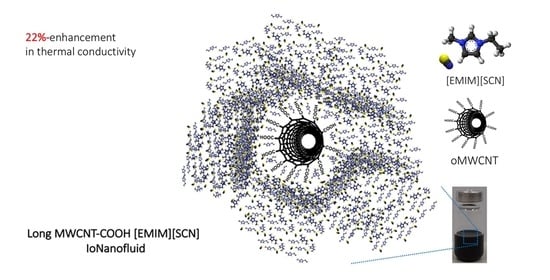
Thermophysical Properties of IoNanofluids Composed of 1-ethyl-3-methylimidazolium Thiocyanate and Carboxyl-functionalized Long Multi-walled Carbon Nanotubes
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Thermal Conductivity
3.2. Viscosity
3.3. Density
4. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nieto de Castro, C.A.; Murshed, S.M.S.; Lourenço, M.J.V.; Santos, F.J.V.; Lopes, M.L.M.; França, J.M.P. Enhanced Thermal Conductivity and Specific Heat Capacity of Carbon Nanotubes Ionanofluids. Int. J. Therm. Sci. 2012, 62, 34–39. [Google Scholar] [CrossRef]
- Murshed, S.M.S.; De Castro, C.N. Superior Thermal Features of Carbon Nanotubes-Based Nanofluids—A Review. Renew. Sustain. Energy Rev. 2014, 37, 155–167. [Google Scholar] [CrossRef]
- França, J.M.P.; Lourenço, M.J.V.; Murshed, S.M.S.; Pádua, A.A.H.; Nieto de Castro, C.A. Thermal Conductivity of Ionic Liquids and IoNanofluids and Their Feasibility as Heat Transfer Fluids. Ind. Eng. Chem. Res. 2018, 57, 6516–6529. [Google Scholar] [CrossRef]
- Oster, K.; Hardacre, C.; Jacquemin, J.; Ribeiro, A.P.C.; Elsinawi, A. Ionic Liquid-Based Nanofluids (Ionanofluids) for Thermal Applications: An Experimental Thermophysical Characterization. Pure Appl. Chem. 2019, 91, 1309–1340. [Google Scholar] [CrossRef]
- Jóźwiak, B.; Dzido, G.; Zorȩbski, E.; Kolanowska, A.; Jȩdrysiak, R.; Dziadosz, J.; Libera, M.; Boncel, S.; Dzida, M. Remarkable Thermal Conductivity Enhancement in Carbon-Based Ionanofluids: Effect of Nanoparticle Morphology. ACS Appl. Mater. Interfaces 2020, 12, 38113–38123. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.P.C.; Vieira, S.I.C.; Goodrich, P.; Hardacre, C.; Lourenço, M.J.V.; De Castro, C.A. Thermal Conductivity of [Cnmim][(CF3SO2)2N] and [C4mim][BF4] IoNanofluids with Carbon Nanotubes—Measurement, Theory and Structural Characterization. J. Nanofluids 2013, 2, 55–62. [Google Scholar] [CrossRef]
- Ferreira, A.G.M.; Simões, P.N.; Ferreira, A.F.; Fonseca, M.A.; Oliveira, M.S.A.; Trino, A.S.M. Transport and Thermal Properties of Quaternary Phosphonium Ionic Liquids and IoNanofluids. J. Chem. Thermodyn. 2013, 64, 80–92. [Google Scholar] [CrossRef]
- Neo, C.Y.; Ouyang, J. Functionalized Carbon Nanotube-Induced Viscosity Reduction of an Ionic Liquid and Performance Improvement of Dye-Sensitized Solar Cells. Electrochim. Acta 2012, 85, 1–8. [Google Scholar] [CrossRef]
