ABHD11-AS1: An Emerging Long Non-Coding RNA (lncRNA) with Clinical Significance in Human Malignancies
Abstract
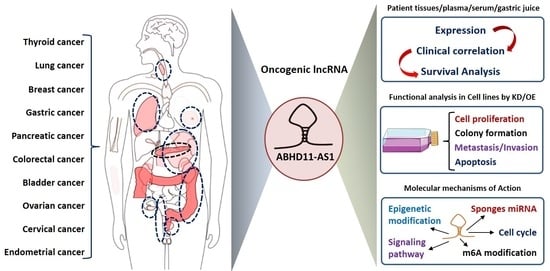
:1. Introduction
2. Oncogenic Role of ABHD11-AS1 lncRNA in Human Cancers
2.1. Gastric Cancer
2.2. Papillary Thyroid Cancer
2.3. Ovarian Cancer
2.4. Colorectal Cancer
2.5. Pancreatic Cancer
2.6. Luminal Breast Cancer
2.7. Non-Small Cell Lung Cancer
2.8. Bladder Cancer
2.9. Endometrial Cancer
2.10. Cervical Cancer
3. Molecular Mechanisms of ABHD11-AS1 lncRNA Dysregulation in Human Malignancies
3.1. ABHD11-AS1 Sponges Multiple miRNAs to Promote Cancer Progression
3.2. Targeting of Key Oncogenic Signaling Pathways by ABHD11-AS1
3.2.1. Phosphoinositide 3 Kinase (PI3K)/Akt Signaling Pathway
3.2.2. Epidermal Growth Factor Receptor (EGFR) Signaling Pathway
3.2.3. Ras Homolog Gene Family Member C (RhoC) Signaling Pathway
3.2.4. Regulation of the Cell Cycle
3.3. Epigenetic Alterations
3.3.1. Dysregulation of Histone Modifications by ABHD11-AS1 in Cancers
3.3.2. Enhanced Stability of ABHD11-AS1 Transcript by N6-methyladenosine (m6A) Modification
4. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Chandra Gupta, S.; Nandan Tripathi, Y. Potential of long non-coding RNAs in cancer patients: From biomarkers to therapeutic targets. Int. J. Cancer 2017, 140, 1955–1967. [Google Scholar] [CrossRef] [PubMed]
- Kung, J.T.; Colognori, D.; Lee, J.T. Long noncoding RNAs: Past, present, and future. Genetics 2013, 193, 651–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ginn, L.; Shi, L.; Montagna, M.; Garofalo, M. LncRNAs in Non-Small-Cell Lung Cancer. Noncoding RNA 2020, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Marchese, F.P.; Raimondi, I.; Huarte, M. The multidimensional mechanisms of long noncoding RNA function. Genome Biol. 2017, 18, 206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sebastian-delaCruz, M.; Gonzalez-Moro, I.; Olazagoitia-Garmendia, A.; Castellanos-Rubio, A.; Santin, I. The Role of lncRNAs in Gene Expression Regulation through mRNA Stabilization. Noncoding RNA 2021, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Statello, L.; Guo, C.J.; Chen, L.L.; Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, A.M.; Chang, H.Y. Long Noncoding RNAs in Cancer Pathways. Cancer Cell 2016, 29, 452–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, Y.; Fullwood, M.J. Roles, Functions, and Mechanisms of Long Non-coding RNAs in Cancer. Genom. Proteom. Bioinform. 2016, 14, 42–54. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.; Yang, M.; Xia, T.; Guo, J. Increased expression of long noncoding RNA ABHD11-AS1 in gastric cancer and its clinical significance. Med. Oncol. 2014, 31, 42. [Google Scholar] [CrossRef] [PubMed]
- Gruber, A.R.; Lorenz, R.; Bernhart, S.H.; Neubock, R.; Hofacker, I.L. The Vienna RNA websuite. Nucleic Acids Res. 2008, 36, W70–W74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iyer, M.K.; Niknafs, Y.S.; Malik, R.; Singhal, U.; Sahu, A.; Hosono, Y.; Barrette, T.R.; Prensner, J.R.; Evans, J.R.; Zhao, S.; et al. The landscape of long noncoding RNAs in the human transcriptome. Nat. Genet. 2015, 47, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Unfried, J.P.; Serrano, G.; Suarez, B.; Sangro, P.; Ferretti, V.; Prior, C.; Boix, L.; Bruix, J.; Sangro, B.; Segura, V.; et al. Identification of Coding and Long Noncoding RNAs Differentially Expressed in Tumors and Preferentially Expressed in Healthy Tissues. Cancer Res. 2019, 79, 5167–5180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francelle, L.; Galvan, L.; Gaillard, M.C.; Petit, F.; Bernay, B.; Guillermier, M.; Bonvento, G.; Dufour, N.; Elalouf, J.M.; Hantraye, P.; et al. Striatal long noncoding RNA Abhd11os is neuroprotective against an N-terminal fragment of mutant huntingtin in vivo. Neurobiol. Aging 2015, 36, 1601.e7–1601.e16. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.S.; Somvanshi, S.; Patel, E.; Chen, T.W.; Singh, V.P.; Zorman, B.; Patil, S.L.; Pan, Y.; Chatterjee, S.S.; Cancer Genome Atlas Research, N.; et al. Pan-Cancer Analysis of lncRNA Regulation Supports Their Targeting of Cancer Genes in Each Tumor Context. Cell Rep. 2018, 23, 297–312.e12. [Google Scholar] [CrossRef]
- Yang, Y.; Shao, Y.; Zhu, M.; Li, Q.; Yang, F.; Lu, X.; Xu, C.; Xiao, B.; Sun, Y.; Guo, J. Using gastric juice lncRNA-ABHD11-AS1 as a novel type of biomarker in the screening of gastric cancer. Tumour Biol. 2016, 37, 1183–1188. [Google Scholar] [CrossRef]
- Xian, H.P.; Zhuo, Z.L.; Sun, Y.J.; Liang, B.; Zhao, X.T. Circulating long non-coding RNAs HULC and ZNFX1-AS1 are potential biomarkers in patients with gastric cancer. Oncol. Lett. 2018, 16, 4689–4698. [Google Scholar] [CrossRef] [Green Version]
- Xin, H.; Yan, Z.; Cao, J. Long non-coding RNA ABHD11-AS1 boosts gastric cancer development by regulating miR-361-3p/PDPK1 signalling. J. Biochem. 2020, 168, 465–476. [Google Scholar] [CrossRef]
- Wen, J.; Wang, H.; Dong, T.; Gan, P.; Fang, H.; Wu, S.; Li, J.; Zhang, Y.; Du, R.; Zhu, Q. STAT3-induced upregulation of lncRNA ABHD11-AS1 promotes tumour progression in papillary thyroid carcinoma by regulating miR-1301-3p/STAT3 axis and PI3K/AKT signalling pathway. Cell Prolif. 2019, 52, e12569. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, X.; Tong, H.; Ding, Y.; Wu, L.; Cai, J.; Si, Y.; Zhang, H.; Shen, M. Long noncoding RNA ABHD11-AS1 functions as a competing endogenous RNA to regulate papillary thyroid cancer progression by miR-199a-5p/SLC1A5 axis. Cell Death Dis. 2019, 10, 620. [Google Scholar] [CrossRef] [Green Version]
- Hou, S.; Zhuang, Y.Y.; Lin, Q.Y.; Chen, Z.; Zhao, H.G.; Zhang, L.; Lin, C.H. Overexpression of serum lncRNA-ABHD11-AS1 as poor prognosis of patients with papillary thyroid carcinoma. Exp. Mol. Pathol. 2021, 121, 104658. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Zhu, C.; Chen, Y.; Ruan, Y.; Fan, L.; Chen, Q.; Wei, Q. LncRNA ABHD11-AS1 promotes tumor progression in papillary thyroid carcinoma by regulating EPS15L1/EGFR signaling pathway. Clin. Transl. Oncol. 2022. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Li, J.; Lin, Y.; Liu, D.; Yang, Q.; Jian, J.; Peng, J. m(6) A transferase METTL3-induced lncRNA ABHD11-AS1 promotes the Warburg effect of non-small-cell lung cancer. J. Cell Physiol. 2021, 236, 2649–2658. [Google Scholar] [CrossRef]
- Qiao, X.; Lv, S.X.; Qiao, Y.; Li, Q.P.; Ye, B.; Wang, C.C.; Miao, L. Long noncoding RNA ABHD11-AS1 predicts the prognosis of pancreatic cancer patients and serves as a promoter by activating the PI3K-AKT pathway. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 8630–8639. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Feng, W.; Liu, W.; Kong, X.; Li, L.; He, J.; Wang, D.; Zhang, M.; Zhou, G.; Xu, W.; et al. Circulating lncRNA ABHD11-AS1 serves as a biomarker for early pancreatic cancer diagnosis. J. Cancer 2019, 10, 3746–3756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, B.; Wang, W.; Sun, S.; Ding, H.; Lan, L.; Li, X.; Han, S. Knockdown of lncRNA ABHD11-AS1 Suppresses the Tumorigenesis of Pancreatic Cancer via Sponging miR-1231. OncoTargets Ther. 2020, 13, 11347–11358. [Google Scholar] [CrossRef]
