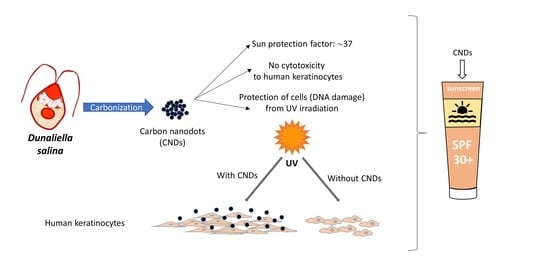
Carbon Nanodots Synthesized from Dunaliella salina as Sun Protection Filters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Instruments
2.3. Dunaliella Salina Culture Conditions
2.4. Synthesis of CNDs
2.5. Sun Protection Factor (SPF) Calculation
2.6. In Vitro Toxicity Study
2.7. In Vitro UV Protection
2.8. Cell Cycle Analysis
2.9. Statistical Analysis
3. Results and Discussions
3.1. CNDs Characterization and Optical Properties
3.2. SPF of CNDs
3.3. In Vitro Assessment of CNDs Toxicity, UV Protection, and Cell Cycle
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Priyanka, S.; Inala, M.S.R.; Nandini, H.; Kutty, A.; Kiranmayee, P. A pilot study on sun protection factor of plant extracts: An observational study. Asian J. Pharm. Clin. Res. 2018, 11, 67–71. [Google Scholar]
- Nicoara, A.I.; Ene, V.L.; Voicu, B.B.; Bucur, M.A.; Neacsu, I.A.; Vasile, B.S.; Iordache, F. Biocompatible ag/fe-enhanced Tio2 nanoparticles as an effective compound in sunscreens. Nanomaterials 2020, 10, 570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Environmental Health Criteria (EHC) 160: Ultraviolet Radiation; WHO: Geneva, Switzerland, 1994; Volume 1. [Google Scholar]
- Kaur, C.D.; Saraf, S. In vitro sun protection factor determination of herbal oils used in cosmetics. Pharmacogn. Res. 2010, 2, 22–25. [Google Scholar]
- Malsawmtluangi, C.; Nath, D.K.; Jamatia, I.; Lianhimgthangi, E.Z.; Pachuau, L. Determination of Sun Protection Factor (SPF) number of some aqueous herbal extracts. J. Appl. Pharm. Sci. 2013, 3, 150–151. [Google Scholar]
- Hess, S.C.; Permatasari, F.A.; Fukazawa, H.; Schneider, E.M.; Balgis, R.; Ogi, T.; Okuyama, K.; Stark, W.J. Direct synthesis of carbon quantum dots in aqueous polymer solution: One-pot reaction and preparation of transparent UV-blocking films. J. Mater. Chem. A 2017, 5, 5187–5194. [Google Scholar] [CrossRef]
- Costa, C.; Detoni, C.B.; Branco, C.; Botura, M.B.; Branco, A. In vitro photoprotective effects of Marcetia taxifolia ethanolic extract and its potential for sunscreen formulations. Rev. Bras. Farm. 2015, 25, 413–418. [Google Scholar] [CrossRef] [Green Version]
- Ebrahimzadeh, M.A.; Enayatifard, R.; Khalili, M.; Ghaffarloo, M.; Saeedi, M.; Charati, J.Y. Correlation between sun protection factor and antioxidant activity, phenol and flavonoid contents of some medicinal plants. Iran. J. Pharm. Res. 2014, 13, 1041–1048. [Google Scholar]
- Chatzimitakos, T.; Stalikas, C. Antimicrobial properties of carbon quantum dots. In Nanotoxicity, 1st ed.; Rajendran, S., Mukherjee, A., Nguyen, T., Godugu, C., Shuklapp, R.K., Eds.; Elsevier GmbH: Munich, Germany, 2020; pp. 301–315. [Google Scholar]
- Kasouni, A.; Chatzimitakos, T.; Stalikas, C. Bioimaging Applications of Carbon Nanodots: A Review. J. Carbon Res. 2019, 5, 19. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Kang, Z.; Liu, Y.; Lee, S.T. Carbon nanodots: Synthesis, properties and applications. J. Mater. Chem. 2012, 22, 24230–24253. [Google Scholar] [CrossRef]
- Wang, J.; Qiu, J. A review of carbon dots in biological applications. J. Mater. Sci. 2016, 51, 4728–4738. [Google Scholar] [CrossRef]
- Zeng, Q.; Shao, D.; He, X.; Ren, Z.; Ji, W.; Shan, C.; Qu, S.; Li, J.; Chen, L.; Li, Q. Carbon dots as a trackable drug delivery carrier for localized cancer therapy: In vivo. J. Mater. Chem. B 2016, 4, 5119–5126. [Google Scholar] [CrossRef]
