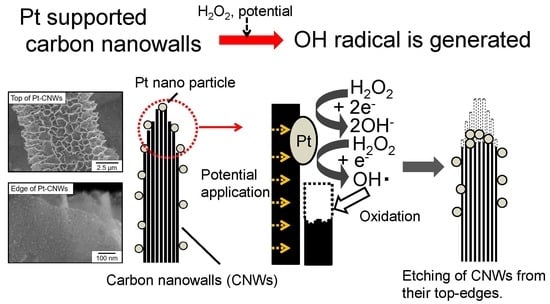
Electrochemical Reaction in Hydrogen Peroxide and Structural Change of Platinum Nanoparticle-Supported Carbon Nanowalls Grown Using Plasma-Enhanced Chemical Vapor Deposition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Prepared CNWs
2.2. Electrochemical Measurements
2.3. Solid Analysis
3. Results
3.1. Observation of Shape of CNWs After Cycle Test
3.2. Observation of Pt NP Supported Form
3.3. Signal Analysis of CV Measurements
3.4. Investigating Factors of Structural Change
3.5. Analysis of the Crystal Structure of CNWs
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hiramatsu, M.; Shiji, K.; Amano, H.; Hori, M. Fabrication of vertically aligned carbon nanowalls using capacitively coupled plasma-enhanced chemical vapor deposition assisted by hydrogen radical injection. Appl. Phys. Lett. 2004, 84, 4708–4710. [Google Scholar] [CrossRef]
- Kobayashi, K.; Tanimura, M.; Nakai, H.; Yoshimura, A.; Yoshimura, H.; Kojima, K.; Tachibana, M. Nanographite domains in carbon nanowalls. J. Appl. Phys. 2007, 101. [Google Scholar] [CrossRef]
- Wu, Y.; Qiao, P.; Chong, T.; Shen, Z. Carbon nanowalls grown by microwave plasma enhanced chemical vapor deposition. Adv. Mater. 2002, 14, 64–67. [Google Scholar] [CrossRef]
- Watanabe, H.; Kondo, H.; Sekine, M.; Hiramatsu, M.; Hori, M. Control of super hydrophobic and super hydrophilic surfaces of carbon nanowalls using atmospheric pressure plasma treatments. Jpn. J. Appl. Phys. 2012, 51, 5–8. [Google Scholar] [CrossRef]
- Gu, L.; Luo, N.; Miley, G.H. Cathode electrocatalyst selection and deposition for a direct borohydride/hydrogen peroxide fuel cell. J. Power Sources 2007, 173, 77–85. [Google Scholar] [CrossRef]
- Sosa, V.; Moline, T.; Somoza, R.; Paciucci, R.; Kondoh, H.; Lleonart, E.M. Oxidative stress and cancer: An overview. Indian J. Clin. Biochem. 2013, 28, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Summers, F.A.; Zhao, B.; Ganini, D.; Mason, R.P. Photooxidation of amplex red to resorufin: Implications of exposing the amplex red assay to light. Methods Enzymol. 2013, 256, 1–17. [Google Scholar]
- Holland, A.L.; Harmony, M.N.; Lunte, M.S. Characterization of an Integrated On—Capillary Dual Electrode for Capillary Electrophoresis—Electrochemistry. Electroanalysis 1999, 11, 327–330. [Google Scholar] [CrossRef]
- Solla-Gullón, J.; Montiel, V.; Aldaz, A.; Clavilier, J. Electrochemical characterization of platinum nanoparticles prepared by microemulsion: How to clean them without loss of crystalline surface structure. J. Electroanal. Chem. 2000, 491, 69–77. [Google Scholar] [CrossRef]
- Ernst, H.; Knoll, M. Electrochemical characterisation of uric acid and ascorbic acid at a platinum electrode. Anal. Chim. Acta 2001, 449, 129–134. [Google Scholar] [CrossRef]
- Sun, Y.; He, K.; Zhang, Z.; Zhou, A.; Duan, H. Real-time electrochemical detection of hydrogen peroxide secretion in live cells by Pt nanoparticles decorated graphene-carbon nanotube hybrid paper electrode. Biosens. Bioelectron. 2015, 68, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Kalambate, P.K.; Sanghavi, B.J.; Karna, S.P.; Srivastava, A.K. Simultaneous voltammetric determination of paracetamol and domperidone based on a graphene/platinum nanoparticles/nafion composite modified glassy carbon electrode. Sens. Actuators B Chem. 2015, 213, 285–294. [Google Scholar] [CrossRef]
- Hussain, S.; Erikson, H.; Kongi, N.; Merisalu, M.; Ritslaid, P.; Sammelselg, V.; Tammeveski, K. Heat-treatment effects on the ORR activity of Pt nanoparticles deposited on multi-walled carbon nanotubes using magnetron sputtering technique. Int. J. Hydrogen Energy 2017, 42, 5958–5970. [Google Scholar] [CrossRef]
- Tomatsu, M.; Hiramatsu, M.; Foord, J.S.; Kondo, H.; Ishikawa, K.; Sekine, M.; Takeda, K.; Hori, M. Hydrogen peroxide sensor based on carbon nanowalls grown by plasma-enhanced chemical vapor deposition. Jpn. J. Appl. Phys. 2017, 56, 1–6. [Google Scholar] [CrossRef]
- Feng, Y.; Zhou, G.; Wang, G.; Qu, M.; Yu, Z. Removal of some impurities from carbon nanotubes. Chem. Phys. Lett. 2003, 375, 645–648. [Google Scholar] [CrossRef]
- Wepasnick, K.A.; Smith, B.A.; Schrote, K.E.; Wilson, H.K.; Diegelmann, S.R.; Fairbrother, D.H. Surface and structural characterization of multi-walled carbon nanotubes following different oxidative treatments. Carbon N. Y. 2011, 49, 24–36. [Google Scholar] [CrossRef]
- Krueger, A.; Lang, D. Functionality is key: Recent progress in the surface modification of nanodiamond. Adv. Funct. Mater. 2012, 22, 890–906. [Google Scholar] [CrossRef]
- De Bruijn, F.A.; Dam, V.A.T.; Janssen, G.J.M. Review: Durability and degradation issues of PEM fuel cell components. Fuel Cells 2008, 8, 3–22. [Google Scholar] [CrossRef]
- Hara, M.; Lee, M.; Liu, C.H.; Chen, B.H.; Yamashita, Y.; Uchida, M.; Uchida, H.; Watanabe, M. Electrochemical and raman spectroscopic evaluation of Pt/graphitized carbon black catalyst durability for the start/stop operating condition of polymer electrolyte fuel cells. Electrochim. Acta 2012, 70, 171–181. [Google Scholar] [CrossRef]
- Fang, B.; Luo, J.; Njoki, P.N.; Loukrakpam, R.; Mott, D.; Wanjala, B.; Hu, X.; Zhong, C.J. Nanostructured PtVFe catalysts: Electrocatalytic performance in proton exchange membrane fuel cells. Electrochem. commun. 2009, 11, 1139–1141. [Google Scholar] [CrossRef]
- Pfrang, A.; Veyret, D.; Sieker, F.; Tsotridis, G. X-ray computed tomography of gas diffusion layers of PEM fuel cells: Calculation of thermal conductivity. Int. J. Hydrogen Energy 2010, 35, 3751–3757. [Google Scholar] [CrossRef]
- Kondo, S.; Hori, M.; Yamakawa, K.; Den, S.; Kano, H.; Hiramatsu, M. Highly reliable growth process of carbon nanowalls using radical injection plasma-enhanced chemical vapor deposition. J. Vac. Sci. Technol. B Microelectron. Nanom. Struct. 2008, 26, 1294. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Miyata, Y.; Maniwa, Y.; Kataura, H. Selective oxidation of semiconducting single-wall carbon nanotubes by hydrogen peroxide. J. Phys. Chem. B 2006, 110, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Tuinstra, F.; Koenig, L.J.J. Raman Spectrum of Graphite. Phys. Chem. 1970, 53, 1126–1130. [Google Scholar] [CrossRef]
- Vidano, R.P.; Fishbach, D.B.; Willis, L.J.; Loehr, T.M. Observation of Raman band shifting with excitation wavelength for carbons and graphites. Solid State Commun. 1981, 39, 341–344. [Google Scholar] [CrossRef]
- Ni, Z.; Wang, Y.; Yu, T.; Shen, Z. Raman spectroscopy and imaging of graphene. Nano Res. 2008, 1, 273–291. [Google Scholar] [CrossRef] [Green Version]
- Lucchese, M.M.; Stavale, F.; Ferreira, E.H.M.; Vilani, C.; Moutinho, M.V.O.; Capaz, R.B.; Achete, C.A.; Jorio, A. Quantifying ion-induced defects and Raman relaxation length in graphene. Carbon N. Y. 2010, 48, 1592–1597. [Google Scholar] [CrossRef]
- Cano-Marquez, A.G.; Rodriguez-Macias, F.J.; Campos-Delgado, J.; Espinosa-Gonzalez, C.G.; Tristan-Lopez, F.; Ramirez-Gonzalez, D.; Cullen, D.A.; Smith, D.J.; Terrones, M.; Vega-Cantu, Y.I. Ex-MWNTs: Graphene Sheets and Ribbons Produced by Lithium Intercalation and Exfoliation of Carbon Nanotubes. Nano Lett. 2009, 9, 1527–1533. [Google Scholar] [CrossRef]
- Bay, C.W.; Kong, H. Electrochemical Synthesis and Characterization of Ferric Chloride-Graphite Intercalation. Science 1998, 36, 383–390. [Google Scholar]
- Onu, A.D.; Iyun, J.F.; Idris, O.S. Kinetics and Stoichiometry of the Reduction of Hydrogen Peroxide by an Aminocarboxylactocobaltate(II) Complex in Aqueous Medium. Open J. Inorg. Chem. 2015, 5, 75–82. [Google Scholar] [CrossRef]