- Seki, S.; Tsuzuki, S.; Hayamizu, K.; Umebayashi, Y.; Serizawa, N.; Takei, K.; Miyashiro, H. Comprehensive Refractive Index Property for Room-Temperature Ionic Liquids. J. Chem. Eng. Data 2012, 57, 2211–2216. [Google Scholar] [CrossRef]
- Ficke, L.E.; Novak, R.R.; Brennecke, J.F. Thermodynamic and Thermophysical Properties of Ionic Liquid + Water Systems. J. Chem. Eng. Data 2010, 55, 4946–4950. [Google Scholar] [CrossRef]
- Rabari, D.; Patel, N.; Joshipura, M.; Banerjee, T. Densities of Six Commercial Ionic Liquids: Experiments and Prediction Using a Cohesion Based Cubic Equation of State. J. Chem. Eng. Data 2014, 59, 571–578. [Google Scholar] [CrossRef]
- Freire, M.G.; Teles, A.R.R.; Rocha, M.A.A.; Schröder, B.; Neves, C.M.S.S.; Carvalho, P.J.; Evtuguin, D.V.; Santos, L.M.N.B.F.; Coutinho, J.A.P. Thermophysical Characterization of Ionic Liquids Able To Dissolve Biomass. J. Chem. Eng. Data 2011, 56, 4813–4822. [Google Scholar] [CrossRef]
- Eves, C.M.S.S.; Adi Kurnia, K.; Coutinho, J.A.P.; Marrucho, I.M.; Canongia Lopes, J.N.; Freire, M.G.; Rebelo, L.P.N. Systematic Study of the Thermophysical Properties of Imidazolium-Based Ionic Liquids with Cyano-Functionalized Anions. J. Phys. Chem. B 2013, 117, 10271–10283. [Google Scholar] [CrossRef]
- Zorębski, E.; Musiał, M.; Bałuszyńska, K.; Zorębski, M.; Dzida, M. Isobaric and Isochoric Heat Capacities as Well as Isentropic and Isothermal Compressibilities of Di- and Trisubstituted Imidazolium-Based Ionic Liquids as a Function of Temperature. Ind. Eng. Chem. Res. 2018, 57, 5161–5172. [Google Scholar] [CrossRef]
- Vataščin, E.; Dohnal, V. Thermodynamic Properties of Aqueous Solutions of [EMIM] Thiocyanate and [EMIM] Dicyanamide. J. Chem. Thermodyn. 2017, 106, 262–275. [Google Scholar] [CrossRef]
- Wang, M.; He, L.; Ferreira, I.C.A. Ammonia Absorption in Ionic Liquids-based Mixtures in Plate Heat Exchangers Studied by a Semi-empirical Heat and Mass Transfer Framework. Int. J. Heat Mass Transf. 2019, 134, 1302–1317. [Google Scholar] [CrossRef]
- Larriba, M.; Navarro, P.; García, J.; Rodríguez, F. Selective Extraction of Toluene from n-Heptane Using [emim][SCN] and [bmim][SCN] Ionic Liquids as Solvents. J. Chem. Thermodyn. 2014, 79, 266–271. [Google Scholar] [CrossRef]





| This Work | Literature | |
|---|---|---|
| ρ (kg⋅m−3) | 1116.1 ± 0.1 | 1115.20 [5], 1115.5 [9], 1115.9 [10,11], 1117 [12,13], 1122.26 [14] |
| η (mPa·s) | 22.70 ± 3.9% | 22.68 [15], 22.64 [5], 23.77 [16], 23.79 [17], 24.50 [12] |
| λ (W·m−1·K−1) | 0.179 ± 5% | 0.177 [5], 0.184 [3] |
| Name | Average Length (μm) | Average Diameter (nm) | Aspect Ratio (-) | Specific Surface Area (m2⋅g−1) | COOH Content (mmol⋅g−1) | OH Content (mmol⋅g−1) |
|---|---|---|---|---|---|---|
| in-house 24 h oMWCNTs | <500 | ~50 | 10,000 | >22 | 1.0 | 0.4 |
| T (°C) | λ (W·m−1·K−1) | |||||||
|---|---|---|---|---|---|---|---|---|
| Series 1 | Series 2 | Series 3 | Mean | Series 1 | Series 2 | Series 3 | Mean | |
| [C2C1im][SCN] | 0.1 wt% in-house 24 h oMWCNTs + [C2C1im][SCN] | |||||||
| 25 | 0.178 | 0.179 | 0.179 | 0.179 | 0.184 | 0.184 | 0.184 | 0.184 |
| 30 | 0.178 | 0.178 | 0.179 | 0.178 | 0.184 | 0.184 | 0.184 | 0.184 |
| 35 | 0.178 | 0.178 | 0.178 | 0.178 | 0.184 | 0.185 | 0.184 | 0.184 |
| 40 | 0.177 | 0.177 | 0.176 | 0.177 | 0.183 | 0.183 | 0.184 | 0.183 |
| 45 | 0.177 | 0.177 | 0.177 | 0.177 | 0.182 | 0.183 | 0.183 | 0.183 |
| 50 | 0.176 | 0.176 | 0.176 | 0.176 | 0.182 | 0.181 | 0.181 | 0.181 |
| 0.005 wt% in-house 24 h oMWCNTs + [C2C1im][SCN] | 0.5 wt% in-house 24 h oMWCNTs + [C2C1im][SCN] | |||||||
| 25 | 0.179 | 0.180 | 0.179 | 0.179 | 0.200 | 0.199 | 0.200 | 0.200 |
| 30 | 0.179 | 0.179 | 0.180 | 0.179 | 0.200 | 0.200 | 0.200 | 0.200 |
| 35 | 0.179 | 0.179 | 0.179 | 0.179 | 0.199 | 0.199 | 0.200 | 0.199 |
| 40 | 0.179 | 0.178 | 0.178 | 0.178 | 0.199 | 0.199 | 0.199 | 0.199 |
| 45 | 0.177 | 0.178 | 0.177 | 0.177 | 0.199 | 0.199 | 0.197 | 0.198 |
| 50 | 0.176 | 0.176 | 0.177 | 0.176 | 0.198 | 0.196 | 0.197 | 0.197 |
| 0.01 wt% in-house 24 h oMWCNTs + [C2C1im][SCN] | 1 wt% in-house 24 h oMWCNTs + [C2C1im][SCN] | |||||||
| 25 | 0.180 | 0.180 | 0.180 | 0.180 | 0.220 | 0.217 | 0.218 | 0.218 |
| 30 | 0.180 | 0.180 | 0.180 | 0.180 | 0.217 | 0.218 | 0.217 | 0.217 |
| 35 | 0.180 | 0.180 | 0.179 | 0.180 | 0.217 | 0.217 | 0.218 | 0.217 |
| 40 | 0.179 | 0.179 | 0.179 | 0.179 | 0.217 | 0.217 | 0.217 | 0.217 |
| 45 | 0.179 | 0.178 | 0.178 | 0.178 | 0.216 | 0.217 | 0.217 | 0.217 |
| 50 | 0.178 | 0.178 | 0.176 | 0.177 | 0.216 | 0.217 | 0.215 | 0.216 |
| 0.05 wt% in-house 24 h oMWCNTs + [C2C1im][SCN] | ||||||||
| 25 | 0.182 | 0.182 | 0.182 | 0.182 | ||||
| 30 | 0.183 | 0.182 | 0.182 | 0.182 | ||||
| 35 | 0.182 | 0.181 | 0.182 | 0.182 | ||||
| 40 | 0.181 | 0.181 | 0.181 | 0.181 | ||||
| 45 | 0.181 | 0.180 | 0.180 | 0.180 | ||||
| 50 | 0.179 | 0.179 | 0.178 | 0.179 | ||||
| T (°C) | ρ (kg·m−3) | T (°C) | ρ (kg·m−3) | T (°C) | ρ (kg·m−3) |
|---|---|---|---|---|---|
| [C2C1im][SCN] | 0.01 wt% in-house 24 h oMWCNTs + [C2C1im][SCN] | 0.1 wt% in-house 24 h oMWCNTs + [C2C1im][SCN] | |||
