. [Google Scholar] [CrossRef] [PubMed]
- Amorim, C.A.; Van Langendonckt, A.; David, A.; Dolmans, M.-M.; Donnez, J. Survival of human pre-antral follicles after cryopreservation of ovarian tissue, follicular isolation and in vitro culture in a calcium alginate matrix. Hum. Reprod. 2009, 24, 92–99. [Google Scholar] [CrossRef] [Green Version]
- Luyckx, V.; Dolmans, M.M.; Vanacker, J.; Legat, C.; Fortuño Moya, C.; Donnez, J.; Amorim, C.A. A new step toward the artificial ovary: Survival and proliferation of isolated murine follicles after autologous transplantation in a fibrin scaffold. Fertil. Steril. 2014, 101, 1149–1156. [Google Scholar] [CrossRef]
- Gu, Q.; Tomaskovic-Crook, E.; Wallace, G.G.; Crook, J.M. 3D bioprinting human induced pluripotent stem cell constructs for in situ cell proliferation and successive multilineage differentiation. Adv. Healthc. Mater. 2017, 6, 1700175. [Google Scholar] [CrossRef] [Green Version]




| Sample Name | CS-Ph Concentration (wt%) | SF Concentration (wt%) | Gelation Time |
|---|---|---|---|
| CS-0.5 | 0.5 | 0 | 56 ± 4 s |
| CS-1 | 1 | 0 | 35 ± 4 s |
| CS-1.5 | 1.5 | 0 | 22 ± 2 s |
| CS-2 | 2 | 0 | 10 ± 2 s |
| SF-1 | 0 | 1 | 188 ± 13 min |
| SF-1.75 | 0 | 1.75 | 153 ± 10 min |
| SF-2.5 | 0 | 2.5 | 117 ± 14 min |
| SF-4 | 0 | 4 | 77 ± 6 min |
| Sample Name | Mass Ratio of Cs to SF | Cs-Ph Concentration (wt%) | SF Concentration (wt%) | Gelation Time (s) |
|---|---|---|---|---|
| Cs-SF-1 | 1:1 | 1 | 1 | 99 ± 5 |
| Cs-SF-2 | 0.57:1 | 1 | 1.75 | 93 ± 5 |
| Cs-SF-3 | 0.4:1 | 1 | 2.5 | 89 ± 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jafari, H.; Dadashzadeh, A.; Moghassemi, S.; Zahedi, P.; Amorim, C.A.; Shavandi, A. Ovarian Cell Encapsulation in an Enzymatically Crosslinked Silk-Based Hydrogel with Tunable Mechanical Properties. Gels 2021, 7, 138. https://doi.org/10.3390/gels7030138
Jafari H, Dadashzadeh A, Moghassemi S, Zahedi P, Amorim CA, Shavandi A. Ovarian Cell Encapsulation in an Enzymatically Crosslinked Silk-Based Hydrogel with Tunable Mechanical Properties. Gels. 2021; 7(3):138. https://doi.org/10.3390/gels7030138
Chicago/Turabian StyleJafari, Hafez, Arezoo Dadashzadeh, Saeid Moghassemi, Payam Zahedi, Christiani A. Amorim, and Amin Shavandi. 2021. "Ovarian Cell Encapsulation in an Enzymatically Crosslinked Silk-Based Hydrogel with Tunable Mechanical Properties" Gels 7, no. 3: 138. https://doi.org/10.3390/gels7030138
APA StyleJafari, H., Dadashzadeh, A., Moghassemi, S., Zahedi, P., Amorim, C. A., & Shavandi, A. (2021). Ovarian Cell Encapsulation in an Enzymatically Crosslinked Silk-Based Hydrogel with Tunable Mechanical Properties. Gels, 7(3), 138. https://doi.org/10.3390/gels7030138