Cryopreservation of Cell Sheets for Regenerative Therapy: Application of Vitrified Hydrogel Membranes
Abstract
:1. Introduction
2. The History of Tissue Culture and Tissue Engineering
3. Regenerative Medicine and Cultured Human Skin
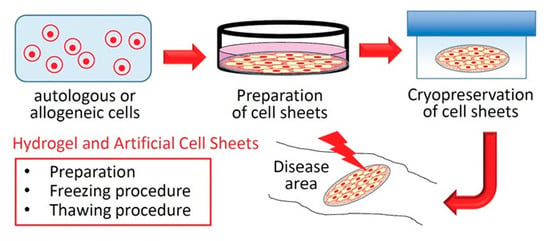
4. Hydrogel and Artificial Cell Sheets
4.1. Extracellular Matrix (ECM)
4.1.1. Collagen and Gelatin
4.1.2. Elastin
4.1.3. Fibronectin
4.1.4. Laminin
4.1.5. Hyaluronan (Hyaluronic Acid)
4.1.6. Alginic Acid
4.1.7. Synthetic Polymer
4.2. Thermoresponsive Polymer
4.3. Vitrified Hydrogel Membranes
5. Artificial Cell Sheets and Cryopreservation
5.1. Cryopreservation of Cellular and Tissue-Based Products and Simple Cell Suspensions
5.2. Cryopreservation of Tissues and Cell Sheets
6. Conclusions and Future Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rheinwald, J.G.; Green, H. Serial cultivation of strains of human epidermal keratinocytes: The formation of keratinizing colonies from single cells. Cell 1975, 6, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Green, H.; Kehinde, O.; Thomas, J. Growth of cultured human epidermal cells into multiple epithelia suitable for grafting. Proc. Natl. Acad. Sci. USA 1979, 76, 5665–5668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Connor, N.E.; Mulliken, J.B.; Banks-Schlegel, S.; Kehinde, O.; Green, H. Grafting of burns with cultured epithelium prepared from autologous epidermal cells. Lancet 1981, 1, 75–78. [Google Scholar] [CrossRef]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [Green Version]
- Vacanti, C.A.; Vacanti, J.P. The history and scope of tissue engineering. In Principles of Tissue Engineering, 3rd ed.; Lanza, R., Langer, R., Vacanti, J.P., Eds.; Academic Press, Elsevier: Waltham, MA, USA, 2007; pp. 3–6. [Google Scholar]
- Yamada, N.; Okano, T.; Sakai, H.; Karikusa, F.; Sawasaki, Y.; Sakurai, Y. Thermo-responsive polymeric surfaces; control of attachment and detachment of cultured cells. Makromol. Chem. Rapid Commun. 1990, 11, 571–576. [Google Scholar] [CrossRef]
- Sawa, Y.; Miyagawa, S.; Sakaguchi, T.; Fujita, T.; Matsuyama, A.; Saito, A.; Shimizu, T.; Okano, T. Tissue engineered myoblast sheets improved cardiac function sufficiently to discontinue LVAS in a patient with DCM: Report of a case. Surg. Today 2012, 42, 181–184. [Google Scholar] [CrossRef]
- Järveläinen, H.; Sainio, A.; Koulu, M.; Wight, T.N.; Penttinen, R. Extracellular matrix molecules: Potential targets in pharmacotherapy. Pharmacol. Rev. 2009, 61, 198–223. [Google Scholar] [CrossRef] [Green Version]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123 Pt 24, 4195–4200. [Google Scholar] [CrossRef] [Green Version]
- Takezawa, T.; Ozaki, K.; Nitani, A.; Takabayashi, C.; Shimo-Oka, T. Collagen vitrigel: A novel scaffold that can facilitate a three-dimensional culture for reconstructing organoids. Cell Transplant. 2004, 13, 463–473. [Google Scholar] [CrossRef]
- Harrison, R.G. Observations on the living developing nerve fiber. Proc. Soc. Exp. Biol. Med. 1907, 4, 140–143. [Google Scholar] [CrossRef]
- Rous, P.; Jones, F.S. A method for obtaining suspensions of living cells from the fixed tissues, and for the plating out of individual cells. J. Exp. Med. 1916, 23, 549–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Earle, W.R. Changes Induced in a Strain of Fibro blasts From a Strain C3H Mouse by the Action of 20-Methylcholanthrene (Preliminary Report). J. Natl. Cancer Inst. 1943, 3, 555–558. [Google Scholar]
- Earle, W.R.; Schilling, E.L.; Stark, T.H.; Straus, N.P.; Brown, M.F.; Shelton, E. Production of Malignancy in Vitro. IV. The Mouse Fibroblast Cultures and Changes Seen in the Living Cells. J. Natl. Cancer Inst. 1943, 4, 165–212. [Google Scholar]
- Gey, G.O.; Coffman, W.D.; Kubicek, M.T. Tissue culture studies of the proliferative capacity of cervical carcinoma and normal epithelium. Cancer Res. 1952, 12, 264–265. [Google Scholar]
- Enders, J.F.; Weller, T.H.; Robbins, F.C. Cultivation of the Lansing Strain of Poliomyelitis Virus in Cultures of Various Human Embryonic Tissues. Science 1949, 109, 85–87. [Google Scholar] [CrossRef]
- Salk, J.E.; Youngner, J.S.; Ward, E.N. Use of color change of phenol red as the indicator in titrating poliomyelitis virus or its antibody in a tissue-culture system. Am. J. Hyg. 1954, 60, 214–230. [Google Scholar] [CrossRef]
- Salk, J.E.; Krech, U.; Youngner, J.S.; Bennett, B.L.; Lewis, L.J.; Bazeley, P.L. Formaldehyde treatment and safety testing of experimental poliomyelitis vaccines. Am. J. Public Health Nations Health 1954, 44, 563–570. [Google Scholar] [CrossRef]
- Salk, J.E.; Bazeley, P.L.; Bennett, B.L.; Krech, U.; Lewis, L.J.; Ward, E.N.; Youngner, J.S. Studies in human subjects on active immunization against poliomyelitis. II. A practical means for inducing and maintaining antibody formation. Am. J. Public Health Nations Health 1954, 44, 994–1009. [Google Scholar] [CrossRef]
