Design and Numerical Simulation of Biomimetic Structures to Capture Particles in a Microchannel
Abstract
:1. Introduction
2. Materials and Methods
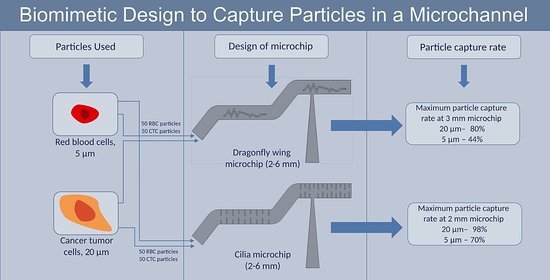
2.1. Design of a Microchip with Corrugated Dragonfly Wing Structure
2.2. Design of a Microchip with Cilia Wall Structure
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sollier, E.; Go, D.E.; Che, J.; Gossett, D.R.; O’Byrne, S.; Weaver, W.M.; Kummer, N. Size-selective collection of circulating tumor cells using Vortex technology. Lab Chip 2014, 14, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xue, C.; Chen, X.; Shan, L.; Tian, Y.; Hu, G. Size-based separation of particles and cells utilizing viscoelastic effects in straight microchannels. Anal. Chem. 2015, 87, 6041–6048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhagat, A.A.S.; Kuntaegowdanahalli, S.S.; Papautsky, I. Continuous particle separation in spiral microchannels using dean flows and differential migration. Lab Chip 2008, 8, 1906–1914. [Google Scholar] [CrossRef]
- Shiriny, A.; Bayareh, M. Inertial focusing of CTCs in a novel spiral microchannel. Chem. Eng. Sci. 2021, 229, 116102. [Google Scholar] [CrossRef]
- Hsu, C.H.; Carlo, D.D.; Chen, C.; Irimia, D.; Toner, M. Microvortex for focusing, guiding and sorting of particles. Lab Chip 2008, 8, 2128–2134. [Google Scholar] [CrossRef] [Green Version]
- Bhagat, A.A.S.; Bow, H.; Hou, H.W.; Tan, S.J.; Han, J.; Lim, C.T. Microfluidics for cell separation. Med. Biol. Eng. Comput. 2020, 48, 999–1014. [Google Scholar] [CrossRef]
- Tang, W.; Jiang, D.; Li, Z.; Zhu, L.; Shi, J.; Yang, J.; Xiang, N. Recent advances in microfluidic cell sorting techniques based on both physical and biochemical principles. Electrophoresis 2019, 40, 930–954. [Google Scholar] [CrossRef]
- Geissler, M.; Voisin, B.; Veres, T. Air stream-mediated vortex agitation of microlitre entities on a fluidic chip. Lab Chip 2011, 11, 1717–1720. [Google Scholar] [CrossRef] [Green Version]
- Haddadi, H.; Naghsh-Nilchi, H.; Carlo, D.D. Separation of cancer cells using vortical microfluidic flows. Biomicrofluidics 2018, 12, 014112. [Google Scholar] [CrossRef]
- Haller, A.; Spittler, A.; Brandhoff, L.; Zirath, H.; Puchberger-Enengl, D.; Keplinger, F.; Vellekoop, M.J. Microfluidic vortex enhancement for on-chip sample preparation. Micromachines 2015, 6, 239–251. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Liu, C.C.; Li, H. Microfluidic chip for blood cell separation and collection based on crossflow filtration. Sens. Actuators B Chem. 2008, 130, 216–221. [Google Scholar] [CrossRef]
- Xuan, X.; Zhu, J.; Church, C. Particle focusing in microfluidic devices. Microfluid. Nanofluidics 2010, 9, 1–16. [Google Scholar] [CrossRef]