- Karyakin, A.A.; Karyakina, E.E.; Gorton, L. On the mechanism of H2O2 reduction at Prussian Blue modified electrodes. Electrochem. Commun. 1999, 1, 78–82. [Google Scholar] [CrossRef]
- Oldfield, L.F. The oxidation-reduction reactions of hydrogen peroxide at inert metal electrodes and mercury cathodes. Trans. Faraday Soc. 1955, 51, 249–259. [Google Scholar] [CrossRef]
- Yue, Q.; Zhang, K.; Chen, X.; Wang, L.; Zhao, J.; Liu, J.; Jia, J. Generation of OH radicals in oxygen reduction reaction at Pt-Co nanoparticles supported on graphene in alkaline solutions. Chem. Commun. 2010, 46, 3369–3371. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Hidai, S.; Niwa, H.; Harada, Y.; Oshima, M.; Horikawa, Y.; Tokushima, T.; Shin, S.; Nakamori, Y.; Aoki, T. Co oxidation accompanied by degradation of Pt-Co alloy cathode catalysts in polymer electrolyte fuel cells. Phys. Chem. Chem. Phys. 2009, 11, 8226–8230. [Google Scholar] [CrossRef] [PubMed]
- Allen, B.L.; Kotchey, G.P.; Chen, Y.; Yanamala, N.V.K.; Klein-Seetharaman, J.; Kagan, V.E.; Star, A. Mechanistic investigations of horseradish peroxidase-catalyzed degradation of single-walled carbon nanotubes. J. Am. Chem. Soc. 2009, 131, 17194–17205. [Google Scholar] [CrossRef]
- Yoshida, K.; Arai, S.; Matsumoto, H.; Shirai, M.; Tanaka, N. Catalytic Etching of Multi-Walled Carbon Nanotubes Controlled by Oxygen Gas Pressure. ChemCatChem 2018, 10, 2205–2209. [Google Scholar] [CrossRef]
- Jiang, D.E.; Sumpter, B.G.; Dai, S. Unique chemical reactivity of a graphene nanoribbon’s zigzag edge. J. Chem. Phys. 2007, 126. [Google Scholar] [CrossRef]
- Yuan, W.; Zhou, Y.; Li, Y.; Li, C.; Peng, H.; Zhang, J.; Liu, Z.; Dai, L.; Shi, G. The edge- and basal-plane-specific electrochemistry of a single-layer graphene sheet. Sci. Rep. 2013, 3, 2248. [Google Scholar] [CrossRef] [Green Version]
- Martín, A.; Escarpa, A. Graphene: The cutting-edge interaction between chemistry and electrochemistry. TrAC—Trends Anal. Chem. 2014, 56, 13–26. [Google Scholar] [CrossRef]






| Pt-CNWs | Pt-CNWs H2O2 3000 Cycles | Pt-CNWs PBS 3000 Cycles | Pt-CNWs after Immersion H2O2 | Pt-CNWs after Immersion PBS | CFP | |
|---|---|---|---|---|---|---|
| ID/IG | 2.12 | 1.83 | 2.32 | 2.38 | 2.24 | 0.29 |
| ID’/IG | 0.13 | 0.1 | 0.13 | 0.13 | 0.13 | 0.05 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomatsu, M.; Hiramatsu, M.; Kondo, H.; Ishikawa, K.; Tsutsumi, T.; Sekine, M.; Hori, M. Electrochemical Reaction in Hydrogen Peroxide and Structural Change of Platinum Nanoparticle-Supported Carbon Nanowalls Grown Using Plasma-Enhanced Chemical Vapor Deposition. C 2019, 5, 7. https://doi.org/10.3390/c5010007
Tomatsu M, Hiramatsu M, Kondo H, Ishikawa K, Tsutsumi T, Sekine M, Hori M. Electrochemical Reaction in Hydrogen Peroxide and Structural Change of Platinum Nanoparticle-Supported Carbon Nanowalls Grown Using Plasma-Enhanced Chemical Vapor Deposition. C. 2019; 5(1):7. https://doi.org/10.3390/c5010007
Chicago/Turabian StyleTomatsu, Masakazu, Mineo Hiramatsu, Hiroki Kondo, Kenji Ishikawa, Takayoshi Tsutsumi, Makoto Sekine, and Masaru Hori. 2019. "Electrochemical Reaction in Hydrogen Peroxide and Structural Change of Platinum Nanoparticle-Supported Carbon Nanowalls Grown Using Plasma-Enhanced Chemical Vapor Deposition" C 5, no. 1: 7. https://doi.org/10.3390/c5010007
APA StyleTomatsu, M., Hiramatsu, M., Kondo, H., Ishikawa, K., Tsutsumi, T., Sekine, M., & Hori, M. (2019). Electrochemical Reaction in Hydrogen Peroxide and Structural Change of Platinum Nanoparticle-Supported Carbon Nanowalls Grown Using Plasma-Enhanced Chemical Vapor Deposition. C, 5(1), 7. https://doi.org/10.3390/c5010007