- Morgan, J.F.; Morton, H.J.; Parker, R.C. Nutrition of animal cells in tissue culture; initial studies on a synthetic medium. Proc. Soc. Exp. Biol. Med. 1950, 73, 1–8. [Google Scholar] [CrossRef]
- Eagle, H. Amino acid metabolism in mammalian cell cultures. Science 1959, 130, 432–437. [Google Scholar] [CrossRef]
- Dulbecco, R.; Freeman, G. Plaque production by the polyoma virus. Virology 1959, 8, 396–397. [Google Scholar] [CrossRef] [PubMed]
- Van Wezel, A.L. Growth of cell-strains and primary cells on micro-carriers in homogeneous culture. Nature 1967, 216, 64–65. [Google Scholar] [CrossRef] [PubMed]
- Knazek, R.A.; Gullino, P.M.; Kohler, P.O.; Dedrick, R.L. Cell culture on artificial capillaries: An approach to tissue growth in vitro. Science 1972, 178, 65–66. [Google Scholar] [CrossRef] [PubMed]
- Kleinman, H.K.; McGarvey, M.L.; Hassell, J.R.; Star, V.L.; Cannon, F.B.; Laurie, G.W.; Martin, G.R. Basement membrane complexes with biological activity. Biochemistry 1986, 25, 312–318. [Google Scholar] [CrossRef]
- Bell, E.; Ivarsson, B.; Merrill, C. Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc. Natl. Acad. Sci. USA 1979, 76, 1274–1278. [Google Scholar] [CrossRef] [Green Version]
- Bell, E.; Ehrlich, H.P.; Buttle, D.J.; Nakatsuji, T. Living tissue formed in vitro and accepted as skin-equivalent tissue of full thickness. Science 1981, 211, 1052–1054. [Google Scholar] [CrossRef]
- Berthiaume, F.; Moghe, P.V.; Toner, M.; Yarmush, M.L. Effect of extracellular matrix topology on cell structure, function, and physiological responsiveness: Hepatocytes cultured in a sandwich configuration. FASEB J. 1996, 10, 1471–1484. [Google Scholar] [CrossRef]
- Takezawa, T.; Takeuchi, T.; Nitani, A.; Takayama, Y.; Kino-Oka, M.; Taya, M.; Enosawa, S. Collagen vitrigel membrane useful for paracrine assays in vitro and drug delivery systems in vivo. J. Biotechnol. 2007, 131, 76–83. [Google Scholar] [CrossRef]
- Takezawa, T.; Nishikawa, K.; Wang, P.C. Development of a human corneal epithelium model utilizing a collagen vitrigel membrane and the changes of its barrier function induced by exposing eye irritant chemicals. Toxicol. In Vitro 2011, 25, 1237–1241. [Google Scholar] [CrossRef]
- Kadler, K.E.; Baldock, C.; Bella, J.; Boot-Handford, R.P. Collagens at a glance. J. Cell Sci. 2007, 120 Pt 12, 1955–1958. [Google Scholar] [CrossRef] [Green Version]
- Kotch, F.W.; Raines, R.T. Self-assembly of synthetic collagen triple helices. Proc. Natl. Acad. Sci. USA 2006, 103, 3028–3033. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.Y.; Mooney, D.J. Hydrogels for Tissue Engineering. Chem. Rev. 2001, 101, 1869–1880. [Google Scholar] [CrossRef]
- Sarrigiannidis, S.O.; Rey, J.M.; Dobre, O.; González-García, C.; Dalby, M.J.; Salmeron-Sanchez, M. A tough act to follow: Collagen hydrogel modifications to improve mechanical and growth factor loading capabilities. Mater. Today Bio 2021, 10, 100098. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Y.; Li, Y.; Yang, Y.; Jin, M.; Lin, X.; Zhuang, Z.; Guo, K.; Zhang, T.; Tan, W. Application of Collagen-Based Hydrogel in Skin Wound Healing. Gels 2023, 9, 185. [Google Scholar] [CrossRef]
- Schussler, O.; Falcoz, P.E.; Chachques, J.C.; Alifano, M.; Lecarpentier, Y. Possible Treatment of Myocardial Infarct Based on Tissue Engineering Using a Cellularized Solid Collagen Scaffold Functionalized with Arg-Glyc-Asp (RGD) Peptide. Int. J. Mol. Sci. 2021, 22, 12563. [Google Scholar] [CrossRef]
- Saotome, T.; Shimada, N.; Matsuno, K.; Nakamura, K.; Tabata, Y. Gelatin hydrogel nonwoven fabrics of a cell culture scaffold to formulate 3-dimensional cell constructs. Regen. Ther. 2021, 18, 418–429. [Google Scholar] [CrossRef]
- Barreto, M.E.V.; Medeiros, R.P.; Shearer, A.; Fook, M.V.L.; Montazerian, M.; Mauro, J.C. Gelatin and Bioactive Glass Composites for Tissue Engineering: A Review. J. Funct. Biomater. 2022, 14, 23. [Google Scholar] [CrossRef]
- Mahmood, A.; Patel, D.; Hickson, B.; DesRochers, J.; Hu, X. Recent Progress in Biopolymer-Based Hydrogel Materials for Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 1415. [Google Scholar] [CrossRef]
- Rodgers, U.R.; Weiss, A.S. Cellular interactions with elastin. Pathol. Biol. 2005, 53, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Mithieux, S.M.; Weiss, A.S. Elastin. Adv. Protein Chem. 2005, 70, 437–461. [Google Scholar] [PubMed]
- Urry, D.W. Free energy transduction in polypeptides and proteins based on inverse temperature transitions. Prog. Biophys. Mol. Biol. 1992, 57, 23–57. [Google Scholar] [CrossRef] [PubMed]
- Urry, D.W. Molecular Machines: How Motion and other Functions of Living Organisms can Result from Reversible Chemical Changes. Angew. Chem. Int. Ed. 1993, 32, 819–841. [Google Scholar] [CrossRef]