- Zhou, J.; Kulasinghe, A.; Bogseth, A.; O’Byrne, K.; Punyadeera, C.; Papautsky, I. Isolation of circulating tumor cells in non-small-cell-lung-cancer patients using a multi-flow microfluidic channel. Microsyst. Nanoeng. 2019, 5, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Cao, B.; Sun, B.; Cao, Y.; Yang, K.; Lin, Y.S. Highly-sensitive capture of circulating tumor cells using micro-ellipse filters. Sci. Rep. 2017, 7, 610. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, J.; Papautsky, I. Vortex-aided inertial microfluidic device for continuous particle separation with high size-selectivity, efficiency, and purity. Biomicrofluidics 2013, 7, 044119. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.; You, H.; Gao, N.; Chang, J.; Gao, Q.; Xie, Y.; Xie, Y.; Xu, R.X. Design and fabrication of a microfluidic chip to detect tumor markers. RSC Adv. 2020, 10, 39779–39785. [Google Scholar] [CrossRef]
- Hosseini, F.; Rahimi, M. Computational fluid dynamics and experimental investigations on liquid–liquid mass transfer in T-type microchannels with different mixing channel barrier shapes. Sep. Sci. Technol. 2020, 55, 3502–3516. [Google Scholar] [CrossRef]
- Ciou, J.S. New Vortex-Based Flow Chips to Capture Particles. Master’s Thesis, Mechanical and Electromechanical Engineering, Tamkang University, Tamkang, Taiwan, 2016. [Google Scholar]
- Wang, P.L.; Ciou, J.S.; Yang, L.J.; Chung, Y.C.; Kapri, N.; Esakki, B. A new vortex-based device using dragonfly wing to reduce the chip size. In Proceedings of the 12th IEEE International Conference on Nano/Micro Engineered and Molecular Systems (NEMS), Los Angeles, CA, USA, 9–11 April 2017; pp. 81–84. [Google Scholar]
- Yang, L.J.; Kapri, N.; Waikhom, R.; Unnam, N.K. Fabrication, Aerodynamic Measurement and Performance Evaluation of Corrugated Flapping Wings. J. Aeronaut. Astronaut. Aviat 2021, 53, 83–94. [Google Scholar] [CrossRef]
- Wang, P.L. Design of a Reusable Particle-Captured Microchannel with Dual Dragonfly Wings. Master’s Thesis, Mechanical and Electromechanical Engineering, Tamkang University, Tamkang, Taiwan, 2018. [Google Scholar]
- Hao, S.J.; Wan, Y.; Xia, Y.Q.; Zou, X.; Zheng, S.Y. Size-based separation methods of circulating tumor cells. Adv. Drug Deliv. Rev. 2018, 125, 3–20. [Google Scholar] [CrossRef]
- Den Toonder, J.; Bos, F.; Broer, D.; Filippini, L.; Gillies, M.; de Goede, J.; Mol, T.; Reijme, M.; Talen, W.; Wilderbeek, H.; et al. Artificial cilia for active micro-fluidic mixing. Lab Chip 2008, 8, 533–541. [Google Scholar] [CrossRef]
- Chen, C.Y.; Lin, C.Y.; Hu, Y.T. Inducing 3D vortical flow patterns with 2D asymmetric actuation of artificial cilia for high-performance active micromixing. Exp. Fluids 2014, 55, 1765. [Google Scholar] [CrossRef]
- Chen, C.Y.; Lin, C.Y.; Hu, Y.T.; Cheng, L.Y.; Hsu, C.C. Efficient micromixing through artificial cilia actuation with fish-schooling configuration. Chem. Eng. J. 2015, 259, 391–396. [Google Scholar] [CrossRef]
- Chen, C.Y.; Hsu, C.C.; Mani, K.; Panigrahi, B. Hydrodynamic influences of artificial cilia beating behaviors on micromixing. Chem. Eng. Process. Process Intensif. 2016, 99, 33–40. [Google Scholar] [CrossRef]
- Wu, Y.A.; Panigrahi, B.; Chen, C.Y. Hydrodynamically efficient micropropulsion through a new artificial cilia beating concept. Microsyst. Technol. 2017, 23, 5893–5902. [Google Scholar] [CrossRef]
