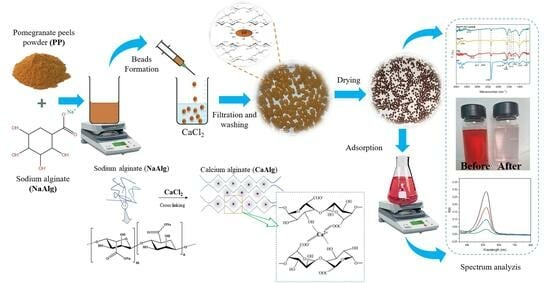
Adsorption of Safranin O Dye by Alginate/Pomegranate Peels Beads: Kinetic, Isotherm and Thermodynamic Studies
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization
2.1.1. Morphology, Size and Water Content of Beads
2.1.2. Swelling Ratio
2.1.3. FTIR Analysis
2.1.4. TGA Analysis
2.2. Adsorption Tests
2.2.1. Effect of Contact Time and Initial Dye Concentration
2.2.2. Effect of Initial pH
2.2.3. Effect of Adsorbent Dosage
2.3. Adsorption Kinetics
2.4. Adsorption Isotherm
2.5. Thermodynamic Study
2.6. Diffusion Coefficients Evaluation
2.7. Mass Transfer Analysis
2.8. Bangham’s and Burt model
2.9. Regeneration Study
2.10. Adsorption Mechanism
3. Conclusions
4. Materials and Methods
4.1. Reagents and Materials
4.2. Preparation of Pomegranate Peels Powder
4.3. Preparation of Beads
4.4. Characterization Methods
4.5. Batch Adsorption Experiments
4.6. Reusability Tests
4.7. Error Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, C.; Liu, H.; Xiong, T.; Xu, A.; Pan, B.; Tang, K. Graphene Oxide Reinforced Alginate/PVA Double Network Hydrogels for Efficient Dye Removal. Polymers 2018, 10, 835. [Google Scholar] [CrossRef]
- Rivera-Arenas, D.; Macías-Quiroga, I.F.; Dávila-Arias, M.T.; Vélez-Upegui, J.J.; Sanabria-González, N.R. Adsorption of Acid Yellow 23 Dye on Organobentonite/Alginate Hydrogel Beads in a Fixed-Bed Column. J. Compos. Sci. 2023, 7, 362. [Google Scholar] [CrossRef]
- Kajekar, A.J.; Dodamani, B.M.; Isloor, A.M.; Karim, Z.A.; Cheer, N.B.; Ismail, A.F.; Shilton, S.J. Preparation and Characterization of Novel PSf/PVP/PANI-Nanofiber Nanocomposite Hollow Fiber Ultrafiltration Membranes and Their Possible Applications for Hazardous Dye Rejection. Desalination 2015, 365, 117–125. [Google Scholar] [CrossRef]
- Lachheb, H.; Puzenat, E.; Houas, A.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.-M. Photocatalytic Degradation of Various Types of Dyes (Alizarin S, Crocein Orange G, Methyl Red, Congo Red, Methylene Blue) in Water by UV-Irradiated Titania. Appl. Catal. B 2002, 39, 75–90. [Google Scholar] [CrossRef]
- Moawed, E.A.; Abulkibash, A.B. Selective Separation of Light Green and Safranin O from Aqueous Solution Using Salvadora Persica (Miswak) Powder as a New Biosorbent. J. Saudi Chem. Soc. 2016, 20, S178–S185. [Google Scholar] [CrossRef]
- Asfaram, A.; Ghaedi, M.; Goudarzi, A.; Rajabi, M. Response Surface Methodology Approach for Optimization of Simultaneous Dye and Metal Ion Ultrasound-Assisted Adsorption onto Mn Doped Fe3O4-NPs Loaded on AC: Kinetic and Isothermal Studies. Dalton Trans. 2015, 44, 14707–14723. [Google Scholar] [CrossRef]
- Fayazi, M.; Afzali, D.; Taher, M.A.; Mostafavi, A.; Gupta, V.K. Removal of Safranin Dye from Aqueous Solution Using Magnetic Mesoporous Clay: Optimization Study. J. Mol. Liq. 2015, 212, 675–685. [Google Scholar] [CrossRef]
- Shao, L.; Cheng, X.Q.; Liu, Y.; Quan, S.; Ma, J.; Zhao, S.Z.; Wang, K.Y. Newly Developed Nanofiltration (NF) Composite Membranes by Interfacial Polymerization for Safranin O and Aniline Blue Removal. J. Memb. Sci. 2013, 430, 96–105. [Google Scholar] [CrossRef]
- Sharma, G.; Gupta, V.K.; Agarwal, S.; Kumar, A.; Thakur, S.; Pathania, D. Fabrication and Characterization of Fe@ MoPO Nanoparticles: Ion Exchange Behavior and Photocatalytic Activity against Malachite Green. J. Mol. Liq. 2016, 219, 1137–1143. [Google Scholar] [CrossRef]
- Ayat, A.; Arris, S.; Abbaz, A.; Aissaoui, H. The Removal of Methylene Blue and COD by Coagulation Using Cactus. Alger. J. Eng. Res. 2022, 5, 1–6. [Google Scholar]
- Bayat, M.; Javanbakht, V.; Esmaili, J. Synthesis of Zeolite/Nickel Ferrite/Sodium Alginate Bionanocomposite via a Co-Precipitation Technique for Efficient Removal of Water-Soluble Methylene Blue Dye. Int. J. Biol. Macromol. 2018, 116, 607–619. [Google Scholar] [CrossRef]
- Hashim, K.S.; Al-Saati, N.H.; Alquzweeni, S.S.; Zubaidi, S.L.; Kot, P.; Kraidi, L.; Hussein, A.H.; Alkhaddar, R.; Shaw, A.; Alwash, R. Decolourization of Dye Solutions by Electrocoagulation: An Investigation of the Effect of Operational Parameters. In Proceedings of the IOP Conference Series: Materials Science and Engineering; IOP Publishing: Najaf, Iraq, 2019; Volume 584, p. 012024. [Google Scholar]
- Nasrullah, A.; Bhat, A.H.; Naeem, A.; Isa, M.H.; Danish, M. High Surface Area Mesoporous Activated Carbon-Alginate Beads for Efficient Removal of Methylene Blue. Int. J. Biol. Macromol. 2018, 107, 1792–1799. [Google Scholar] [CrossRef]
- Sun, L.; Chen, D.; Wan, S.; Yu, Z. Performance, Kinetics, and Equilibrium of Methylene Blue Adsorption on Biochar Derived from Eucalyptus Saw Dust Modified with Citric, Tartaric, and Acetic Acids. Bioresour. Technol. 2015, 198, 300–308. [Google Scholar] [CrossRef]
- Chikri, R.; Elhadiri, N.; Benchanaa, M.; Maguana, Y. Efficiency of Sawdust as Low-Cost Adsorbent for Dyes Removal. J. Chem. 2020, 2020, 8813420. [Google Scholar] [CrossRef]
- Ayat, A.; Arris, S.; Abbaz, A.; Bencheikh-Lehocine, M.; Meniai, A.H. Application of Response Surface Methodology for Modeling and Optimization of a Bio Coagulation Process (Sewage Wastewater Treatment Plant). Environ. Manag. 2021, 67, 489–497. [Google Scholar] [CrossRef]
- Viscusi, G.; Lamberti, E.; Gorrasi, G. Design of Sodium Alginate/Soybean Extract Beads Loaded with Hemp Hurd and Halloysite as Novel and Sustainable Systems for Methylene Blue Adsorption. Polym. Eng. Sci. 2021, 62, 129–144. [Google Scholar] [CrossRef]
- Viscusi, G.; Lamberti, E.; Gorrasi, G. Design of a Hybrid Bio-Adsorbent Based on Sodium Alginate/Halloysite/Hemp Hurd for Methylene Blue Dye Removal: Kinetic Studies and Mathematical Modeling. Colloids Surf. A Physicochem. Eng. Asp. 2022, 633, 127925. [Google Scholar] [CrossRef]
- Ali, R.M.; Hamad, H.A.; Hussein, M.M.; Malash, G.F. Potential of Using Green Adsorbent of Heavy Metal Removal from Aqueous Solutions: Adsorption Kinetics, Isotherm, Thermodynamic, Mechanism and Economic Analysis. Ecol. Eng. 2016, 91, 317–332. [Google Scholar] [CrossRef]
- Fathy, N.A.; El-Shafey, O.I.; Khalil, L.B. Effectiveness of Alkali-Acid Treatment in Enhancement the Adsorption Capacity for Rice Straw: The Removal of Methylene Blue Dye. ISRN Phys. Chem. 2013, 2013, 208087. [Google Scholar] [CrossRef]
- Arris, S.; Abbaz, I.; Kassir, M.; Abbaz, A.; Ayat, A.; Bencheikh Lehocine, M. Optimization of Phenol Sorption in Aqueous Solution by Date Stone through Response Surface Methodology. Alger. J. Eng. Res. 2022, 5, 28–34. [Google Scholar]
- Tang, Y.; Lin, T.; Jiang, C.; Zhao, Y.; Ai, S. Renewable Adsorbents from Carboxylate-Modified Agro-Forestry Residues for Efficient Removal of Methylene Blue Dye. J. Phys. Chem. Solids 2021, 149, 109811. [Google Scholar] [CrossRef]
- Viscusi, G.; Lamberti, E.; Gorrasi, G. Hemp Fibers Modified with Graphite Oxide as Green and Efficient Solution for Water Remediation: Application to Methylene Blue. Chemosphere 2021, 288, 132614. [Google Scholar] [CrossRef]
- Chaukura, N.; Murimba, E.C.; Gwenzi, W. Synthesis, Characterisation and Methyl Orange Adsorption Capacity of Ferric Oxide–Biochar Nano-Composites Derived from Pulp and Paper Sludge. Appl. Water Sci. 2017, 7, 2175–2186. [Google Scholar] [CrossRef]
- Deng, H.; Lu, J.; Li, G.; Zhang, G.; Wang, X. Adsorption of Methylene Blue on Adsorbent Materials Produced from Cotton Stalk. Chem. Eng. J. 2011, 172, 326–334. [Google Scholar] [CrossRef]
- Yusufa, M.; Elfghi, F.M.; Mallak, S.K. Kinetic Studies of Safranin-O Removal from Aqueous Solutions Using Pineapple Peels. Iran. J. Energy Environ. 2015, 6, 2079–2115. [Google Scholar] [CrossRef]
- Elsherif, K.M.; El-Dali, A.; Ewlad-Ahmed, A.M.; Treban, A.A.; Alqadhi, H.; Alkarewi, S. Kinetics and Isotherms Studies of Safranin Adsorption onto Two Surfaces Prepared from Orange Peels. Moroc. J. Chem. 2022, 10, 639–651. [Google Scholar] [CrossRef]
- Januário, E.F.D.; Vidovix, T.B.; de Araújo, L.A.; Bergamasco Beltran, L.; Bergamasco, R.; Vieira, A.M.S. Investigation of Citrus Reticulata Peels as an Efficient and Low-Cost Adsorbent for the Removal of Safranin Orange Dye. Environ. Technol. 2022, 43, 4315–4329. [Google Scholar] [CrossRef]
- Ghosh, I.; Kar, S.; Chatterjee, T.; Bar, N.; Das, S.K. Adsorptive Removal of Safranin-O Dye from Aqueous Medium Using Coconut Coir and Its Acid-Treated Forms: Adsorption Study, Scale-up Design, MPR and GA-ANN Modeling. Sustain. Chem. Pharm. 2021, 19, 100374. [Google Scholar] [CrossRef]
- Ghosal, D.; Singh, V.K. Study of the Removal of Safranine-O Dye from Wastewater Using Waste Derived Biosorbent. Recent Innov. Chem. Eng. 2020, 13, 248–260. [Google Scholar] [CrossRef]
- Phuong, D.T.M.; Loc, N.X. Rice Straw Biochar and Magnetic Rice Straw Biochar for Safranin O Adsorption from Aqueous Solution. Water 2022, 14, 186. [Google Scholar] [CrossRef]
- Nehaba, S.S.; Abdullah, R.H.; Oda, A.M.; Omran, A.R.; Mottaleb, A.S. Evaluation of the Efficiency of Tea Waste Powder to Remove the Safranin O Dye Compared to the Activated Carbon As Adsorbent. Orient. J. Chem. 2019, 35, 1201–1207. [Google Scholar] [CrossRef]
- Muftah Elsherif, K.; El-Dali, A.; Mabrok Ewlad-Ahmed, A.; Treban, A.; Alttayib, I. Removal of Safranin Dye from Aqueous Solution by Adsorption onto Olive Leaves Powder. J. Mater. Environ. Sci. 2021, 12, 418–430. [Google Scholar]
- Bellahsen, N.; Varga, G.; Halyag, N.; Kertész, S.; Tombácz, E.; Hodúr, C. Pomegranate Peel as a New Low-Cost Adsorbent for Ammonium Removal. Int. J. Environ. Sci. Technol. 2021, 18, 711–722. [Google Scholar] [CrossRef]
- Hashem, A.; Aniagor, C.O.; Fikry, M.; Taha, G.M.; Badawy, S.M. Characterization and Adsorption of Raw Pomegranate Peel Powder for Lead (II) Ions Removal. J. Mater. Cycles Waste Manag. 2023, 25, 2087–2100. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Kyzas, G.Z. Agricultural Peels for Dye Adsorption: A Review of Recent Literature. J. Mol. Liq. 2014, 200, 381–389. [Google Scholar] [CrossRef]
- Saad, H.; Charrier-El Bouhtoury, F.; Pizzi, A.; Rode, K.; Charrier, B.; Ayed, N. Characterization of Pomegranate Peels Tannin Extractives. Ind. Crops Prod. 2012, 40, 239–246. [Google Scholar] [CrossRef]
- Solangi, N.H.; Kumar, J.; Mazari, S.A.; Ahmed, S.; Fatima, N.; Mubarak, N.M. Development of Fruit Waste Derived Bio-Adsorbents for Wastewater Treatment: A Review. J. Hazard Mater. 2021, 416, 125848. [Google Scholar] [CrossRef]
- Afaq, F.; Saleem, M.; Krueger, C.G.; Reed, J.D.; Mukhtar, H. Anthocyanin- and Hydrolyzable Tannin-Rich Pomegranate Fruit Extract Modulates MAPK and NF-ΚB Pathways and Inhibits Skin Tumorigenesis in CD-1 Mice. Int. J. Cancer 2005, 113, 423–433. [Google Scholar] [CrossRef]
- Kahramanoglu, I. Trends in Pomegranate Sector: Production, Postharvest Handling and Marketing. Int. J. Agric. For. Life Sci. 2019, 3, 239–246. [Google Scholar]
- Talekar, S.; Patti, A.F.; Vijayraghavan, R.; Arora, A. Complete Utilization of Waste Pomegranate Peels to Produce a Hydrocolloid, Punicalagin Rich Phenolics, and a Hard Carbon Electrode. ACS Sustain. Chem. Eng. 2018, 6, 16363–16374. [Google Scholar] [CrossRef]
- Hadrich, F.; Cher, S.; Gargouri, Y.T.; Adel, S. Antioxidant and Lipase Inhibitory Activities and Essential Oil Composition of Pomegranate Peel Extracts. J. Oleo Sci. 2014, 63, 515–525. [Google Scholar] [CrossRef]
- Ara, K.M.; Raofie, F. Application of Response Surface Methodology for the Optimization of Supercritical Fluid Extraction of Essential Oil from Pomegranate (Punica granatum L.) Peel. J. Food Sci. Technol. 2016, 53, 3113–3121. [Google Scholar] [CrossRef]
- Ventura, J.; Alarcón-Aguilar, F.; Roman-Ramos, R.; Campos-Sepulveda, E.; Reyes-Vega, M.L.; Boone-Villa, V.D.; Jasso-Villagómez, E.I.; Aguilar, C.N. Quality and Antioxidant Properties of a Reduced-Sugar Pomegranate Juice Jelly with an Aqueous Extract of Pomegranate Peels. Food Chem. 2013, 136, 109–115. [Google Scholar] [CrossRef]
- Grabež, M.; Škrbić, R.; Stojiljković, M.P.; Rudić-Grujić, V.; Paunović, M.; Arsić, A.; Petrović, S.; Vučić, V.; Mirjanić-Azarić, B.; Šavikin, K. Beneficial Effects of Pomegranate Peel Extract on Plasma Lipid Profile, Fatty Acids Levels and Blood Pressure in Patients with Diabetes Mellitus Type-2: A Randomized, Double-Blind, Placebo-Controlled Study. J. Funct. Foods 2020, 64, 103692. [Google Scholar] [CrossRef]
- Demiray, E.; Ertuğrul Karatay, S.; Dönmez, G. Efficient Bioethanol Production from Pomegranate Peels by Newly Isolated Kluyveromyces Marxianus. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 42, 709–718. [Google Scholar] [CrossRef]
- Jain, K.; Suryawanshi, P.; Chaudhari, A. Recovery of Acerbic Anaerobic Digester for Biogas Production from Pomegranate Shells Using Organic Loading Approach. Indian J. Biochem. Biophys. 2020, 57, 86–91. [Google Scholar]
- Tezcan, F.; Günister, E.; Özen, G.; Erim, F.B. Biocomposite Films Based on Alginate and Organically Modified Clay. Int. J. Biol. Macromol. 2012, 50, 1165–1168. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, P.; Hussain, Z.; Zhang, H.; Wang, H.; Chang, N.; Li, F. Modification and Characterization of Hydrogel Beads and Its Used as Environmentally Friendly Adsorbent for the Removal of Reactive Dyes. J. Clean Prod. 2022, 342, 130789. [Google Scholar] [CrossRef]
- Garg, D.; Majumder, C.B.; Kumar, S.; Sarkar, B. Removal of Direct Blue-86 Dye from Aqueous Solution Using Alginate Encapsulated Activated Carbon (PnsAC-Alginate) Prepared from Waste Peanut Shell. J. Environ. Chem. Eng. 2019, 7, 103365. [Google Scholar] [CrossRef]
- Ajeel, S.J.; Beddai, A.A.; Almohaisen, A.M.N. Preparation of Alginate/Graphene Oxide Composite for Methylene Blue Removal. In Proceedings of the Materials Today: Proceedings; Elsevier Ltd.: Amsterdam, The Netherlands, 2021; Volume 51, pp. 289–297. [Google Scholar]
- Işık, B.; Uğraşkan, V. Adsorption of Methylene Blue on Sodium Alginate–Flax Seed Ash Beads: Isotherm, Kinetic and Thermodynamic Studies. Int. J. Biol. Macromol. 2021, 167, 1156–1167. [Google Scholar] [CrossRef]
- Aichour, A.; Zaghouane-Boudiaf, H.; Iborra, C.V.; Polo, M.S. Bioadsorbent Beads Prepared from Activated Biomass/Alginate for Enhanced Removal of Cationic Dye from Water Medium: Kinetics, Equilibrium and Thermodynamic Studies. J. Mol. Liq. 2018, 256, 533–540. [Google Scholar] [CrossRef]
- Oussalah, A.; Boukerroui, A. Alginate-Bentonite Beads for Efficient Adsorption of Methylene Blue Dye. EuroMediterr. J. Environ. Integr. 2020, 5, 31. [Google Scholar] [CrossRef]
- Belhouchat, N.; Zaghouane-Boudiaf, H.; Viseras, C. Removal of Anionic and Cationic Dyes from Aqueous Solution with Activated Organo-Bentonite/Sodium Alginate Encapsulated Beads. Appl. Clay Sci. 2017, 135, 9–15. [Google Scholar] [CrossRef]
- Ohemeng-Boahen, G.; Sewu, D.D.; Woo, S.H. Preparation and Characterization of Alginate-Kelp Biochar Composite Hydrogel Bead for Dye Removal. Environ. Sci. Pollut. Res. 2019, 26, 33030–33042. [Google Scholar] [CrossRef]
- Isik, Z.; Saleh, M.; Bilici, Z.; Dizge, N. Remazol Brilliant Blue R (RBBR) Dye and Phosphate Adsorption by Calcium Alginate Beads Modified with Polyethyleneimine. Water Environ. Res. 2021, 93, 2780–2794. [Google Scholar] [CrossRef]
- Zhao, D.; Shen, Z.; Shen, X. Dual-Functional Calcium Alginate Hydrogel Beads for Disinfection Control and Removal of Dyes in Water. Int. J. Biol. Macromol. 2021, 188, 253–262. [Google Scholar] [CrossRef]
- Abdollahi, M.; Alboofetileh, M.; Rezaei, M.; Behrooz, R. Comparing Physico-Mechanical and Thermal Properties of Alginate Nanocomposite Films Reinforced with Organic and/or Inorganic Nanofillers. Food Hydrocoll. 2013, 32, 416–424. [Google Scholar] [CrossRef]
- Kumar, M.; Tamilarasan, R.; Sivakumar, V. Adsorption of Victoria Blue by Carbon/Ba/Alginate Beads: Kinetics, Thermodynamics and Isotherm Studies. Carbohydr. Polym. 2013, 98, 505–513. [Google Scholar] [CrossRef]
- Vairavel, P.; Ramachandra Murty, V. Optimization, Kinetics, Equilibrium Isotherms, and Thermodynamics Studies for Congo Red Dye Adsorption Using Calcium Alginate Beads Immobilized with Dual Adsorbent (Neurospora Crassa Dead Fungal Biomass and Wheat Bran). Desalination Water Treat. 2017, 97, 338–362. [Google Scholar] [CrossRef]
- Flores-Céspedes, F.; Villafranca-Sánchez, M.; Fernández-Pérez, M. Alginate-Bentonite-Based Hydrogels Designed to Obtain Controlled-Release Formulations of Dodecyl Acetate. Gels 2023, 9, 388. [Google Scholar] [CrossRef]
- Yadav, S.; Asthana, A.; Chakraborty, R.; Jain, B.; Singh, A.K.; Carabineiro, S.A.C.; Susan, M.A.B.H. Cationic Dye Removal Using Novel Magnetic/Activated Charcoal/β-Cyclodextrin/Alginate Polymer Nanocomposite. Nanomaterials 2020, 10, 170. [Google Scholar] [CrossRef]
- Viscusi, G.; Gorrasi, G. Facile Preparation of Layered Double Hydroxide (LDH)-Alginate Beads as Sustainable System for the Triggered Release of Diclofenac: Effect of PH and Temperature on Release Rate. Int. J. Biol. Macromol. 2021, 184, 271–281. [Google Scholar] [CrossRef]
- Nitta, S.K.; Numata, K. Biopolymer-Based Nanoparticles for Drug/Gene Delivery and Tissue Engineering. Int. J. Mol. Sci. 2013, 14, 1629–1654. [Google Scholar] [CrossRef]
- Senturk Parreidt, T.; Müller, K.; Schmid, M. Alginate-Based Edible Films and Coatings for Food Packaging Applications. Foods 2018, 7, 170. [Google Scholar] [CrossRef]
- Vijayaraghavan, G.; Shanthakumar, S. Effective Removal of Acid Black 1 Dye in Textile Effluent Using Alginate from Brown Algae as a Coagulant. Iran. J. Chem. Chem. Eng. 2018, 37, 145–151. [Google Scholar]
- Asadi, S.; Eris, S.; Azizian, S. Alginate-Based Hydrogel Beads as a Biocompatible and Efficient Adsorbent for Dye Removal from Aqueous Solutions. ACS Omega 2018, 3, 15140–15148. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, X.; Hu, S.; Zhang, X.; Luo, X. Core–Shell Zeolite@ Alg–Ca Particles for Removal of Strontium from Aqueous Solutions. RSC Adv. 2016, 6, 73959–73973. [Google Scholar] [CrossRef]
- Yu, F.; Cui, T.; Yang, C.; Dai, X.; Ma, J. κ-Carrageenan/Sodium Alginate Double-Network Hydrogel with Enhanced Mechanical Properties, Anti-Swelling, and Adsorption Capacity. Chemosphere 2019, 237, 124417. [Google Scholar] [CrossRef]
- Hassan, A.F.; Abdel-Mohsen, A.M.; Fouda, M.M.G. Comparative Study of Calcium Alginate, Activated Carbon, and Their Composite Beads on Methylene Blue Adsorption. Carbohydr. Polym. 2014, 102, 192–198. [Google Scholar] [CrossRef]
- Boukhalfa, N.; Boutahala, M.; Djebri, N.; Idris, A. Maghemite/Alginate/Functionalized Multiwalled Carbon Nanotubes Beads for Methylene Blue Removal: Adsorption and Desorption Studies. J. Mol. Liq. 2019, 275, 431–440. [Google Scholar] [CrossRef]
- El-Qelish, M.; Elgarahy, A.M.; Ibrahim, H.S.; El-Kholly, H.K.; Gad, M.; Ali, M.E.M. Multi-Functional Core-Shell Pomegranate Peel Amended Alginate Beads for Phenol Decontamination and Bio-Hydrogen Production: Synthesis, Characterization, and Kinetics Investigation. Biochem. Eng. J. 2023, 195, 108932. [Google Scholar] [CrossRef]
- Zhong, Z.-Y.; Yang, Q.; Li, X.-M.; Luo, K.; Liu, Y.; Zeng, G.-M. Preparation of Peanut Hull-Based Activated Carbon by Microwave-Induced Phosphoric Acid Activation and Its Application in Remazol Brilliant Blue R Adsorption. Ind. Crops Prod. 2012, 37, 178–185. [Google Scholar] [CrossRef]
- Deng, A.; Wu, S.; Hao, J.; Pan, H.; Li, M.; Gao, X. Photocatalytic Removal of Cr (VI) by Thiourea Modified Sodium Alginate/Biochar Composite Gel. Gels 2022, 8, 293. [Google Scholar] [CrossRef]
- Siddiqui, M.T.H.; Nizamuddin, S.; Mubarak, N.M.; Shirin, K.; Aijaz, M.; Hussain, M.; Baloch, H.A. Characterization and Process Optimization of Biochar Produced Using Novel Biomass, Waste Pomegranate Peel: A Response Surface Methodology Approach. Waste Biomass Valorization 2019, 10, 521–532. [Google Scholar] [CrossRef]
- Ben-Ali, B.; Jaouali, I.; Souissi-Najar, S.; Ouederni, A. Characterization and Adsorption Capacity of Raw Pomegranate Peel Biosorbent for Copper Removal. J. Clean Prod. 2017, 142, 3809–3821. [Google Scholar] [CrossRef]