- Urry, D.W. Physical Chemistry of Biological Free Energy Transduction as Demonstrated by Elastic Protein-Based Polymers. J. Phys. Chem. B 1997, 101, 11007–11028. [Google Scholar] [CrossRef]
- Lim, D.W.; Trabbic-Carlson, K.; Mackay, J.A.; Chilkoti, A. Improved non-chromatographic purification of a recombinant protein by cationic elastin-like polypeptides. Biomacromolecules 2007, 8, 1417–1424. [Google Scholar] [CrossRef] [Green Version]
- Le, D.H.; Hanamura, R.; Pham, D.H.; Kato, M.; Tirrell, D.A.; Okubo, T.; Sugawara-Narutaki, A. Self-assembly of elastin-mimetic double hydrophobic polypeptides. Biomacromolecules 2013, 14, 1028–1034. [Google Scholar] [CrossRef] [Green Version]
- Lim, D.W.; Nettles, D.L.; Setton, L.A.; Chilkoti, A. Rapid cross-linking of elastin-like polypeptides with (hydroxymethyl)phosphines in aqueous solution. Biomacromolecules 2007, 8, 1463–1470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trabbic-Carlson, K.; Setton, L.A.; Chilkoti, A. Swelling and mechanical behaviors of chemically cross-linked hydrogels of elastin-like polypeptides. Biomacromolecules 2003, 4, 572–580. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Avery, R.K.; Vallmajo-Martin, Q.; Assmann, A.; Vegh, A.; Memic, A.; Olsen, B.D.; Annabi, N.; Khademhosseini, A. A Highly Elastic and Rapidly Crosslinkable Elastin-Like Polypeptide-Based Hydrogel for Biomedical Applications. Adv. Funct. Mater. 2015, 25, 4814–4826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saiki, I. Cell adhesion molecules and cancer metastasis. Jpn. J. Pharmacol. 1997, 75, 215–242. [Google Scholar] [CrossRef] [Green Version]
- Kaur, J.; Reinhardt, D.P. Extracellular Matrix (ECM) Molecules. In Stem Cell Biology and Tissue Engineering in Dental Sciences; Vishwakarma, A., Sharpe, P., Shi, S., Ramalingam, M., Eds.; Academic Press, Elsevier Inc.: Waltham, MA, USA, 2015; pp. 25–45. [Google Scholar]
- Trujillo, S.; Gonzalez-Garcia, C.; Rico, P.; Reid, A.; Windmill, J.; Dalby, M.J.; Salmeron-Sanchez, M. Engineered 3D hydrogels with full-length fibronectin that sequester and present growth factors. Biomaterials 2020, 252, 120104. [Google Scholar] [CrossRef]
- Nakagawa, M.; Taniguchi, Y.; Senda, S.; Takizawa, N.; Ichisaka, T.; Asano, K.; Morizane, A.; Doi, D.; Takahashi, J.; Nishizawa, M.; et al. A novel efficient feeder-free culture system for the derivation of human induced pluripotent stem cells. Sci. Rep. 2014, 4, 3594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodin, S.; Domogatskaya, A.; Ström, S.; Hansson, E.M.; Chien, K.R.; Inzunza, J.; Hovatta, O.; Tryggvason, K. Long-term self-renewal of human pluripotent stem cells on human recombinant laminin-511. Nat. Biotechnol. 2010, 28, 611–615. [Google Scholar] [CrossRef] [Green Version]
- Jury, M.; Matthiesen, I.; Rasti Boroojeni, F.; Ludwig, S.L.; Civitelli, L.; Winkler, T.E.; Selegård, R.; Herland, A.; Aili, D. Bioorthogonally Cross-Linked Hyaluronan-Laminin Hydrogels for 3D Neuronal Cell Culture and Biofabrication. Adv. Healthc. Mater. 2022, 11, e2102097. [Google Scholar] [CrossRef]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Toole, B.P. Hyaluronan in morphogenesis. J. Intern. Med. 1997, 242, 35–40. [Google Scholar] [CrossRef] [Green Version]
- Toole, B.P. Hyaluronan: From extracellular glue to pericellular cue. Nat. Rev. Cancer 2004, 4, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Chung, C.; Khetan, S.; Burdick, J.A. Hydrolytically degradable hyaluronic acid hydrogels with controlled temporal structures. Biomacromolecules 2008, 9, 1088–1092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic acid hydrogels for biomedical applications. Adv. Mater. 2011, 23, H41–H56. [Google Scholar] [CrossRef] [PubMed]
- Horder, H.; Guaza Lasheras, M.; Grummel, N.; Nadernezhad, A.; Herbig, J.; Ergün, S.; Teßmar, J.; Groll, J.; Fabry, B.; Bauer-Kreisel, P.; et al. Bioprinting and Differentiation of Adipose-Derived Stromal Cell Spheroids for a 3D Breast Cancer-Adipose Tissue Model. Cells. 2021, 10, 803. [Google Scholar] [CrossRef]
- Abka-Khajouei, R.; Tounsi, L.; Shahabi, N.; Patel, A.K.; Abdelkafi, S.; Michaud, P. Structures, Properties and Applications of Alginates. Mar. Drugs 2022, 20, 364. [Google Scholar] [CrossRef]
- Mørch, Y.A.; Donati, I.; Strand, B.L.; Skjåk-Braek, G. Effect of Ca2+, Ba2+, and Sr2+ on alginate microbeads. Biomacromolecules 2006, 7, 1471–1480. [Google Scholar] [CrossRef] [PubMed]
- Draget, K.I.; Strand, B.; Hartmann, M.; Valla, S.; Smidsrød, O.; Skjåk-Braek, G. Ionic and Acid Gel Formation of Epimerised Alginates; the Effect of Alge4. Int. J. Biol. Macromol. 2000, 27, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Ching, S.H.; Bansal, N.; Bhandari, B. Alginate Gel Particles—A Review of Production Techniques and Physical Properties. Crit. Rev. Food Sci. Nutr. 2017, 57, 1133–1152. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Tong, T.; Yu, C.; Wang, Q. Advances in Algin and Alginate-Hybrid Materials for Drug Delivery and Tissue Engineering. Mar. Drugs 2022, 21, 14. [Google Scholar] [CrossRef]