- Chen, C.Y.; Cheng, L.Y.; Hsu, C.C.; Mani, K. Microscale flow propulsion through bioinspired and magnetically actuated artificial cilia. Biomicrofluidics 2015, 9, 034105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakashima, M.; Yamada, M.; Seki, M. Pinched flow fractionation (PFF) for continuous particle separation in a microfluidic device. In Proceedings of the 17th IEEE International Conference on Micro Electro Mechanical Systems. Maastricht MEMS 2004 Technical Digest, Maastricht, The Netherlands, 25–29 January 2004; pp. 33–36. [Google Scholar]
- Unnam, N.K.; Yang, L.J.; Joseph, V.J.A.; Chang, P.Z. A transparent face mask with microchannel against virus via aerosol. In Proceedings of the 25th International Conference on Miniaturized Systems for Chemistry and Lift Sciences (μTAS 2021), Palm Springs, CA, USA, 10–14 October 2021. [Google Scholar]














| Size of Microchip | Particle Size | Outlet 1 | Outlet 2 | Inside Microchannel |
|---|---|---|---|---|
| 2 mm | 5 µm | 56% | 32% | 12% |
| 20 µm | 76% | 0 | 24% | |
| 3 mm | 5 µm | 56% | 32% | 12% |
| 20 µm | 80% | 16% | 4% | |
| 4 mm | 5 µm | 58% | 36% | 6% |
| 20 µm | 74% | 18% | 8% | |
| 5 mm | 5 µm | 56% | 38% | 6% |
| 20 µm | 76% | 20% | 4% | |
| 6 mm | 5 µm | 54% | 36% | 10% |
| 20 µm | 72% | 22% | 6% |
| Size of Microchip | Particle Size | Outlet 1 | Outlet 2 | Inside Microchannel |
|---|---|---|---|---|
| 2 mm | 5 µm | 30% | 58% | 12% |
| 20 µm | 98% | 0% | 2% | |
| 3 mm | 5 µm | 32% | 58% | 10% |
| 20 µm | 96% | 0% | 4% | |
| 4 mm | 5 µm | 36% | 54% | 10% |
| 20 µm | 88% | 10% | 2% | |
| 5 mm | 5 µm | 38% | 58% | 4% |
| 20 µm | 92% | 4% | 4% | |
| 6 mm | 5 µm | 36% | 62% | 2% |
| 20 µm | 98% | 0% | 2% |
| Size of Microchip | Particle Size | Vortex Chambers | Inside Microchannel | Outlet |
|---|---|---|---|---|
| 17.5 mm | 5 µm | 10% | 2% | 88% |
| 20 µm | 28% | 14% | 58% |
| Sollier [1] | Dragonfly Wing | Cilia | |
|---|---|---|---|
| Total size of microchip | 17.5 mm | 3 mm | 2 mm |
| Driving pressure | 185,695 Pa | 17,340 Pa | 35,564 Pa |
| Maximum filtering/separation performance index of bigger particles at outlet 1 | 58% | 80% | 98% |
| Maximum filtering/separation performance index of smaller particles at outlet 1 | 88% | 56% | 30% |
| Maximum filtering/separation performance index of smaller particles at outlet 2 | - | 46% | 70% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.-J.; Joseph, V.-J.; Unnam, N.-K.; Esakki, B. Design and Numerical Simulation of Biomimetic Structures to Capture Particles in a Microchannel. Fluids 2022, 7, 32. https://doi.org/10.3390/fluids7010032
Yang L-J, Joseph V-J, Unnam N-K, Esakki B. Design and Numerical Simulation of Biomimetic Structures to Capture Particles in a Microchannel. Fluids. 2022; 7(1):32. https://doi.org/10.3390/fluids7010032
Chicago/Turabian StyleYang, Lung-Jieh, Vivek-Jabaraj Joseph, Neethish-Kumar Unnam, and Balasubramanian Esakki. 2022. "Design and Numerical Simulation of Biomimetic Structures to Capture Particles in a Microchannel" Fluids 7, no. 1: 32. https://doi.org/10.3390/fluids7010032
APA StyleYang, L. -J., Joseph, V. -J., Unnam, N. -K., & Esakki, B. (2022). Design and Numerical Simulation of Biomimetic Structures to Capture Particles in a Microchannel. Fluids, 7(1), 32. https://doi.org/10.3390/fluids7010032