- Lei, X.; Li, L.; Duan, X. Long non-coding RNA ABHD11-AS1 promotes colorectal cancer development through regulation of miR-133a/SOX4 axis. Biosci. Rep. 2018, 38, BSR20181386. [Google Scholar] [CrossRef] [Green Version]
- He, D.; Yue, Z.; Liu, L.; Fang, X.; Chen, L.; Han, H. Long noncoding RNA ABHD11-AS1 promote cells proliferation and invasion of colorectal cancer via regulating the miR-1254-WNT11 pathway. J. Cell Physiol. 2019, 234, 12070–12079. [Google Scholar] [CrossRef]
- Luo, J.; Jiang, Y.; Wu, L.; Zhuo, D.; Zhang, S.; Jiang, X.; Sun, Y.; Huang, Y. Long non-coding RNA ABHD11-AS1 promotes colorectal cancer progression and invasion through targeting the integrin subunit alpha 5/focal adhesion kinase/phosphoinositide 3 kinase/Akt signaling pathway. Aging 2021, 13, 20179–20191. [Google Scholar] [CrossRef]
- Wu, D.D.; Chen, X.; Sun, K.X.; Wang, L.L.; Chen, S.; Zhao, Y. Role of the lncRNA ABHD11-AS1 in the tumorigenesis and progression of epithelial ovarian cancer through targeted regulation of RhoC. Mol. Cancer 2017, 16, 138. [Google Scholar] [CrossRef]
- Zeng, X.Y.; Jiang, X.Y.; Yong, J.H.; Xie, H.; Yuan, J.; Zeng, D.; Dou, Y.Y.; Xiao, S.S. lncRNA ABHD11-AS1, regulated by the EGFR pathway, contributes to the ovarian cancer tumorigenesis by epigenetically suppressing TIMP2. Cancer Med. 2019, 8, 7074–7085. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Huang, X.; Shi, J. EZH2-mediated lncRNA ABHD11-AS1 promoter regulates the progression of ovarian cancer by targeting miR-133a-3p. Anticancer Drugs 2021, 32, 269–277. [Google Scholar] [CrossRef]
- Chen, M.; Li, J.; Zhuang, C.; Cai, Z. Increased lncRNA ABHD11-AS1 represses the malignant phenotypes of bladder cancer. Oncotarget 2017, 8, 28176–28186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehrpour Layeghi, S.; Arabpour, M.; Shakoori, A.; Naghizadeh, M.M.; Mansoori, Y.; Tavakkoly Bazzaz, J.; Esmaeili, R. Expression profiles and functional prediction of long non-coding RNAs LINC01133, ZEB1-AS1 and ABHD11-AS1 in the luminal subtype of breast cancer. J. Transl. Med. 2021, 19, 364. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, L.L.; Chen, S.; Zong, Z.H.; Guan, X.; Zhao, Y. LncRNA ABHD11-AS1 promotes the development of endometrial carcinoma by targeting cyclin D1. J. Cell Mol. Med. 2018. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Zhang, X.; Yang, J. Long non-coding RNA ABHD11-AS1 facilitates the progression of cervical cancer by competitively binding to miR-330-5p and upregulating MARK2. Exp. Cell Res. 2022, 410, 112929. [Google Scholar] [CrossRef]
- Zhu, D.; Hao, Q.; Qian, M.; Hu, Y.; Wu, F. LncRNA ABHD11-AS1 Participates in the Progression of Cervical Carcinoma by Targeting miR-1254 and Is the Key to the Diagnosis and Treatment of Cervical Carcinoma in the Future. J. Healthc. Eng. 2022, 2022, 8387458. [Google Scholar] [CrossRef]
- Song, Z.; Wu, Y.; Yang, J.; Yang, D.; Fang, X. Progress in the treatment of advanced gastric cancer. Tumour Biol. 2017, 39, 1010428317714626. [Google Scholar] [CrossRef] [Green Version]
- Kelley, J.R.; Duggan, J.M. Gastric cancer epidemiology and risk factors. J. Clin. Epidemiol. 2003, 56, 1–9. [Google Scholar] [CrossRef]
- Karimi, P.; Islami, F.; Anandasabapathy, S.; Freedman, N.D.; Kamangar, F. Gastric cancer: Descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol. Biomark. Prev. 2014, 23, 700–713. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.L.; Zhang, L.; Cui, X.F. Downregulation of long noncoding RNA LINC01419 inhibits cell migration, invasion, and tumor growth and promotes autophagy via inactivation of the PI3K/Akt1/mTOR pathway in gastric cancer. Ther. Adv. Med. Oncol. 2019, 11, 1758835919874651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xuan, Y.; Wang, Y. Long non-coding RNA SNHG3 promotes progression of gastric cancer by regulating neighboring MED18 gene methylation. Cell Death Dis. 2019, 10, 694. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, J.W.; Ren, J.Y.; Guo, M.; Guo, C.W.; Ning, S.W.; Yu, S. Long noncoding RNAs in gastric cancer: From molecular dissection to clinical application. World J. Gastroenterol. 2020, 26, 3401–3412. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Zhou, Q.; Yi, H.; Ma, S.; Li, D.; Xu, Y.; Wang, J.; Yin, S. A novel lncRNA n384546 promotes thyroid papillary cancer progression and metastasis by acting as a competing endogenous RNA of miR-145-5p to regulate AKT3. Cell Death Dis. 2019, 10, 433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitahara, C.M.; Sosa, J.A. The changing incidence of thyroid cancer. Nat. Rev. Endocrinol. 2016, 12, 646–653. [Google Scholar] [CrossRef]
- Peng, X.; Zhang, K.; Ma, L.; Xu, J.; Chang, W. The Role of Long Non-Coding RNAs in Thyroid Cancer. Front. Oncol. 2020, 10, 941. [Google Scholar] [CrossRef] [PubMed]
- Javed, Z.; Ahmed Shah, F.; Rajabi, S.; Raza, Q.; Iqbal, Z.; Ullah, M.; Ahmad, T.; Salehi, B.; Sharifi-Rad, M.; Pezzani, R.; et al. LncRNAs as Potential Therapeutic Targets in Thyroid Cancer. Asian Pac. J. Cancer Prev. 2020, 21, 281–287. [Google Scholar] [CrossRef]
- Jayson, G.C.; Kohn, E.C.; Kitchener, H.C.; Ledermann, J.A. Ovarian cancer. Lancet 2014, 384, 1376–1388. [Google Scholar] [CrossRef]
- Chen, Y.; Bi, F.; An, Y.; Yang, Q. Identification of pathological grade and prognosis-associated lncRNA for ovarian cancer. J. Cell Biochem. 2019, 120, 14444–14454. [Google Scholar] [CrossRef]
- Zhan, L.; Li, J.; Wei, B. Long non-coding RNAs in ovarian cancer. J. Exp. Clin. Cancer Res. 2018, 37, 120. [Google Scholar] [CrossRef] [Green Version]
- Salamini-Montemurri, M.; Lamas-Maceiras, M.; Barreiro-Alonso, A.; Vizoso-Vazquez, A.; Rodriguez-Belmonte, E.; Quindos-Varela, M.; Cerdan, M.E. The Challenges and Opportunities of LncRNAs in Ovarian Cancer Research and Clinical Use. Cancers 2020, 12, 1020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Kandimalla, R.; Huang, H.; Zhu, L.; Li, Y.; Gao, F.; Goel, A.; Wang, X. Molecular subtyping of colorectal cancer: Recent progress, new challenges and emerging opportunities. Semin. Cancer Biol. 2019, 55, 37–52. [Google Scholar] [CrossRef]
- He, Q.; Long, J.; Yin, Y.; Li, Y.; Lei, X.; Li, Z.; Zhu, W. Emerging Roles of lncRNAs in the Formation and Progression of Colorectal Cancer. Front. Oncol. 2019, 9, 1542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poursheikhani, A.; Abbaszadegan, M.R.; Kerachian, M.A. Mechanisms of long non-coding RNA function in colorectal cancer tumorigenesis. Asia Pac. J. Clin. Oncol. 2021, 17, 7–23. [Google Scholar] [CrossRef]
- Rawla, P.; Sunkara, T.; Gaduputi, V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J. Oncol. 2019, 10, 10–27. [Google Scholar] [CrossRef]
- Duguang, L.; Jin, H.; Xiaowei, Q.; Peng, X.; Xiaodong, W.; Zhennan, L.; Jianjun, Q.; Jie, Y. The involvement of lncRNAs in the development and progression of pancreatic cancer. Cancer Biol. Ther. 2017, 18, 927–936. [Google Scholar] [CrossRef]