- Zhang, Q.; Gonelle-Gispert, C.; Li, Y.; Geng, Z.; Gerber-Lemaire, S.; Wang, Y.; Buhler, L. Islet Encapsulation: New Developments for the Treatment of Type 1 Diabetes. Front. Immunol. 2022, 13, 869984. [Google Scholar] [CrossRef]
- Sun, S.; Cui, Y.; Yuan, B.; Dou, M.; Wang, G.; Xu, H.; Wang, J.; Yin, W.; Wu, D.; Peng, C. Drug delivery systems based on polyethylene glycol hydrogels for enhanced bone regeneration. Front. Bioeng. Biotechnol. 2023, 11, 1117647. [Google Scholar] [CrossRef]
- Yin, L.; Pang, Y.; Shan, L.; Gu, J. The In Vivo Pharmacokinetics of Block Copolymers Containing Polyethylene Glycol Used in Nanocarrier Drug Delivery Systems. Drug Metab. Dispos. 2022, 50, 827–836. [Google Scholar] [CrossRef]
- Ju, H.J.; Ji, Y.B.; Kim, S.; Yun, H.W.; Kim, J.H.; Min, B.H.; Kim, M.S. Development and In Vivo Assessment of an Injectable Crosslinked Cartilage Acellular Matrix-PEG Hydrogel Scaffold Derived from Porcine Cartilage for Tissue Engineering. Macromol. Biosci. 2023, e2300029. [Google Scholar] [CrossRef]
- Friend, N.E.; McCoy, A.J.; Stegemann, J.P.; Putnam, A.J. A combination of matrix stiffness and degradability dictate microvascular network assembly and remodeling in cell-laden poly(ethylene glycol) hydrogels. Biomaterials 2023, 295, 122050. [Google Scholar] [CrossRef]
- Mongia, N.K.; Anseth, K.S.; Peppas, N.A. Mucoadhesive poly(vinyl alcohol) hydrogels produced by freezing/thawing processes: Applications in the development of wound healing systems. J. Biomater. Sci. Polym. Ed. 1996, 7, 1055–1064. [Google Scholar] [CrossRef]
- Zhu, C.; Huang, C.; Zhang, W.; Ding, X.; Yang, Y. Biodegradable-Glass-Fiber Reinforced Hydrogel Composite with Enhanced Mechanical Performance and Cell Proliferation for Potential Cartilage Repair. Int. J. Mol. Sci. 2022, 23, 8717. [Google Scholar] [CrossRef]
- Singhvi, M.S.; Zinjarde, S.S.; Gokhale, D.V. Polylactic acid: Synthesis and biomedical applications. J. Appl. Microbiol. 2019, 127, 1612–1626. [Google Scholar] [CrossRef] [Green Version]
- Savioli Lopes, M.; Jardini, A.L.; Maciel Filho, R. Poly (Lactic Acid) Production for Tissue Engineering Applications. Procedia Eng. 2012, 42, 1402–1413. [Google Scholar] [CrossRef] [Green Version]
- Narayanan, G.; Vernekar, V.N.; Kuyinu, E.L.; Laurencin, C.T. Poly (lactic acid)-based biomaterials for orthopaedic regenerative engineering. Adv. Drug Deliv. Rev. 2016, 107, 247–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sekine, H.; Shimizu, T.; Sakaguchi, K.; Dobashi, I.; Wada, M.; Yamato, M.; Kobayashi, E.; Umezu, M.; Okano, T. In vitro fabrication of functional three-dimensional tissues with perfusable blood vessels. Nat. Commun. 2013, 4, 1399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishida, K.; Yamato, M.; Hayashida, Y.; Watanabe, K.; Yamamoto, K.; Adachi, E.; Nagai, S.; Kikuchi, A.; Maeda, N.; Watanabe, H.; et al. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N. Engl. J. Med. 2004, 351, 1187–1196. [Google Scholar] [CrossRef] [Green Version]
- Ohki, T.; Yamato, M.; Murakami, D.; Takagi, R.; Yang, J.; Namiki, H.; Okano, T.; Takasaki, K. Treatment of oesophageal ulcerations using endoscopic transplantation of tissue-engineered autologous oral mucosal epithelial cell sheets in a canine model. Gut 2006, 55, 1704–1710. [Google Scholar] [CrossRef] [Green Version]
- Ohki, T.; Yamato, M.; Ota, M.; Takagi, R.; Murakami, D.; Kondo, M.; Sasaki, R.; Namiki, H.; Okano, T.; Yamamoto, M. Prevention of esophageal stricture after endoscopic submucosal dissection using tissue-engineered cell sheets. Gastroenterology 2012, 143, 582–588.e2. [Google Scholar] [CrossRef] [PubMed]
- Iwata, T.; Yamato, M.; Tsuchioka, H.; Takagi, R.; Mukobata, S.; Washio, K.; Okano, T.; Ishikawa, I. Periodontal regeneration with multi-layered periodontal ligament-derived cell sheets in a canine model. Biomaterials 2009, 30, 2716–2723. [Google Scholar] [CrossRef] [PubMed]
- Takushi, E.; Asato, L.; Nakada, T. Edible eyeballs from fish. Nature 1990, 345, 298–299. [Google Scholar] [CrossRef]
- Takezawa, T.; Nitani, A.; Shimo-Oka, T.; Takayama, Y. A protein-permeable scaffold of a collagen vitrigel membrane useful for reconstructing crosstalk models between two different cell types. Cells Tissues Organs 2007, 185, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Puleo, C.M.; Ambrose, W.M.; Takezawa, T.; Elisseeff, J.; Wang, T.H. Integration and application of vitrified collagen in multilayered microfluidic devices for corneal microtissue culture. Lab Chip 2009, 9, 3221–3227. [Google Scholar] [CrossRef]