- Li, Z.F.; Zhang, C.J.; Cui, L.; Zhu, P.; Yan, C.; Liu, Y. Fire Retardant and Thermal Degradation Properties of Cotton Fabrics Based on APTES and Sodium Phytate through Layer-by-Layer Assembly. J. Anal. Appl. Pyrolysis 2017, 123, 216–223. [Google Scholar] [CrossRef]
- Shao, W.; Liu, H.; Liu, X.; Wang, S.; Wu, J.; Zhang, R.; Min, H.; Huang, M. Development of Silver Sulfadiazine Loaded Bacterial Cellulose/Sodium Alginate Composite Films with Enhanced Antibacterial Property. Carbohydr. Polym. 2015, 132, 351–358. [Google Scholar] [CrossRef]
- Demiral, I.; Eryazici, A.; Şensöz, S. Bio-Oil Production from Pyrolysis of Corncob (Zea mays L.). Biomass Bioenergy 2012, 36, 43–49. [Google Scholar] [CrossRef]
- Li, P.; Yang, C.; Xu, X.; Miao, C.; He, T.; Jiang, B.; Wu, W. Preparation of Bio-Based Aerogel and Its Adsorption Properties for Organic Dyes. Gels 2022, 8, 755. [Google Scholar] [CrossRef]
- Li, H.; Liu, L.; Cui, J.; Cui, J.; Wang, F.; Zhang, F. High-Efficiency Adsorption and Regeneration of Methylene Blue and Aniline onto Activated Carbon from Waste Edible Fungus Residue and Its Possible Mechanism. RSC Adv. 2020, 10, 14262–14273. [Google Scholar] [CrossRef]
- Azimvand, J.; Didehban, K.; Mirshokraie, S.A. Safranin-O Removal from Aqueous Solutions Using Lignin Nanoparticle-g-Polyacrylic Acid Adsorbent: Synthesis, Properties, and Application. Adsorpt. Sci. Technol. 2018, 36, 1422–1440. [Google Scholar] [CrossRef]
- Maurya, N.S.; Mittal, A.K. Removal Mechanism of Cationic Dye (Safranin O) from the Aqueous Phase by Dead Macro Fungus Biosorbent. Water Sci. Technol. 2013, 68, 1048–1054. [Google Scholar] [CrossRef]
- Lagergren, S.K. About the Theory of So-Called Adsorption of Soluble Substances. Sven. Vetenskapsakad. Handingarl 1898, 24, 1–39. [Google Scholar]
- Ho, Y.-S.; McKay, G. Pseudo-Second Order Model for Sorption Processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Weber Jr, W.J.; Morris, J.C. Kinetics of Adsorption on Carbon from Solution. J. Sanit. Eng. Div. 1963, 89, 31–59. [Google Scholar] [CrossRef]
- Kumar, P.S.; Ramalingam, S.; Kirupha, S.D.; Murugesan, A.; Vidhyadevi, T.; Sivanesan, S. Adsorption Behavior of Nickel (II) onto Cashew Nut Shell: Equilibrium, Thermodynamics, Kinetics, Mechanism and Process Design. Chem. Eng. J. 2011, 167, 122–131. [Google Scholar] [CrossRef]
- Pholosi, A.; Naidoo, E.B.; Ofomaja, A.E. Intraparticle Diffusion of Cr(VI) through Biomass and Magnetite Coated Biomass: A Comparative Kinetic and Diffusion Study. S. Afr. J. Chem. Eng. 2020, 32, 39–55. [Google Scholar] [CrossRef]
- Langmuir, I. The Adsorption of Gases on Plane Surfaces of Glass, Mica and Platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Over the Adsorption in Solution. J. Phys. Chem. 1906, 57, 1100–1107. [Google Scholar]
- Temkin, M.I. Kinetics of Ammonia Synthesis on Promoted Iron Catalysts. Acta Physiochim. URSS 1940, 12, 327–356. [Google Scholar]
- Dubinin, M.M. The Equation of the Characteristic Curve of Activated Charcoal. Dokl. Akad. Nauk. SSSR 1947, 55, 327–329. [Google Scholar]
- Kundu, S.; Gupta, A.K. Arsenic Adsorption onto Iron Oxide-Coated Cement (IOCC): Regression Analysis of Equilibrium Data with Several Isotherm Models and Their Optimization. Chem. Eng. J. 2006, 122, 93–106. [Google Scholar] [CrossRef]
- Andersson, K.I.; Eriksson, M.; Norgren, M. Removal of Lignin from Wastewater Generated by Mechanical Pulping Using Activated Charcoal and Fly Ash: Adsorption Isotherms and Thermodynamics. Ind. Eng. Chem. Res. 2011, 50, 7722–7732. [Google Scholar] [CrossRef]
- Bhattacharya, K.G.; Sharma, A. Kinetics and Thermodynamics of Methylene Blue Adsorption on Neem (Azadirachta Indica) Leaf Powder. Dye. Pigment. 2005, 65, 51–59. [Google Scholar] [CrossRef]
- Oladipo, A.A.; Ifebajo, A.O. Highly Efficient Magnetic Chicken Bone Biochar for Removal of Tetracycline and Fluorescent Dye from Wastewater: Two-Stage Adsorber Analysis. J. Environ. Manag. 2018, 209, 9–16. [Google Scholar] [CrossRef]
- Fan, S.; Wang, Y.; Wang, Z.; Tang, J.; Tang, J.; Li, X. Removal of Methylene Blue from Aqueous Solution by Sewage Sludge-Derived Biochar: Adsorption Kinetics, Equilibrium, Thermodynamics and Mechanism. J. Environ. Chem. Eng. 2017, 5, 601–611. [Google Scholar] [CrossRef]
- Sun, C.L.; Wang, C.S. Estimation on the Intramolecular Hydrogen-Bonding Energies in Proteins and Peptides by the Analytic Potential Energy Function. J. Mol. Struct. Theochem. 2010, 956, 38–43. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Clarendon Press: Oxford, UK, 1979; ISBN 3804204422. [Google Scholar]
- McKay, G.; Otterburn, M.S.; Sweeney, A.G. Surface Mass Transfer Processes during Colour Removal from Effluent Using Silica. Water Res. 1981, 15, 327–331. [Google Scholar] [CrossRef]
- Mehmood, A.; Bano, S.; Fahim, A.; Parveen, R.; Khurshid, S. Efficient Removal of Crystal Violet and Eosin B from Aqueous Solution Using Syzygium Cumini Leaves: A Comparative Study of Acidic and Basic Dyes on a Single Adsorbent. Korean J. Chem. Eng. 2015, 32, 882–895. [Google Scholar] [CrossRef]
- Liu, L.; Gao, Z.Y.; Su, X.P.; Chen, X.; Jiang, L.; Yao, J.M. Adsorption Removal of Dyes from Single and Binary Solutions Using a Cellulose-Based Bioadsorbent. ACS Sustain. Chem. Eng. 2015, 3, 432–442. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, Y.; Bai, H.; Zhang, T.; Ibarra-Galvan, V.; Song, S. Methylene Blue Removal from Water Using the Hydrogel Beads of Poly(Vinyl Alcohol)-Sodium Alginate-Chitosan-Montmorillonite. Carbohydr. Polym. 2018, 198, 518–528. [Google Scholar] [CrossRef]
- Sahu, M.K.; Sahu, U.K.; Patel, R.K. Adsorption of Safranin-O Dye on CO2 Neutralized Activated Red Mud Waste: Process Modelling, Analysis and Optimization Using Statistical Design. RSC Adv. 2015, 5, 42294–42304. [Google Scholar] [CrossRef]
- Çetinkaya, S.; Kaya, S.; Aksu, A.; Çetintaş, H.İ.; Jalbani, N.S.; Erkan, S.; Marzouki, R. Equilibrium and DFT Modeling Studies for the Biosorption of Safranin O Dye from Water Samples Using Bacillus Subtilis Biosorbent. J. Mol. Struct. 2023, 1276, 134761. [Google Scholar] [CrossRef]
- Cheriaa, J.; Khaireddine, M.; Rouabhia, M.; Bakhrouf, A. Removal of Triphenylmethane Dyes by Bacterial Consortium. Sci. World J. 2012, 2012, 512454. [Google Scholar] [CrossRef]
- Ozdemir, N.C.; Saleh, M.; Bilici, Z.; Arslan, H.; Dizge, N. Preparation of Leonardite Powder-Embedded Calcium Alginate Beads and Adsorption of Safranin-O Dye. Water Pract. Technol. 2023, 18, 1711–1726. [Google Scholar] [CrossRef]
- Bayazit, Ş.S. Investigation of Safranin O Adsorption on Superparamagnetic Iron Oxide Nanoparticles (SPION) and Multi-Wall Carbon Nanotube/SPION Composites. Desalination Water Treat. 2014, 52, 6966–6975. [Google Scholar] [CrossRef]
- Dwivedi, M.K.; Jain, N.; Sharma, P.; Alawa, C. Adsorption of Safranin from Wastewater Using Coal Fly Ash. IOSR J. Appl. Chem. 2015, 8, 27–35. [Google Scholar]
- Al-Mammar, D.E. Decolorization of the Aqueous Safranin O Dye Solution Using Thuja Orientalis as Biosorbent. Iraqi J. Sci. 2014, 55, 886–898. [Google Scholar]
- Witono, J.R.; Noordergraaf, I.W.; Heeres, H.J.; Janssen, L. Water Absorption, Retention and the Swelling Characteristics of Cassava Starch Grafted with Polyacrylic Acid. Carbohydr. Polym. 2014, 103, 325–332. [Google Scholar] [CrossRef]
- Thagira Banu, H.; Karthikeyan, P.; Meenakshi, S. Lanthanum (III) Encapsulated Chitosan-Montmorillonite Composite for the Adsorptive Removal of Phosphate Ions from Aqueous Solution. Int. J. Biol. Macromol. 2018, 112, 284–293. [Google Scholar] [CrossRef]



















| Kinetic Model | Coefficients | 50 mg/L | 100 mg/L | 300 mg/L |
|---|---|---|---|---|
| (mg g−1) | 3.992 | 8.7957 | 24.7377 | |
| Pseudo-first-order | (mg g−1) | 0.9217 | 4.6039 | 12.9416 |
| (min−1) | 0.0209 | 0.0288 | 0.01813 | |
| 0.8856 | 0.6071 | 0.9387 | ||
| 2.5103 | 23.232 | 0.9574 | ||
| 0.3961 | 1.2050 | 2.2446 | ||
| Pseudo-second-order | (mg g−1) | 4.0541 | 9.1166 | 26.1917 |
| (g mg−1 min−1) | 0.0595 | 0.0141 | 0.0026 | |
| 0.9997 | 0.9988 | 0.9966 | ||
| 0.7446 | 0.7340 | 0.2573 | ||
| 0.2092 | 0.2078 | 0.1230 | ||
| Elovich | (mg g−1) | 0.4074 | 1.3367 | 4.3697 |
| α (mg/g min) | 54.8413 | 7.2104 | 7.4085 | |
| (g/mg) | 2.4542 | 0.748 | 0.2288 | |
| 0.8304 | 0.8278 | 0.9603 | ||
| 0.5671 | 6.2137 | 13.300 | ||
| 0.1826 | 0.6045 | 0.8845 | ||
| Intraparticle diffusion | (mg g−1 min−1/2) | 0.5251 | 1.1135 | 2.9248 |
| (mg g−1) | 1.1566 | 1.2241 | 2.7464 | |
| 0.7865 | 0.5325 | 0.8812 | ||
| 0.1889 | 2.7481 | 2.9092 | ||
| 0.4346 | 1.6577 | 1.7056 | ||
| (mg g−1 min−1/2) | 0.1973 | 1.2676 | 1.5126 | |
| (mg g−1) | 2.43 | 0.2905 | 8.7234 | |
| 0.628 | 0.9580 | 0.9040 | ||
| 0.0153 | 0.7066 | 3.7221 | ||
| 0.1236 | 0.8406 | 0.8628 | ||
| (mg g−1 min−1/2) | 0.0302 | 0.1176 | 0.3266 | |
| (mg g−1) | 3.5733 | 7.2205 | 19.8407 | |
| 0.9395 | 0.9580 | 0.8199 | ||
| 0.0041 | 0.0422 | 0.5109 | ||
| 0.0177 | 0.0569 | 0.2382 | ||
| (cm2 s−1) | 1.2.10−6 | 1.2.10−6 | 6.2.10−7 |
| Isotherm Model | Parameters | Value |
|---|---|---|
| Langmuir | (mg g−1) | 30.769 |
| (L mg−1) | 0.0258 | |
| 0.7725 | ||
| 0.9240 | ||
| 0.0096 | ||
| 0.0568 | ||
| Freundlich | 1.1312 | |
| ((mg g−1) * (L mg−1)1/n) | 0.8334 | |
| 0.8810 | ||
| 0.5090 | ||
| 0.4119 | ||
| Temkin | (L g−1) | 0.3223 |
| 8.1594 | ||
| (kJ mol−1) | 298.55 | |
| 0.8219 | ||
| 69.580 | ||
| 4.8159 | ||
| D-R | (mg g−1) | 12.562 |
| (mol2 kJ−2) | 3.3431 | |
| (kJ mol−1) | 0.3867 | |
| 0.6021 | ||
| 1.7020 | ||
| 0.7532 |
| Temperature (K) | ΔG (kJ mol−1) | ΔH (kJ mol−1) | ΔS (kJ mol−1 K−1) |
|---|---|---|---|
| 293 | −6.82 | 9.30 | |
| 303 | −7.05 | ||
| 313 | −7.28 | 23.30 | |
| 323 | −7.52 | ||
| 333 | −7.75 |
| Parameters | Value | , cm2/s |
|---|---|---|
| pH | 2 | 1.31 ± 0.05 × 10−7 a |
| 4 | 1.60 ± 0.05 × 10−7 a | |
| 6 | 2.67 ± 0.12 × 10−7 a | |
| 8 | 2.71 ± 0.09 × 10−7 b | |
| 10 | 2.79 ± 0.06 × 10−7 c | |
| T | 293 | 2.31 ± 0.04 × 10−7 a |
| 303 | 2.48 ± 0.05 × 10−7 b | |
| 313 | 2.96 ± 0.07 × 10−7 b | |
| 323 | 3.10 ± 0.055 × 10−7 c | |
| 333 | 3.31 ± 0.06 × 10−7 c |
| Concentration, mg/L | β*Ss, s−1 | R2 |
|---|---|---|
| 10 | 0.080 ± 0.008 a | 0.79 |
| 25 | 0.85 ± 0.10 a | 0.81 |
| 50 | 1.03 ± 0.08 ab | 0.39 |
| 100 | 0.97 ± 0.09 bc | 0.79 |
| 200 | 0.78 ± 0.06 c | 0.84 |
| 300 | 0.53 ± 0.03 d | 0.83 |
| Concentration, mg/L | α2 | kb | R2 |
|---|---|---|---|
| 10 | 0.57 | 0.03 | 0.97 |
| 25 | 0.22 | 0.10 | 0.96 |
| 50 | 0.13 | 0.17 | 0.94 |
| 100 | 0.34 | 0.07 | 0.97 |
| 200 | 0.48 | 0.05 | 0.98 |
| 300 | 0.42 | 0.04 | 0.98 |
| Adsorbent | Conditions | Qmax (mg/g) | References | ||
|---|---|---|---|---|---|
| pH | T | C0 (mg/l) | |||
| Leo−Ca−Alg | 8 | 25 | 10–30 | 3.43 | [108] |
| SPION | Natural pH | 25 | 1–15 | 0.79 | [109] |
| MWCNT/SPION | Natural pH | 25 | 1–15 | 8.42 | |
| Coal fly ash | 9 | 30 | 5–50 | 1.76 | [110] |
| Thuja orientalis | 7 | 20 | 5–25 | 0.17 | [111] |
| Alg−PP | Natural pH | 25 | 10–50 | 30.769 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbaz, A.; Arris, S.; Viscusi, G.; Ayat, A.; Aissaoui, H.; Boumezough, Y. Adsorption of Safranin O Dye by Alginate/Pomegranate Peels Beads: Kinetic, Isotherm and Thermodynamic Studies. Gels 2023, 9, 916. https://doi.org/10.3390/gels9110916
Abbaz A, Arris S, Viscusi G, Ayat A, Aissaoui H, Boumezough Y. Adsorption of Safranin O Dye by Alginate/Pomegranate Peels Beads: Kinetic, Isotherm and Thermodynamic Studies. Gels. 2023; 9(11):916. https://doi.org/10.3390/gels9110916
Chicago/Turabian StyleAbbaz, Amina, Sihem Arris, Gianluca Viscusi, Asma Ayat, Halima Aissaoui, and Yasser Boumezough. 2023. "Adsorption of Safranin O Dye by Alginate/Pomegranate Peels Beads: Kinetic, Isotherm and Thermodynamic Studies" Gels 9, no. 11: 916. https://doi.org/10.3390/gels9110916
APA StyleAbbaz, A., Arris, S., Viscusi, G., Ayat, A., Aissaoui, H., & Boumezough, Y. (2023). Adsorption of Safranin O Dye by Alginate/Pomegranate Peels Beads: Kinetic, Isotherm and Thermodynamic Studies. Gels, 9(11), 916. https://doi.org/10.3390/gels9110916