- Lv, Y.; Huang, S. Role of non-coding RNA in pancreatic cancer. Oncol. Lett. 2019, 18, 3963–3973. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.; Chen, L.; Li, C.; Huang, R.; Guo, M.; Ning, S.; Ji, J.; Guo, X.; Lou, G.; Jia, X.; et al. The multifaceted roles of long noncoding RNAs in pancreatic cancer: An update on what we know. Cancer Cell Int. 2020, 20, 41. [Google Scholar] [CrossRef] [Green Version]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Makki, J. Diversity of Breast Carcinoma: Histological Subtypes and Clinical Relevance. Clin. Med. Insights Pathol. 2015, 8, 23–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathias, C.; Zambalde, E.P.; Rask, P.; Gradia, D.F.; de Oliveira, J.C. Long non-coding RNAs differential expression in breast cancer subtypes: What do we know? Clin. Genet. 2019, 95, 558–568. [Google Scholar] [CrossRef] [PubMed]
- Minotti, L.; Agnoletto, C.; Baldassari, F.; Corra, F.; Volinia, S. SNPs and Somatic Mutation on Long Non-Coding RNA: New Frontier in the Cancer Studies? High Throughput 2018, 7, 34. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zhao, Z.; Han, X.; Zhang, Y.; Zhang, Y.; Li, F.; Li, H. Single-Nucleotide Polymorphisms Promote Dysregulation Activation by Essential Gene Mediated Bio-Molecular Interaction in Breast Cancer. Front. Oncol. 2021, 11, 791943. [Google Scholar] [CrossRef] [PubMed]
- Wood, D.E.; Kazerooni, E.A.; Baum, S.L.; Eapen, G.A.; Ettinger, D.S.; Hou, L.; Jackman, D.M.; Klippenstein, D.; Kumar, R.; Lackner, R.P.; et al. Lung Cancer Screening, Version 3.2018, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2018, 16, 412–441. [Google Scholar] [CrossRef]
- Yuan, S.; Xiang, Y.; Guo, X.; Zhang, Y.; Li, C.; Xie, W.; Wu, N.; Wu, L.; Cai, T.; Ma, X.; et al. Circulating Long Noncoding RNAs Act as Diagnostic Biomarkers in Non-Small Cell Lung Cancer. Front. Oncol. 2020, 10, 537120. [Google Scholar] [CrossRef]
- Xue, M.; Pang, H.; Li, X.; Li, H.; Pan, J.; Chen, W. Long non-coding RNA urothelial cancer-associated 1 promotes bladder cancer cell migration and invasion by way of the hsa-miR-145-ZEB1/2-FSCN1 pathway. Cancer Sci. 2016, 107, 18–27. [Google Scholar] [CrossRef]
- Wang, J.; Ma, W.; Liu, Y. Long non-coding RNA HULC promotes bladder cancer cells proliferation but inhibits apoptosis via regulation of ZIC2 and PI3K/AKT signaling pathway. Cancer Biomark. 2017, 20, 425–434. [Google Scholar] [CrossRef]
- Cao, X.; Xu, J.; Yue, D. LncRNA-SNHG16 predicts poor prognosis and promotes tumor proliferation through epigenetically silencing p21 in bladder cancer. Cancer Gene Ther. 2018, 25, 10–17. [Google Scholar] [CrossRef] [Green Version]
- Su, G.; He, Q.; Wang, J. Clinical Values of Long Non-coding RNAs in Bladder Cancer: A Systematic Review. Front. Physiol. 2018, 9, 652. [Google Scholar] [CrossRef] [Green Version]
- Rizzo, S.; Femia, M.; Buscarino, V.; Franchi, D.; Garbi, A.; Zanagnolo, V.; Del Grande, M.; Manganaro, L.; Alessi, S.; Giannitto, C.; et al. Endometrial cancer: An overview of novelties in treatment and related imaging keypoints for local staging. Cancer Imaging 2018, 18, 45. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wan, J.; Chu, J. Long non-coding RNAs and endometrial cancer. Biomed. PharmacoTher. 2019, 119, 109396. [Google Scholar] [CrossRef] [PubMed]
- Li, B.L.; Wan, X.P. The role of lncRNAs in the development of endometrial carcinoma. Oncol. Lett. 2018, 16, 3424–3429. [Google Scholar] [CrossRef] [Green Version]
- Aalijahan, H.; Ghorbian, S. Long non-coding RNAs and cervical cancer. Exp. Mol. Pathol 2019, 106, 7–16. [Google Scholar] [CrossRef]
- Shi, D.; Zhang, C.; Liu, X. Long noncoding RNAs in cervical cancer. J. Cancer Res. Ther. 2018, 14, 745–753. [Google Scholar] [CrossRef]
- He, J.; Huang, B.; Zhang, K.; Liu, M.; Xu, T. Long non-coding RNA in cervical cancer: From biology to therapeutic opportunity. Biomed. Pharmacother. 2020, 127, 110209. [Google Scholar] [CrossRef]
- Zhong, Q.; Lu, M.; Yuan, W.; Cui, Y.; Ouyang, H.; Fan, Y.; Wang, Z.; Wu, C.; Qiao, J.; Hang, J. Eight-lncRNA signature of cervical cancer were identified by integrating DNA methylation, copy number variation and transcriptome data. J. Transl. Med. 2021, 19, 58. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [Green Version]
- Thomson, D.W.; Dinger, M.E. Endogenous microRNA sponges: Evidence and controversy. Nat. Rev. Genet. 2016, 17, 272–283. [Google Scholar] [CrossRef]
- Wang, H.; An, H.; Wang, B.; Liao, Q.; Li, W.; Jin, X.; Cui, S.; Zhang, Y.; Ding, Y.; Zhao, L. miR-133a represses tumour growth and metastasis in colorectal cancer by targeting LIM and SH3 protein 1 and inhibiting the MAPK pathway. Eur. J. Cancer 2013, 49, 3924–3935. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, X.; Wang, C.H.; Wang, D.; Du, J.J. Decreased expression of miR-1254 is associated with cancer aggressiveness and predicts poor outcome in cervical cancer. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 2997–3001. [Google Scholar] [CrossRef]
- Xia, W.; Jie, W. ZEB1-AS1/miR-133a-3p/LPAR3/EGFR axis promotes the progression of thyroid cancer by regulating PI3K/AKT/mTOR pathway. Cancer Cell Int. 2020, 20, 94. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Vega, F.; Mina, M.; Armenia, J.; Chatila, W.K.; Luna, A.; La, K.C.; Dimitriadoy, S.; Liu, D.L.; Kantheti, H.S.; Saghafinia, S.; et al. Oncogenic Signaling Pathways in The Cancer Genome Atlas. Cell 2018, 173, 321–337.e10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayer, I.A.; Arteaga, C.L. The PI3K/AKT Pathway as a Target for Cancer Treatment. Annu. Rev. Med. 2016, 67, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.F.; Zheng, X.; Fan, X.; Lin, A.F. Role of cytoplasmic lncRNAs in regulating cancer signaling pathways. J. Zhejiang Univ. Sci. B 2019, 20, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Psyrri, A.; Kassar, M.; Yu, Z.; Bamias, A.; Weinberger, P.M.; Markakis, S.; Kowalski, D.; Camp, R.L.; Rimm, D.L.; Dimopoulos, M.A. Effect of epidermal growth factor receptor expression level on survival in patients with epithelial ovarian cancer. Clin. Cancer Res. 2005, 11, 8637–8643. [Google Scholar] [CrossRef] [Green Version]
- Normanno, N.; De Luca, A.; Bianco, C.; Strizzi, L.; Mancino, M.; Maiello, M.R.; Carotenuto, A.; De Feo, G.; Caponigro, F.; Salomon, D.S. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene 2006, 366, 2–16. [Google Scholar] [CrossRef]
- Liang, W.; Wu, X.; Fang, W.; Zhao, Y.; Yang, Y.; Hu, Z.; Xue, C.; Zhang, J.; Zhang, J.; Ma, Y.; et al. Network meta-analysis of erlotinib, gefitinib, afatinib and icotinib in patients with advanced non-small-cell lung cancer harboring EGFR mutations. PLoS ONE 2014, 9, e85245. [Google Scholar] [CrossRef]
- Lou, Y.; Jiang, Y.; Liang, Z.; Liu, B.; Li, T.; Zhang, D. Role of RhoC in cancer cell migration. Cancer Cell Int. 2021, 21, 527. [Google Scholar] [CrossRef]
- Stojic, L.; Lun, A.T.L.; Mascalchi, P.; Ernst, C.; Redmond, A.M.; Mangei, J.; Barr, A.R.; Bousgouni, V.; Bakal, C.; Marioni, J.C.; et al. A high-content RNAi screen reveals multiple roles for long noncoding RNAs in cell division. Nat. Commun. 2020, 11, 1851. [Google Scholar] [CrossRef] [Green Version]
- Zhou, B.B.; Elledge, S.J. The DNA damage response: Putting checkpoints in perspective. Nature 2000, 408, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, M.; Kitagawa, K.; Kotake, Y.; Niida, H.; Ohhata, T. Cell cycle regulation by long non-coding RNAs. Cell Mol. Life Sci. 2013, 70, 4785–4794. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; He, C.; Wang, M.; Ma, X.; Mo, F.; Yang, S.; Han, J.; Wei, X. Targeting epigenetic regulators for cancer therapy: Mechanisms and advances in clinical trials. Signal. Transduct. Target. Ther. 2019, 4, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kouzarides, T. Histone methylation in transcriptional control. Curr. Opin. Genet. Dev. 2002, 12, 198–209. [Google Scholar] [CrossRef]
- Sims, R.J., 3rd; Nishioka, K.; Reinberg, D. Histone lysine methylation: A signature for chromatin function. Trends Genet. 2003, 19, 629–639. [Google Scholar] [CrossRef]
- Margueron, R.; Reinberg, D. The Polycomb complex PRC2 and its mark in life. Nature 2011, 469, 343–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Wang, W.; Zhu, W.; Dong, J.; Cheng, Y.; Yin, Z.; Shen, F. Mechanisms and Functions of Long Non-Coding RNAs at Multiple Regulatory Levels. Int. J. Mol. Sci. 2019, 20, 5573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Peng, C.; Chen, J.; Chen, D.; Yang, B.; He, B.; Hu, W.; Zhang, Y.; Liu, H.; Dai, L.; et al. WTAP facilitates progression of hepatocellular carcinoma via m6A-HuR-dependent epigenetic silencing of ETS1. Mol. Cancer 2019, 18, 127. [Google Scholar] [CrossRef] [Green Version]
- Meyer, K.D.; Saletore, Y.; Zumbo, P.; Elemento, O.; Mason, C.E.; Jaffrey, S.R. Comprehensive analysis of mRNA methylation reveals enrichment in 3’ UTRs and near stop codons. Cell 2012, 149, 1635–1646. [Google Scholar] [CrossRef] [Green Version]
- He, L.; Li, H.; Wu, A.; Peng, Y.; Shu, G.; Yin, G. Functions of N6-methyladenosine and its role in cancer. Mol. Cancer 2019, 18, 176. [Google Scholar] [CrossRef] [Green Version]





| Tumor Type | Sample Type | Patient/Control Size | Expression | Clinical Correlation | Function | Biomarker | Relevant to Prognosis | Overall Survival | Year | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| GC | Tissue | 75/75 | Upregulated | Degree of differentiation, Lauren type | Diagnosis | 2014 | [10] | |||
| Tissue | 73/37 | Upregulated | 2016 | [16] | ||||||
| Gastric juice | 39/45 | Upregulated | Gender, tumor size, tumor stage, Lauren type, blood CEA levels | Diagnosis/ Early diagnosis | ||||||
| Plasma | 10/10 | No change | 2018 | [17] | ||||||
| Tissue | 41/41 | Upregulated | Proliferation, apoptosis | 2020 | [18] | |||||
| PTC | Tissue | 82/82 | Upregulated | TNM stage, lymph node metastasis, tumor infiltration | Proliferation, apoptosis, migration | Negative correlation | Poor | 2018 | [19] | |
| Tissue | 80/80 | Upregulated | Proliferation, colony formation, migration, invasion, apoptosis | Diagnosis | 2019 | [20] | ||||
| Serum | 64/50 | Upregulated | Tumor diameter, lymph node metastasis | Proliferation, apoptosis | Diagnosis | Negative correlation | Poor | 2021 | [21] | |
| Tissue | 98/98 | Upregulated | Lymph node metastasis | Proliferation, invasion, migration | Diagnosis | 2022 | [22] | |||
| NSCLC | Tissue | 40/40 | Upregulated | TNM stage | Proliferation, Warburg effect | Negative correlation | Poor | 2020 | [23] | |
| PC | Tissue | 147/147 | Upregulated | TNM stage, distant metastasis, and tumor differentiation | Proliferation, colony formation, migration, invasion, apoptosis | Prognosis | Negative correlation | Poor | 2018 | [24] |
| Plasma | 114/46 | Upregulated | Diagnosis/ Early diagnosis | Negative correlation | Poor | 2019 | [25] | |||
| Tissue/ TCGA | 179/171 | Upregulated | Proliferation, migration, invasion, apoptosis | Poor | 2020 | [26] | ||||
| CRC | Tissue | 132/132 | Upregulated | TNM stage, lymph node metastasis | Proliferation, colony formation, migration, invasion, apoptosis | Negative correlation | Poor | 2018 | [27] | |
| Tissue | 53/53 | Upregulated | Proliferation, colony formation, invasion | 2019 | [28] | |||||
| Tissue | 60/60 | Upregulated | Proliferation, migration, invasion, apoptosis | 2021 | [29] | |||||
| OC | Tissue | 51/13 | Upregulated | Tumor stage, Degree of differentiation | Proliferation, apoptosis, invasion, migration | 2017 | [30] | |||
| Tissue | 53/53 | Upregulated | Proliferation, invasion, migration, colony formation | 2019 | [31] | |||||
| Tissue | 50/50 | Upregulated | Tumor stage, lymph node metastasis | Proliferation, apoptosis, invasion, migration | Poor | 2021 | [32] | |||
| Bladder Cancer | Tissue | 66/66 | Upregulated | TNM stage, Histological grade, tumor invasion depth | Proliferation, apoptosis, migration, | 2017 | [33] | |||
| BC | Tissue | 79/79 | Upregulated | No association | Same | 2021 | [34] | |||
| EC | Tissue | 89/27 | Upregulated | Proliferation, apoptosis, invasion, migration | 2018 | [35] | ||||
| CC | Cell lines | Upregulated | Proliferation, apoptosis, colony formation, invasion, migration | 2021 | [36] | |||||
| Tissue/ Serum | 72/78 | Upregulated | Proliferation, apoptosis, invasion, migration | Diagnosis/ Prognosis | Negative correlation | Poor | 2022 | [37] |
| S.No. | miRNA Name | Target Gene | Cancer Type | Reference |
|---|---|---|---|---|
| 1 | miR-361-3p | 3-Phosphoinositide Dependent Protein Kinase 1 (PDPK1) | GC | [18] |
| 2 | miR-1301-3p | Signal Transducer and Activator of Transcription 3 (STAT3) | PTC | [19] |
| 3 | miR-199a-5p | Solute Carrier Family 1 Member 5 (SLC1A5) | [20] | |
| 4 | miR-330-5p | Microtubule Affinity Regulating Kinase 2 (MARK2) | CC | [36] |
| 5 | miR-1254 | - | [37] | |
| 6 | Wnt Family Member 11 (WNT11) | CRC | [28] | |
| 7 | miR-133a | SRY-Box Transcription Factor 4 (SOX4) | [27] | |
| 8 | miR-1231 | cyclin E1 (CCNE1) | PC | [26] |
| 9 | miR-133a-3p | - | OC | [32] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golla, U.; Sesham, K.; Dallavalasa, S.; Manda, N.K.; Unnam, S.; Sanapala, A.K.; Nalla, S.; Kondam, S.; Kumar, R. ABHD11-AS1: An Emerging Long Non-Coding RNA (lncRNA) with Clinical Significance in Human Malignancies. Non-Coding RNA 2022, 8, 21. https://doi.org/10.3390/ncrna8020021
Golla U, Sesham K, Dallavalasa S, Manda NK, Unnam S, Sanapala AK, Nalla S, Kondam S, Kumar R. ABHD11-AS1: An Emerging Long Non-Coding RNA (lncRNA) with Clinical Significance in Human Malignancies. Non-Coding RNA. 2022; 8(2):21. https://doi.org/10.3390/ncrna8020021
Chicago/Turabian StyleGolla, Upendarrao, Kishore Sesham, Siva Dallavalasa, Naresh Kumar Manda, Sambamoorthy Unnam, Arun Kumar Sanapala, Sharada Nalla, Susmitha Kondam, and Rajesh Kumar. 2022. "ABHD11-AS1: An Emerging Long Non-Coding RNA (lncRNA) with Clinical Significance in Human Malignancies" Non-Coding RNA 8, no. 2: 21. https://doi.org/10.3390/ncrna8020021
APA StyleGolla, U., Sesham, K., Dallavalasa, S., Manda, N. K., Unnam, S., Sanapala, A. K., Nalla, S., Kondam, S., & Kumar, R. (2022). ABHD11-AS1: An Emerging Long Non-Coding RNA (lncRNA) with Clinical Significance in Human Malignancies. Non-Coding RNA, 8(2), 21. https://doi.org/10.3390/ncrna8020021