- Zhao, J.; Shinkai, M.; Takezawa, T.; Ohba, S.; Chung, U.I.; Nagamune, T. Bone regeneration using collagen type I vitrigel with bone morphogenetic protein-2. J. Biosci. Bioeng. 2009, 107, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, J.; Oshikata-Miyazaki, A.; Yokoo, S.; Yamagami, S.; Takezawa, T.; Amano, S. Development and evaluation of porcine atelocollagen vitrigel membrane with a spherical curve and transplantable artificial corneal endothelial grafts. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4975–4981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chae, J.J.; Ambrose, W.M.; Espinoza, F.A.; Mulreany, D.G.; Ng, S.; Takezawa, T.; Trexler, M.M.; Schein, O.D.; Chuck, R.S.; Elisseeff, J.H. Regeneration of corneal epithelium utilizing a collagen vitrigel membrane in rabbit models for corneal stromal wound and limbal stem cell deficiency. Acta Ophthalmol. 2015, 93, e57–e66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okuyama, H.; Ohnishi, H.; Nakamura, R.; Yamashita, M.; Kishimoto, Y.; Tateya, I.; Suehiro, A.; Gotoh, S.; Takezawa, T.; Nakamura, T.; et al. Transplantation of multiciliated airway cells derived from human iPS cells using an artificial tracheal patch into rat trachea. J. Tissue Eng. Regen. Med. 2019, 13, 1019–1030. [Google Scholar] [CrossRef]
- Chae, J.J.; Mulreany, D.G.; Guo, Q.; Lu, Q.; Choi, J.S.; Strehin, I.; Espinoza, F.A.; Schein, O.; Trexler, M.M.; Bower, K.S.; et al. Application of a collagen-based membrane and chondroitin sulfate-based hydrogel adhesive for the potential repair of severe ocular surface injuries. Mil. Med. 2014, 179, 686–694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Kanie, K.; Takezawa, T.; Horikawa, M.; Kaneko, K.; Sugimoto, A.; Yamawaki-Ogata, A.; Narita, Y.; Kato, R. Bi-layered carboxymethyl cellulose-collagen vitrigel dual-surface adhesion-prevention membrane. Carbohydr. Polym. 2022, 285, 119223. [Google Scholar] [CrossRef] [PubMed]
- Zamprogno, P.; Thoma, G.; Cencen, V.; Ferrari, D.; Putz, B.; Michler, J.; Fantner, G.E.; Guenat, O.T. Mechanical Properties of Soft Biological Membranes for Organ-on-a-Chip Assessed by Bulge Test and AFM. ACS Biomater. Sci. Eng. 2021, 7, 2990–2997. [Google Scholar] [CrossRef]
- Stacey, G.N.; Day, J.G. Long-term Ex Situ conservation of biological resources and the role of biological resource centers. In Cryopreservation and Freeze-Drying Protocols, 2nd ed.; Day, J.G., Stacey, G.N., Eds.; Humana Press: Totowa, NJ, USA, 2007; pp. 1–14. [Google Scholar]
- Mazur, P. Kinetics of Water Loss from Cells at Subzero Temperatures and the Likelihood of Intracellular Freezing. J. Gen. Physiol. 1963, 47, 347–369. [Google Scholar] [CrossRef]
- Mazur, P.; Leibo, S.P.; Chu, E.H. A two-factor hypothesis of freezing injury. Evidence from Chinese hamster tissue-culture cells. Exp. Cell Res. 1972, 71, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Pegg, D.E. Principles of cryopreservation. In Cryopreservation and Freeze-Drying Protocols, 2nd ed.; Day, J.G., Stacey, G.N., Eds.; Humana Press: Totowa, NJ, USA, 2007; pp. 39–57. [Google Scholar]
- Taylor, M.J. Biology of cell survival in the cold: The basis for biopreservation of tissues and organs. In Advances in Biopreservation; Baust, J.G., Baust, J.M., Eds.; CRC Press: New York, NY, USA; Taylor and Francis: New York, NY, USA, 2007; pp. 15–62. [Google Scholar]
- Baust, J.G. Concepts in biopreservation. In Advances in Biopreservation; Baust, J.G., Baust, J.M., Eds.; CRC Press: New York, NY, USA; Taylor and Francis: New York, NY, USA, 2007; pp. 1–14. [Google Scholar]
- Acker, J.P. The use of intracellular protectants in cell biopreservation. In Advances in Biopreservation; Baust, J.G., Baust, J.M., Eds.; CRC Press: New York, NY, USA; Taylor and Francis: New York, NY, USA, 2007; pp. 299–320. [Google Scholar]
- Polge, C.; Smith, A.U.; Parkes, A.S. Revival of spermatozoa after vitrification and dehydration at low temperatures. Nature 1949, 164, 666. [Google Scholar] [CrossRef]
- Lovelock, J.E.; Bishop, M.W. Prevention of freezing damage to living cells by dimethyl sulphoxide. Nature 1959, 183, 1394–1395. [Google Scholar] [CrossRef]
- Morris, C.B. Cryopreservation of animal and human cell lines. In Cryopreservation and Freeze-Drying Protocols, 2nd ed.; Day, J.G., Stacey, G.N., Eds.; Humana Press: Totowa, NJ, USA, 2007; pp. 227–236. [Google Scholar]
- U.S. Food and Drug Administration (FDA). Cellular & Gene Therapy Guidances. Available online: https://www.fda.gov/vaccines-blood-biologics/biologics-guidances/cellular-gene-therapy-guidances (accessed on 6 December 2022).
- The Pharmaceuticals and Medical Devices Agency (PMDA). Review Reports: Regenerative Medical Products. Available online: https://www.pmda.go.jp/english/review-services/reviews/approved-information/0004.html (accessed on 6 December 2022).
- The European Medicines Agency (EMA). Human Cell-Based Medicinal Products—Scientific Guideline. Available online: https://www.ema.europa.eu/en/human-cell-based-medicinal-products-scientific-guideline (accessed on 6 December 2022).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ (accessed on 6 December 2022).