- Chatzimitakos, T.G.; Kasouni, A.I.; Troganis, A.N.; Stalikas, C.D. Carbonization of Human Fingernails: Toward the Sustainable Production of Multifunctional Nitrogen and Sulfur Codoped Carbon Nanodots with Highly Luminescent Probing and Cell Proliferative/Migration Properties. ACS Appl. Mater. Interfaces 2018, 10, 16024–16032. [Google Scholar] [CrossRef]
- Chatzimitakos, T.G.; Kasouni, A.I.; Troganis, A.N.; Stalikas, C.D. Exploring the antibacterial potential and unraveling the mechanism of action of non-doped and heteroatom-doped carbon nanodots. J. Nanopart. Res. 2020, 22, 36. [Google Scholar] [CrossRef]
- Turner, M.F. Dunaliella: Physiology, biochemistry, and biotechnology. J. Exp. Mar. Bio. Ecol. 1993, 171, 142–144. [Google Scholar] [CrossRef]
- Ben-Amotz, A.; Avron, M. The biotechnology of mass culturing Dunaliella for products of commercial interest. In Algal and Cyanobacterial Biotechnology; Cresswell, R.C., Rees, T.A.V., Shah, N., Eds.; Longman Scientific and Technical: New York, NY, USA, 1989; pp. 91–114. [Google Scholar]
- Rocio Gomez, J.O. Apoptosis comparison effects between synthetic and natural β-carotene from dunaliella salina on MDA-MB-231 Brest Cancer Cells. J. Microb. Biochem. Technol. 2015, 7, 51–56. [Google Scholar] [CrossRef] [Green Version]
- Magarelli, M.; Passamonti, P.; Renieri, C. Purification, characterization and analysis of sepia melanin from Purification, characterization and analysis of sepia melanin from. Rev. Ces Med. Vet. y Zootec. 2010, 5, 18–29. [Google Scholar]
- Solano, F. Photoprotection and skin pigmentation: Melanin-related molecules and some other new agents obtained from natural sources. Molecules 2020, 25, 1537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walne, P.R. Studies on the Food Value of Nineteen Genera of Algae to Juvenile Bivalves of the Genera Ostrea, Crassostrea, Mercenaria and Mytilus. Fish. Invest. Ser. 1970, 26, 62p. [Google Scholar]
- Sayre, R.M.; Agin, P.P.; LeVee, G.J.; Marlowe, E. A comparison of in vivo and in vitro testing of sunscreening formulas. Photochem. Photobiol. 1979, 29, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Chatzimarkou, A.; Chatzimitakos, T.G.; Kasouni, A.; Sygellou, L.; Avgeropoulos, A.; Stalikas, C.D. Selective FRET-based sensing of 4-nitrophenol and cell imaging capitalizing on the fluorescent properties of carbon nanodots from apple seeds. Sens. Actuators B Chem. 2018, 258, 1152–1160. [Google Scholar] [CrossRef]
- Chatzimitakos, T.; Kasouni, A.; Sygellou, L.; Avgeropoulos, A.; Troganis, A.; Stalikas, C. Two of a kind but different: Luminescent carbon quantum dots from Citrus peels for iron and tartrazine sensing and cell imaging. Talanta 2017, 175, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Chatzimitakos, T.; Kasouni, A.; Sygellou, L.; Leonardos, I.; Troganis, A.; Stalikas, C. Human fingernails as an intriguing precursor for the synthesis of nitrogen and sulfur-doped carbon dots with strong fluorescent properties: Analytical and bioimaging applications. Sens. Actuators B Chem. 2018, 267, 494–501. [Google Scholar] [CrossRef]
- Prasannan, A.; Imae, T. One-pot synthesis of fluorescent carbon dots from orange waste peels. Ind. Eng. Chem. Res. 2013, 52, 15673–15678. [Google Scholar] [CrossRef]
- Edison, T.N.J.I.; Atchudan, R.; Sethuraman, M.G.; Shim, J.J.; Lee, