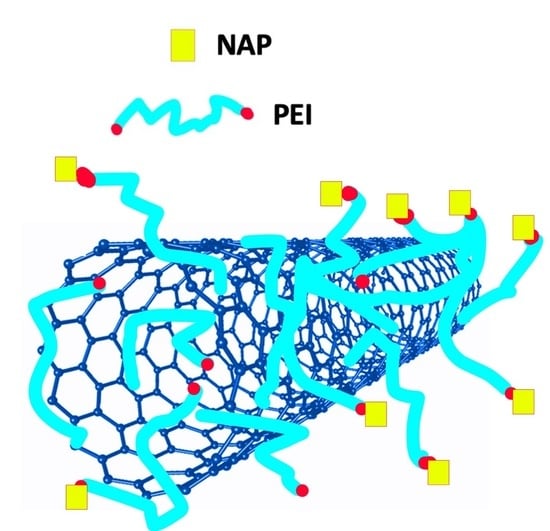
Facile Method to Prepare pH-Sensitive PEI-Functionalized Carbon Nanotubes as Rationally Designed Vehicles for Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Synthetic Remarks
2.2. Shortening and Functionalization of Mag-CNTs (NL002)
2.3. PEI-Functionalization of Carboxylated Mag-CNTs (NL003)
2.4. Indirect Coupling of NAP on Mag-CNTs@PEI (NL004)
2.5. FT-IR/UV–Vis Spectra
2.6. Raman Measurements
2.7. Drug-Release Protocol
2.8. Preparation of CT DNA Solution for DNA-Binding Studies
2.9. Albumin and DNA-Binding Studies
3. Results
3.1. Synthetic Considerations
3.2. Magnetic Properties
3.3. Raman Studies
3.4. Interaction with BSA
3.5. Interaction with DNA
3.6. Drug-Release Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dalton, A.B.; Collins, S.; Munoz, E.; Razal, J.M.; Ebron, V.H.; Ferraris, J.P.; Coleman, J.N.; Kim, B.G.; Baughman, R.H. Super-tough carbon-nanotube fibres. Nature 2003, 423, 703. [Google Scholar] [CrossRef]
- Hibino, N.; Suzuki, S.; Wakahara, H.; Kobayashi, Y.; Sato, T.; Maki, H. Short-Wavelength Electroluminescence from Single-Walled Carbon Nanotubes with High Bias Voltage. ACS Nano 2011, 5, 1215–1222. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Shi, Z.; Li, N.; Gu, Z.; Zhuang, Q. Investigation of the Electrochemical and Electrocatalytic Behavior of Single-Wall Carbon Nanotube Film on a Glassy Carbon Electrode. Anal. Chem. 2001, 73, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Shim, M.; Kam, N.W.S.; Chen, R.J.; Li, Y.M.; Dai, H.J. Functionalization of Carbon Nanotubes for Biocompatibility and Biomolecular Recognition. Nano Lett. 2002, 2, 285–288. [Google Scholar] [CrossRef]
- Chen, J.; Chen, S.; Zhao, X.; Kuznetsova, L.V.; Wong, S.S.; Ojima, I. Functionalized Single-Walled Carbon Nanotubes as Rationally Designed Vehicles for Tumor-Targeted Drug Delivery. J. Am. Chem. Soc. 2008, 130, 16778–16785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, G.; Zhang, J.; Xie, L.; Jandt, K.D. Biomimetic growth of hydroxyapatite on super water-soluble carbon nanotube-protein hybrid nanofibers. Carbon 2011, 49, 2216–2226. [Google Scholar] [CrossRef]
- Tasis, D.; Tagmatarchis, N.; Bianco, A.; Prato, M. Chemistry of Carbon Nanotubes. Chem. Rev. 2006, 106, 1105–1136. [Google Scholar] [CrossRef]
- Wei, G.; Pan, C.; Reichert, J.; Jandt, K.D. Controlled assembly of protein-protected gold nanoparticles on noncovalent functionalized carbon nanotubes. Carbon 2010, 48, 645–653. [Google Scholar] [CrossRef]
- Wei, G.; Xu, F.; Li, Z.; Jandt, K.D. Protein-Promoted Synthesis of Pt Nanoparticles on Carbon Nanotubes for Electrocatalytic Nanohybrids with Enhanced Glucose Sensing. J. Phys. Chem. C 2011, 115, 11453–11460. [Google Scholar] [CrossRef]
- Foillard, S.; Zuber, G.; Doris, E. Polyethylenimine–carbon nanotube nanohybrids for siRNA-mediated gene silencing at cellular level. Nanoscale 2011, 3, 1461–1464. [Google Scholar] [CrossRef]