- Yuan, C.; Gao, J.; Guo, J.; Bai, L.; Marshall, C.; Cai, Z.; Wang, L.; Xiao, M. Dimethyl sulfoxide damages mitochondrial integrity and membrane potential in cultured astrocytes. PLoS ONE 2014, 9, e107447. [Google Scholar] [CrossRef] [Green Version]
- Miyamoto, Y.; Teramoto, N.; Hayashi, S.; Enosawa, S. An improvement in the attaching capability of cryopreserved human hepatocytes by a proteinaceous high molecule, sericin, in the serum-free solution. Cell Transplant. 2010, 19, 701–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyamoto, Y.; Suzuki, S.; Nomura, K.; Enosawa, S. Improvement of hepatocyte viability after cryopreservation by supplementation of long-chain oligosaccharide in the freezing medium in rats and humans. Cell Transplant. 2006, 15, 911–919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyamoto, Y.; Oishi, K.; Yukawa, H.; Noguchi, H.; Sasaki, M.; Iwata, H.; Hayashi, S. Cryopreservation of human adipose tissue-derived stem/progenitor cells using the silk protein sericin. Cell Transplant. 2012, 21, 617–622. [Google Scholar] [CrossRef]
- Yamatoya, K.; Nagai, Y.; Teramoto, N.; Kang, W.; Miyado, K.; Nakata, K.; Yagi, T.; Miyamoto, Y. Cryopreservation of undifferentiated and differentiated human neuronal cells. Regen. Ther. 2022, 19, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.J. Sub-zero preservation and the prospect of long-term storage of multicellular tissues and organs. In Transplantation Immunology: Clinical and Experimental; Calne, R.Y., Ed.; Oxford University Press: Oxford, UK, 1984; pp. 360–390. [Google Scholar]
- Brockbank, K.G.M.; Taylor, M.J. Tissue preservation. In Advances in Biopreservation; Baust, J.G., Baust, J.M., Eds.; CRC Press: New York, NY, USA; Taylor and Francis: New York, NY, USA, 2007; pp. 157–196. [Google Scholar]
- Taylor, M.J.; Song, Y.C.; Brockbank, K.G.M. Vitrification in tissue preservation: New developments. In Life in the Frozen State; Fuller, B.J., Lane, N., Benson, E.E., Eds.; CRC Press: London, UK; Taylor and Francis: London, UK, 2004; pp. 603–641. [Google Scholar]
- Karlsson, J.O.; Toner, M. Long-term storage of tissues by cryopreservation: Critical issues. Biomaterials 1996, 17, 243–256. [Google Scholar] [CrossRef]
- Takahashi, T.; Hirsh, A.; Erbe, E.F.; Bross, J.B.; Steere, R.L.; Williams, R.J. Vitrification of human monocytes. Cryobiology 1986, 23, 103–115. [Google Scholar] [CrossRef]
- Jutte, N.H.; Heyse, P.; Jansen, H.G.; Bruining, G.J.; Zeilmaker, G.H. Vitrification of mouse islets of Langerhans: Comparison with a more conventional freezing method. Cryobiology 1987, 24, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Jutte, N.H.; Heyse, P.; Jansen, H.G.; Bruining, G.J.; Zeilmaker, G.H. Vitrification of human islets of Langerhans. Cryobiology 1987, 24, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Van Wagtendonk-De Leeuw, A.M.; Den Daas, J.H.; Kruip, T.A.; Rall, W.F. Comparison of the efficacy of conventional slow freezing and rapid cryopreservation methods for bovine embryos. Cryobiology 1995, 32, 157–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohlendorf, C.; Tomford, W.W.; Mankin, H.J. Chondrocyte survival in cryopreserved osteochondral articular cartilage. J. Orthop. Res. 1996, 14, 413–416. [Google Scholar] [CrossRef] [PubMed]
- Tomford, W.W.; Fredericks, G.R.; Mankin, H.J. Studies on cryopreservation of articular cartilage chondrocytes. J. Bone Jt. Surg. Am. 1984, 66, 253–259. [Google Scholar] [CrossRef]
- Song, Y.C.; An, Y.H.; Kang, Q.K.; Li, C.; Boggs, J.M.; Chen, Z.; Taylor, M.J.; Brockbank, K.G. Vitreous preservation of articular cartilage grafts. J. Investig. Surg. 2004, 17, 65–70. [Google Scholar] [CrossRef]
- Chong, Y.K.; Toh, T.B.; Zaiden, N.; Poonepalli, A.; Leong, S.H.; Ong, C.E.; Yu, Y.; Tan, P.B.; See, S.J.; Ng, W.H.; et al. Cryopreservation of neurospheres derived from human glioblastoma multiforme. Stem Cells 2009, 27, 29–39. [Google Scholar] [CrossRef] [Green Version]
- Spurrier, R.G.; Speer, A.L.; Grant, C.N.; Levin, D.E.; Grikscheit, T.C. Vitrification preserves murine and human donor cells for generation of tissue-engineered intestine. J. Surg. Res. 2014, 190, 399–406. [Google Scholar] [CrossRef]
- Mukherjee, N.; Chen, Z.; Sambanis, A.; Song, Y. Effects of cryopreservation on cell viability and insulin secretion in a model tissue-engineered pancreatic substitute (TEPS). Cell Transplant. 2005, 14, 449–456. [Google Scholar] [CrossRef] [Green Version]
- Kearney, J.N. Guidelines on processing and clinical use of skin allografts. Clin. Dermatol. 2005, 23, 357–364. [Google Scholar] [CrossRef]
- Kinoshita, S.; Nakamura, T. Development of cultivated mucosal epithelial sheet transplantation for ocular surface reconstruction. Artif. Organs 2004, 28, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Kito, K.; Kagami, H.; Kobayashi, C.; Ueda, M.; Terasaki, H. Effects of cryopreservation on histology and viability of cultured corneal epithelial cell sheets in rabbit. Cornea 2005, 24, 735–741. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, W.; Wu, W.; Jin, Y.; Cen, L.; Kretlow, J.D.; Gao, W.; Dai, Z.; Wang, J.; Zhou, G.; et al. Cryopreservation of tissue-engineered epithelial sheets in trehalose. Biomaterials 2011, 32, 8426–8435. [Google Scholar] [CrossRef] [PubMed]
- Oliva, J.; Florentino, A.; Bardag-Gorce, F.; Niihara, Y. Vitrification and storage of oral mucosa epithelial cell sheets. J. Tissue Eng. Regen. Med. 2019, 13, 1153–1163. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Shao, J.; Zhuang, J.; Zhou, S.; Yin, S.; Wu, F.; Hou, J.; Wang, X. Biobanked human foreskin epithelial cell sheets reduce inflammation and promote wound healing in a nude mouse model. BMC Biotechnol. 2021, 21, 11. [Google Scholar] [CrossRef]
- Maehara, M.; Sato, M.; Watanabe, M.; Matsunari, H.; Kokubo, M.; Kanai, T.; Sato, M.; Matsumura, K.; Hyon, S.H.; Yokoyama, M.; et al. Development of a novel vitrification method for chondrocyte sheets. BMC Biotechnol. 2013, 13, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, A.; Maehara, M.; Uchikura, A.; Matsunari, H.; Matsumura, K.; Hyon, S.H.; Sato, M.; Nagashima, H. Development of an efficient vitrification method for chondrocyte sheets for clinical application. Regen. Ther. 2020, 14, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Ohkawara, H.; Miyagawa, S.; Fukushima, S.; Yajima, S.; Saito, A.; Nagashima, H.; Sawa, Y. Development of a vitrification method for preserving human myoblast cell sheets for myocardial regeneration therapy. BMC Biotechnol. 2018, 18, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ochiai, J.; Niihara, Y.; Oliva, J. Measurement of the Adipose Stem Cells Cell Sheets Transmittance. Bioengineering 2021, 8, 93. [Google Scholar] [CrossRef]
- Li, M.; Feng, C.; Gu, X.; He, Q.; Wei, F. Effect of cryopreservation on proliferation and differentiation of periodontal ligament stem cell sheets. Stem Cell Res. Ther. 2017, 8, 77. [Google Scholar] [CrossRef] [Green Version]
- Motoike, S.; Kajiya, M.; Komatsu, N.; Takewaki, M.; Horikoshi, S.; Matsuda, S.; Ouhara, K.; Iwata, T.; Takeda, K.; Fujita, T.; et al. Cryopreserved clumps of mesenchymal stem cell/extracellular matrix complexes retain osteogenic capacity and induce bone regeneration. Stem Cell Res. Ther. 2018, 9, 73. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, K.; Mukobata, S.; Utoh, R.; Yamashita, S.; Masuda, T.; Sakai, H.; Okano, T. Production of islet cell sheets using cryopreserved islet cells. Transplant. Proc. 2011, 43, 3188–3191. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Akahane, M.; Ueha, T.; Kido, A.; Omokawa, S.; Kobata, Y.; Murata, K.; Kawate, K.; Tanaka, Y. Osteogenesis of cryopreserved osteogenic matrix cell sheets. Cryobiology 2013, 66, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Ike, S.; Ueno, K.; Yanagihara, M.; Mizoguchi, T.; Harada, T.; Suehiro, K.; Kurazumi, H.; Suzuki, R.; Kondo, T.; Murata, T.; et al. Cryopreserved allogenic fibroblast sheets: Development of a promising treatment for refractory skin ulcers. Am. J. Transl. Res. 2022, 14, 3879–3892. [Google Scholar]
- Sheikhi, M.; Hultenby, K.; Niklasson, B.; Lundqvist, M.; Hovatta, O. Clinical grade vitrification of human ovarian tissue: An ultrastructural analysis of follicles and stroma in vitrified tissue. Hum. Reprod. 2011, 26, 594–603. [Google Scholar] [CrossRef]
- Marco-Jimenez, F.; Garcia-Dominguez, X.; Jimenez-Trigos, E.; Vera-Donoso, C.D.; Vicente, J.S. Vitrification of kidney precursors as a new source for organ transplantation. Cryobiology 2015, 70, 278–282. [Google Scholar] [CrossRef]
- Li, T.; Zhou, C.; Liu, C.; Mai, Q.; Zhuang, G. Bulk vitrification of human embryonic stem cells. Hum. Reprod. 2008, 23, 358–364. [Google Scholar] [CrossRef] [Green Version]
- Miyamoto, Y.; Enosawa, S.; Takeuchi, T.; Takezawa, T. Cryopreservation in situ of cell monolayers on collagen vitrigel membrane culture substrata: Ready-to-use preparation of primary hepatocytes and ES cells. Cell Transplant. 2009, 18, 619–626. [Google Scholar] [CrossRef]
- Martinez-Garcia, F.D.; Fischer, T.; Hayn, A.; Mierke, C.T.; Burgess, J.K.; Harmsen, M.C. A Beginner's Guide to the Characterization of Hydrogel Microarchitecture for Cellular Applications. Gels 2022, 8, 535. [Google Scholar] [CrossRef]
- Revete, A.; Aparicio, A.; Cisterna, B.A.; Revete, J.; Luis, L.; Ibarra, E.; Segura González, E.A.; Molino, J.; Reginensi, D. Advancements in the Use of Hydrogels for Regenerative Medicine: Properties and Biomedical Applications. Int. J. Biomater. 2022, 2022, 3606765. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, Y.; Zhang, L.; Wu, L.; Chen, Y.; Xie, D.; Chen, W. Hydrogel Cryopreservation System: An Effective Method for Cell Storage. Int. J. Mol. Sci. 2018, 19, 3330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manferdini, C.; Gabusi, E.; Saleh, Y.; Lenzi, E.; D'Atri, G.; Ricotti, L.; Lisignoli, G. Mesenchymal Stromal Cells Laden in Hydrogels for Osteoarthritis Cartilage Regeneration: A Systematic Review from In Vitro Studies to Clinical Applications. Cells 2022, 11, 3969. [Google Scholar] [CrossRef] [PubMed]
- Campbell, L.H.; Brockbank, K.G.M. Development of a Vitrification Preservation Process for Bioengineered Epithelial Constructs. Cells 2022, 11, 1115. [Google Scholar] [CrossRef] [PubMed]

| Cells | Origin | Species | Sheet Preparation | In Vivo Test | Year of Publication | Reference |
|---|---|---|---|---|---|---|