| 25 | 1116.10 | 25 | 1116.37 | 25 | 1116.89 |
| 30 | 1113.05 | 30 | 1113.33 | 30 | 1113.85 |
| 35 | 1110.02 | 35 | 1110.29 | 35 | 1110.82 |
| 40 | 1107.00 | 40 | 1107.27 | 40 | 1107.80 |
| 45 | 1104.01 | 45 | 1104.27 | 45 | 1104.82 |
| 50 | 1101.02 | 50 | 1101.28 | 50 | 1101.81 |
| 0.005 wt% in-house 24 h oMWCNTs + [C2C1im][SCN] | 0.05 wt% in-house 24 h oMWCNTs + [C2C1im][SCN] | 0.5 wt% in-house 24 h oMWCNTs + [C2C1im][SCN] | |||
| 25 | 1116.11 | 25 | 1116.61 | 25 | 1118.23 |
| 30 | 1113.12 | 30 | 1113.57 | 30 | 1115.18 |
| 35 | 1110.08 | 35 | 1110.54 | 35 | 1112.15 |
| 40 | 1107.06 | 40 | 1107.52 | 40 | 1109.13 |
| 45 | 1104.06 | 45 | 1104.52 | 45 | 1106.12 |
| 50 | 1101.07 | 50 | 1101.53 | 50 | 1103.13 |
| 1 wt% in-house 24 h oMWCNTs + [C2C1im][SCN] | |||||
| 25 | 1119.95 | ||||
| 30 | 1116.91 | ||||
| 35 | 1113.83 | ||||
| 40 | 1110.85 | ||||
| 45 | 1107.85 | ||||
| 50 | 1104.87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jóźwiak, B.; Dziadosz, J.; Golba, A.; Cwynar, K.; Dzido, G.; Zorębski, E.; Kolanowska, A.; Jędrysiak, R.; Gancarz, P.; Scheller, Ł.; et al. Thermophysical Properties of IoNanofluids Composed of 1-ethyl-3-methylimidazolium Thiocyanate and Carboxyl-functionalized Long Multi-walled Carbon Nanotubes. Fluids 2020, 5, 214. https://doi.org/10.3390/fluids5040214
Jóźwiak B, Dziadosz J, Golba A, Cwynar K, Dzido G, Zorębski E, Kolanowska A, Jędrysiak R, Gancarz P, Scheller Ł, et al. Thermophysical Properties of IoNanofluids Composed of 1-ethyl-3-methylimidazolium Thiocyanate and Carboxyl-functionalized Long Multi-walled Carbon Nanotubes. Fluids. 2020; 5(4):214. https://doi.org/10.3390/fluids5040214
Chicago/Turabian StyleJóźwiak, Bertrand, Justyna Dziadosz, Adrian Golba, Krzysztof Cwynar, Grzegorz Dzido, Edward Zorębski, Anna Kolanowska, Rafał Jędrysiak, Paweł Gancarz, Łukasz Scheller, and et al. 2020. "Thermophysical Properties of IoNanofluids Composed of 1-ethyl-3-methylimidazolium Thiocyanate and Carboxyl-functionalized Long Multi-walled Carbon Nanotubes" Fluids 5, no. 4: 214. https://doi.org/10.3390/fluids5040214
APA StyleJóźwiak, B., Dziadosz, J., Golba, A., Cwynar, K., Dzido, G., Zorębski, E., Kolanowska, A., Jędrysiak, R., Gancarz, P., Scheller, Ł., Boncel, S., & Dzida, M. (2020). Thermophysical Properties of IoNanofluids Composed of 1-ethyl-3-methylimidazolium Thiocyanate and Carboxyl-functionalized Long Multi-walled Carbon Nanotubes. Fluids, 5(4), 214. https://doi.org/10.3390/fluids5040214