- McNicholas, T.P.; Yum, K.; Ahn, J.H.; Mu, B.; Plettenburg, O.; Gooderman, A.; Natesan, S.; Strano, M.S. Structure and function of glucose binding protein-single walled carbon nanotube complexes. Small 2012, 8, 3510–3516. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H. The enhanced permeability and retention (EPR) effect in tumor vasculature: The key role of tumor-selective macromolecular drug targeting. Adv. Enzyme Regul. 2001, 41, 189–207. [Google Scholar] [CrossRef]
- Iyer, A.K.; Khaled, G.; Fang, J.; Maeda, H. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov. Today 2006, 11, 812–818. [Google Scholar] [CrossRef]
- Kostarelos, K.; Lacerda, L.; Pastorin, G.; Wu, W.; Wieckowski, S.; Luangsivilay, J.; Godefroy, S.; Pantarotto, D.; Briand, J.P.; Muller, S.; et al. Cellular uptake of functionalized carbon nanotubes is independent of functional group and cell type. Nat. Nanotechnol. 2007, 2, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Lacerda, L.; Russier, J.; Pastorin, G.; Herrero, M.A.; Venturelli, E.; Dumortier, H.; Al-Jamal, K.T.; Prato, M.; Kostarelos, K.; Bianco, A. Translocation mechanisms of chemically functionalized carbon nanotubes across plasma membranes. Biomaterials 2012, 33, 3334–3343. [Google Scholar] [CrossRef]
- Boncel, S.; Zajac, P.; Koziol, K.K. Liberation of drugs from multi-wall carbon nanotube carriers. J. Control Release 2013, 169, 126–140. [Google Scholar] [CrossRef]
- Karousis, N.; Tagmatarchis, N.; Tasis, D. Current Progress on the Chemical Modification of Carbon Nanotubes. Chem. Rev. 2010, 110, 5366–5397. [Google Scholar] [CrossRef]
- Wu, H.C.; Chang, X.; Liu, L.; Zhao, F.; Zhao, Y. Chemistry of carbon nanotubes in biomedical applications. J. Mater. Chem. 2010, 20, 1036–1052. [Google Scholar] [CrossRef]
- Singh, P.S.; Campidelli, S.; Giordani, S.; Bonifazi, D.; Bianco, A.; Prato, M. Organic functionalisation and characterisation of single-walled carbon nanotubes. Chem. Soc. Rev. 2009, 38, 2214–2230. [Google Scholar] [CrossRef]
- Peng, X.; Wong, S.S. Functional Covalent Chemistry of Carbon Nanotube Surfaces. Adv. Mater. 2009, 21, 625–642. [Google Scholar] [CrossRef]
- Lee, Y.; Geckeler, K.E. Carbon Nanotubes in the Biological Interphase: The Relevance of Noncovalence. Adv. Mater. 2010, 22, 4076–4083. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Stoddart, J.F. Noncovalent Functionalization of Single-Walled Carbon Nanotubes. Acc. Chem. Res. 2009, 42, 1161–1171. [Google Scholar] [CrossRef]
- Bianco, A.; Kostarelos, K.; Prato, M. Making carbon nanotubes biocompatible and biodegradable. Chem. Commun. 2011, 47, 10182–10188. [Google Scholar] [CrossRef]
- Fabbro, C.; Ali-Boucetta, H.; Ros, T.D.; Kostarelos, K.; Bianco, A.; Prato, M. Targeting carbon nanotubes against cancer. Chem. Commun. 2012, 48, 3911–3926. [Google Scholar] [CrossRef]
- Hong, H.; Gao, T.; Cai, W. Molecular Imaging with Single-Walled Carbon Nanotubes. Nano Today 2009, 4, 252–261. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Yang, K.; Lee, S.T. Single-walled carbon nanotubes in biomedical imaging. J. Mater. Chem. 2011, 21, 586–598. [Google Scholar] [CrossRef]
- Kuznik, N.; Tomczyk, M.M. Multiwalled carbon nanotube hybrids as MRI contrast agents. Beilstein J. Nanotechnol. 2016, 7, 1086–1103. [Google Scholar] [CrossRef]
- Vittorio, O.; Duce, S.L.; Pietrabissa, A.; Cuschieri, A. Multiwall carbon nanotubes as MRI contrast agents for tracking stem cells. Nanotechnology 2011, 22, 095706. [Google Scholar] [CrossRef]
- Masotti, A.; Caporali, A. Preparation of Magnetic Carbon Nanotubes (Mag-CNTs) for Biomedical and Biotechnological Applications. Int. J. Mol. Sci. 2013, 14, 24619–24642. [Google Scholar] [CrossRef] [Green Version]
- Battigelli, A.; Ménard-Moyon, C.; Ros, T.D.; Prato, M.; Bianco, A. Endowing carbon nanotubes with biological and biomedical properties by chemical modifications. Adv. Drug Deliv. Rev. 2013, 65, 1899–1920. [Google Scholar] [CrossRef]
- Zeinabad, H.A.; Zarrabian, A.; Saboury, A.A.; Alizadeh, A.M.; Falahati, M. Interaction of single and multiwall carbon nanotubes with the biological systems: Tau protein and PC12 cells as targets. Sci. Rep. 2016, 6, 26508. [Google Scholar] [CrossRef]
- Meek, L.I.; van der Laar, A.F.J.M.; Vonkeman, E.H. Non Steroidal Anti-Inflammatory Drugs: An overview of Cardiovascular Risks. Pharmaceuticals 2010, 3, 2146–2162. [Google Scholar] [CrossRef] [Green Version]
- Weder, J.E.; Dillon, C.T.; Hambley, T.W.; Kennedy, B.J.; Lay, P.A.; Biffin, J.R.; Regtop, H.L.; Davies, N.M. Copper Complexes of Non-steroidal Anti-inflammatory Drugs: An Opportunity yet to be Realized. Coord. Chem. Rev. 2002, 232, 95–126. [Google Scholar] [CrossRef]
- Psomas, G.; Kessissoglou, D.P. Quinolones and Non-Steroidal Antiinflammatory Drugs Interacting with Copper(II), Nickel(II), Cobalt(II) and Zinc(II): Structural Features, Biological Evaluation and Perspectives. Dalton Trans. 2013, 42, 6252–6276. [Google Scholar] [CrossRef]
- Banti, C.N.; Hadjikakou, S.K. Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) in Metal Complexes and Their Effect at the Cellular Level. Eur. J. Inorg. Chem. 2016, 3048–3071. [Google Scholar] [CrossRef]
- Psomas, G. Copper(II) and zinc(II) coordination compounds of non-steroidal anti-inflammatory drugs: Structural features and antioxidant activity. Coord. Chem. Rev. 2020, 412, 213259. [Google Scholar] [CrossRef]
- Habibizadeh, M.; Rostamizadeh, K.; Dalali, N.; Ramazani, A. Preparation and characterization of PEGylated multiwall carbon nanotubes as covalently conjugated and non-covalent drug carrier: A comparative study. Mat. Sci. Eng. C 2017, 74, 1–9. [Google Scholar] [CrossRef]
- Marmur, J. A procedure for the isolation of deoxyribonucleic acid from micro-organisms. J. Mol. Biol. 1961, 3, 208–218. [Google Scholar] [CrossRef]
- Reichmann, M.F.; Rice, S.A.; Thomas, C.A.; Doty, P. A Further Examination of the Molecular Weight and Size of Desoxypentose Nucleic Acid. J. Am. Chem. Soc. 1954, 76, 3047–3053. [Google Scholar] [CrossRef]
- Dimiza, F.; Perdih, F.; Tangoulis, V.; Turel, I.; Kessissoglou, D.P.; Psomas, G. Interaction of copper(II) with the non-steroidal anti-inflammatory drugs naproxen and diclofenac: Synthesis, structure, DNA- and albumin-binding. J. Inorg. Biochem. 2011, 105, 476–489. [Google Scholar] [CrossRef]
- Dimiza, F.; Raptopoulou, C.P.; Psycharis, V.; Papadopoulos, A.N.; Psomas, G. Manganese(II) complexes with the non-steroidal anti-inflammatory drugs naproxen and mefenamic acid. Synthesis, structure, antioxidant capacity, interaction with albumins and DNA. N. J. Chem. 2018, 42, 16666–16681. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Plenum Press: New York, NY, USA, 2006. [Google Scholar]
- Stella, L.; Capodilupo, A.L.; Bietti, M. A reassessment of the association between azulene and [60]fullerene. Possible pitfalls in the determination of binding constants through fluorescence spectroscopy. Chem. Commun. 2008, 4744–4746. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, H.; Zhang, G.; Tao, W.; Tang, S. Interaction of the flavonoid hesperidin with bovine serum albumin: A fluorescence quenching study. J. Lumin. 2007, 126, 211–218. [Google Scholar] [CrossRef]
- Wolfe, A.; Shimer, G.; Meehan, T. Polycyclic aromatic hydrocarbons physically intercalate into duplex regions of denatured DNA. Biochemistry 1987, 26, 6392–6396. [Google Scholar] [CrossRef]
- Zhao, G.; Lin, H.; Zhu, S.; Sun, H.; Chen, Y. Dinuclear palladium(II) complexes containing two monofunctional [Pd(en)(pyridine)Cl]+ units bridged by Se or S. Synthesis, characterization, cytotoxicity and kinetic studies of DNA-binding. J. Inorg. Biochem. 1998, 70, 219–226. [Google Scholar] [CrossRef]
- Heller, D.P.; Greenstock, C.L. Fluorescence lifetime analysis of DNA intercalated ethidium bromide and quenching by free dye. Biophys. Chem. 1994, 50, 305–312. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, D.C.; Zhang, W.D.; Jiang, X.; He, C.B.; Chung, T.S.; Goh, S.H.; Leong, K.W. Polyethylenimine-grafted multiwalled carbon nanotubes for secure noncovalent immobilization and efficient delivery of DNA. Angew. Chem. Int. Ed. Engl. 2005, 44, 4782–4785. [Google Scholar] [CrossRef]
- Wang, L.; Shi, J.; Zhang, H.; Li, H.; Gao, Y.; Wang, Z.; Wang, H.; Li, L.; Zhang, C.; Chen, C.; et al. Synergistic anticancer effect of RNAi and photothermal therapy mediated by functionalized single-walled carbon nanotubes. Biomaterials 2013, 34, 262–274. [Google Scholar] [CrossRef]
- Huang, Y.P.; Lin, I.J.; Chen, C.C.; Hsu, Y.C.; Chang, C.C.; Lee, M.J. Delivery of small interfering RNAs in human cervical cancer cells by polyethylenimine-functionalized carbon nanotubes. Nanoscale Res. Lett. 2013, 8, 267–278. [Google Scholar] [CrossRef] [Green Version]
- Georgiadou, V.; Makris, G.; Papagiannopoulou, D.; Vourlias, G.; Dendrinou-Samara, C. Octadecylamine-Mediated Versatile Coating of CoFe2O4 NPs for the Sustained Release of Anti-inflammatory Drug Naproxen and in vivo Target Selectivity. Appl. Mater. Interfaces 2016, 8, 9345–9360. [Google Scholar] [CrossRef]
- Zhang, X.X.; Wen, G.H.; Huang, S.; Dai, L.; Gao, R.; Wang, Z.L. Magnetic properties of Fe nanoparticles trapped at the tips of the aligned carbon nanotubes. J. Magn. Magn. Mater. 2001, 231, L9–L12. [Google Scholar] [CrossRef]
- Gozzi, D.; Latini, A.; Capannelli, G.; Canepa, F.; Napoletano, M.; Cimberle, M.R.; Tropeano, M. Synthesis and magnetic characterization of Ni nanoparticles and Ni nanoparticles in multiwalled carbon nanotubes. J. Alloys Comp. 2006, 419, 32–39. [Google Scholar] [CrossRef]
- Zhang, X.X.; Hernàndez, J.M.; Tejada, J.; Solé, R.; Ruiz, X. Magnetic properties and domain-wall motion in single-crystal BaFe10.2Sn0.74Co0.66O19. Phys. Rev. B 2006, 53, 3336–3340. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Yusuf, S.M. The phenomenon of negative magnetization and its implications. Phys. Rep. 2015, 556, 1–34. [Google Scholar] [CrossRef]
- Sarkar, B.; Dalal, B.; De, S.K. Temperature induced magnetization reversal in SrRuO3. Appl. Phys. Lett. 2013, 103, 252403. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Dresselhaus, G.; Saito, R.; Jorio, A. Raman spectroscopy of carbon nanotubes. Phys. Rep. 2005, 409, 47–99. [Google Scholar] [CrossRef]
- Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S.; Cançado, L.G.; Jorio, A.; Saito, R. Studying disorder in graphite-based systems by Raman spectroscopy. Phys. Chem. Chem. Phys. 2007, 9, 1276–1291. [Google Scholar] [CrossRef]
- Dimiza, F.; Papadopoulos, A.N.; Tangoulis, V.; Psycharis, V.; Raptopoulou, C.P.; Kessissoglou, D.P.; Psomas, G. Biological evaluation of cobalt(II) complexes with non-steroidal anti-inflammatory drug naproxen. J. Inorg. Biochem. 2012, 107, 54–64. [Google Scholar] [CrossRef]
- Totta, X.; Hatzidimitriou, A.G.; Papadopoulos, A.N.; Psomas, G. Nickel(II)–naproxen Mixed-Ligand Complexes: Synthesis, Structure, Antioxidant Activity and Interaction with Albumins and Calf-Thymus DNA. New J. Chem. 2017, 41, 4478–4492. [Google Scholar] [CrossRef]
- Rajendiran, V.; Karthik, R.; Palaniandavar, M.; Stoeckli-Evans, H.; Periasamy, V.S.; Akbarsha, M.A.; Srinag, B.S.; Krishnamurthy, H. Mixed-Ligand Copper(II)-phenolate Complexes: Effect of Coligand on Enhanced DNA and Protein Binding, DNA Cleavage, and Anticancer Activity. Inorg. Chem. 2007, 46, 8208–8221. [Google Scholar] [CrossRef]
- Roy, S.; Banerjee, R.; Sarkar, M. Direct binding of Cu(II)-complexes of oxicam NSAIDs with DNA backbone. J. Inorg. Biochem. 2006, 100, 1320–1331. [Google Scholar] [CrossRef] [PubMed]
- Pyle, A.M.; Rehmann, J.P.; Meshoyrer, R.; Kumar, C.V.; Turro, N.J.; Barton, J.K. Mixed-ligand complexes of ruthenium(II): Factors governing binding to DNA. J. Am. Chem. Soc. 1989, 111, 3051–3058. [Google Scholar] [CrossRef]
- Dimitrakopoulou, A.; Dendrinou-Samara, C.; Pantazaki, A.A.; Alexiou, M.; Nordlander, E.; Kessissoglou, D.P. Synthesis, structure and interactions with DNA of novel tetranuclear, [Mn4(II/II/II/IV)] mixed valence complexes. J. Inorg. Biochem. 2008, 102, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Pratviel, G.; Bernadou, J.; Meunier, B. DNA and RNA Cleavage by Metal Complexes. Adv. Inorg. Chem. 1998, 45, 251–262. [Google Scholar]
- Garcia-Gimenez, J.L.; Gonzalez-Alvarez, M.; Liu-Gonzalez, M.; Macias, B.; Borras, J.; Alzuet, G. Toward the development of metal-based synthetic nucleases: DNA binding and oxidative DNA cleavage of a mixed copper(II) complex with N -(9H -purin-6-yl)benzenesulfonamide and 1,10-phenantroline. Antitumor activity in human Caco-2 cells and Jurkat T lymphocytes. Evaluation of p53 and Bcl-2 proteins in the apoptotic mechanism. J. Inorg. Biochem. 2009, 103, 923–934. [Google Scholar]
- Wilson, W.D.; Ratmeyer, L.; Zhao, M.; Strekowski, L.; Boykin, D. The search for structure-specific nucleic acid-interactive drugs: Effects of compound structure on RNA versus DNA interaction strength. Biochemistry 1993, 32, 4098–4104. [Google Scholar] [CrossRef]
- Sousa, C.T.; Nunes, C.; Proenca, M.P.; Leitao, D.C.; Lima, J.L.; Reis, S.; Araujo, J.P.; Lucio, M. pH sensitive silica nanotubes as rationally designed vehicles for NSAIDs delivery. Colloids Surf. B Biointerfaces 2012, 94, 288–295. [Google Scholar] [CrossRef]
- Tozuka, Y.; Yokohama, C.; Higashi, K.; Moribe, K.; Yamamoto, K. Adsorption state of naphthoic acids on folded sheets mesoporous materials with different pore sizes. J. Drug Del. Sci. Tech. 2009, 19, 401–404. [Google Scholar] [CrossRef]









| D (Pos, FWHM, A) * | G (Pos, FWHM, A) | D’ (Pos, FWHM, A) | ID/IG | |
|---|---|---|---|---|
| NL001 | (1302.2, 71.0, 82.4) | (1579.5, 61.9, 38.7) | (1600.1, 33.1, 13.5) | 2.13 |
| NL002 | (1307.3, 67.5, 152.9) | (1580.8, 56.7, 59.5) | (1606.5, 30.4, 25.8) | 2.57 |
| NL004 | (1303.9, 69.0, 102.8) | (1575.7, 58.1, 41.8) | (1601.0, 29.2, 14.8) | 2.46 |
| Spectroscopic Data and Constants | NL004 a | NAP b |
|---|---|---|
| BSA fluorescence quenching (ΔI/Io, %) | 29.1 | 23.0 |
| KSV (M−1) | 2.05(±0.10) × 104 | 1.18(±0.06) × 104 |
| kq (M−1s−1) | 2.05(±0.10) × 1012 | 1.18(±0.06) × 1012 |
| K (M−1) | 7.84(±0.35) × 104 | 5.35(±0.42) × 103 |
| N | 0.40 | 2.14 |
| Spectroscopic Data and Constants | NL004 a | NAP b |
|---|---|---|
| λ(nm) (ΔA/Ao(%), Δλ(nm) c | 265 (−27, 0); 311 (−40, elm d); 324 (−39, +6); 355 (−40, elm) | 325(+22, +2) |
| Kb (M−1) | 8.66(±0.07) × 104 | 2.67(±0.22) × 104 |
| EB–DNA fluorescence quenching (ΔI/Io, %) | 50.7 | 82.0 |
| KSV (M−1) | 3.37(±0.10) × 104 | 1.47(±0.04) × 105 |
| kq (M−1s−1) | 1.47(±0.04) × 1012 | 6.39(±0.17) × 1012 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tangoulis, V.; Lalioti, N.; Parthenios, J.; Langford, N.; Valsami-Jones, E.; Kakoulidou, C.; Psomas, G.; Bekiari, V. Facile Method to Prepare pH-Sensitive PEI-Functionalized Carbon Nanotubes as Rationally Designed Vehicles for Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) Delivery. C 2020, 6, 62. https://doi.org/10.3390/c6040062
Tangoulis V, Lalioti N, Parthenios J, Langford N, Valsami-Jones E, Kakoulidou C, Psomas G, Bekiari V. Facile Method to Prepare pH-Sensitive PEI-Functionalized Carbon Nanotubes as Rationally Designed Vehicles for Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) Delivery. C. 2020; 6(4):62. https://doi.org/10.3390/c6040062
Chicago/Turabian StyleTangoulis, Vassilis, Nikolia Lalioti, John Parthenios, Nathan Langford, Eugenia Valsami-Jones, Chrisoula Kakoulidou, George Psomas, and Vlasoula Bekiari. 2020. "Facile Method to Prepare pH-Sensitive PEI-Functionalized Carbon Nanotubes as Rationally Designed Vehicles for Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) Delivery" C 6, no. 4: 62. https://doi.org/10.3390/c6040062
APA StyleTangoulis, V., Lalioti, N., Parthenios, J., Langford, N., Valsami-Jones, E., Kakoulidou, C., Psomas, G., & Bekiari, V. (2020). Facile Method to Prepare pH-Sensitive PEI-Functionalized Carbon Nanotubes as Rationally Designed Vehicles for Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) Delivery. C, 6(4), 62. https://doi.org/10.3390/c6040062