| Epithelial cells | Oral mucosa | Rabbit | Oral mucosal epithelium cells were cultured with MMC-treated NIH/3T3 feeder cells. | YES | 2019 | [129] |
| Epithelial cells | Foreskin keratinocytes | Human | Human epithelial cell sheets (ECSs) were cultured on plastic dishes. | YES | 2021 | [130] |
| Chondrocytes | Knee cartilage | Rabbit | Primary cultured cells derived from the knee cartilage were plated onto temperature-responsive culture dishes (UpCell®, CellSeed). | 2020 | [132] | |
| Myoblast cells | Vastus medialis muscle | Human | Cell sheets consisting of myoblast cells were prepared using temperature-responsive culture dishes (UpCell®, CellSeed). | YES | 2018 | [133] |
| Mesenchymal stem cells (MSCs) | The bone marrow of femurs | Rat | Cell sheets consisting of mesenchymal stem cells were prepared using temperature-responsive culture dishes. | YES | 2018 | [136] |
| Fibroblasts | Tail skin | Mouse | Primary fibroblasts were inoculated on plastic dishes | YES | 2022 | [139] |
| Hepatocytes | Liver | Rat | Primary hepatocytes were inoculated onto collagen vitrigel membranes. | 2009 | [143] | |
| Embryonic stem cells (ESCs) | Fertilized egg | Mouse | 1st step: Primary mouse embryonic fibroblasts (MEF feeder cells) were inoculated onto UV-irradiated collagen vitrigels. 2nd step: A mouse ES cell culture was performed with mitomycin C-treated MEF feeder cell layers. | 2009 | [143] | |
| Embryonic fibroblasts | Fetal tissues | Mouse | Primary mouse embryonic fibroblasts (MEF feeder cells) were inoculated onto collagen vitrigel membranes. | 2009 | [143] |
| Cells | Freezing Procedure | Thawing Procedure | Reference |
|---|---|---|---|
| Epithelial cells | Vitrification of epithelial cell sheets Vitrification Procedures (1, and 2) in solutions Vitrification Procedures (3) in bulk The plastic containers were placed in the liquid nitrogen freezer | Stepwise The cell sheets were thawed in four steps using the solutions (culture media, culture media supplemented with 0.2, and 0.1 M sucrose) at 37 °C. | [129] |
| Epithelial cells | Programmed freezing Epithelial cells were cryopreserved for storage in KGM and 10 μM Y27632 at 4 °C. Or cryochamber (Planer KRYO 10 Series III Freezer, UK) was run by the pre-set cooling program. | Rapid thawing Thawing was carried out rapidly by holding in air for 1 min to boil off any liquid nitrogen and swirling in a water bath at 40 °C. | [130] |
| Chondrocytes | Vitrification of the chondrocyte sheets The chondrocyte sheets in the circulating vitrification bag were held 1 cm above the surface of liquid nitrogen vapor (approximately −150 °C). | Rapid thawing The circulating vitrification bag was placed on a heating plate at 45 °C. The gel was placed on top of the cell sheet to thaw it rapidly. | [132] |
| Myoblast cells | Vitrification of the myoblast sheets The myoblast sheets were detached from the dish at 22 °C. The cell sheets were placed in thin polyethylene films and held 1 cm above the surface of liquid nitrogen vapor. | Rapid thawing Thin polyethylene films was placed on a heating plate at 37 °C. The gel was placed on top of the cell sheet to thaw it rapidly. | [133] |
| Mesenchymal stem cells (MSCs) | Slow freezing MSCs sheets were placed directly into a deep-freezer set at −80 °C. | Rapid thawing MSCs sheets were placed in a 37 °C water bath for rapid thawing until almost no ice was detectable. | [136] |
| Fibroblasts | Stepwise The fibroblasts sheets were placed into a 3D freezer (Koga Sangyo Co., Ltd.) at −35 °C for 20 min to freeze the cells and then transferred to a −80 °C deep freezer. | Rapid thawing The fibroblasts sheets were placed in a 37 °C water bath for rapid thawing until almost no ice was detectable. | [139] |
| Hepatocytes | Programmed freezing The hepatocytes sheets were placed in a controlled rate freezer (Kryo10, Planer) and frozen at a rate of 1 °C/min [108]. | Rapid thawing To thaw the cells, 2 mL of culture medium warmed to 37 °C was added. | [143] |
| Embryonic stem cells (ESCs) | Programmed freezing The ESCs sheets were placed in a controlled rate freezer (Kryo10, Planer) and frozen at a rate of 1 °C/min [108]. | Rapid thawing To thaw the cells, 2 mL of culture medium warmed to 37 °C was added. | [143] |
| Embryonic fibroblast | Programmed freezing The embryonic fibroblast sheets were placed in a controlled rate freezer (Kryo10, Planer) and frozen at a rate of 1 °C/min [108]. | Rapid thawing To thaw the cells, 2 mL of culture medium warmed to 37 °C was added. | [143] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyamoto, Y. Cryopreservation of Cell Sheets for Regenerative Therapy: Application of Vitrified Hydrogel Membranes. Gels 2023, 9, 321. https://doi.org/10.3390/gels9040321
Miyamoto Y. Cryopreservation of Cell Sheets for Regenerative Therapy: Application of Vitrified Hydrogel Membranes. Gels. 2023; 9(4):321. https://doi.org/10.3390/gels9040321
Chicago/Turabian StyleMiyamoto, Yoshitaka. 2023. "Cryopreservation of Cell Sheets for Regenerative Therapy: Application of Vitrified Hydrogel Membranes" Gels 9, no. 4: 321. https://doi.org/10.3390/gels9040321
APA StyleMiyamoto, Y. (2023). Cryopreservation of Cell Sheets for Regenerative Therapy: Application of Vitrified Hydrogel Membranes. Gels, 9(4), 321. https://doi.org/10.3390/gels9040321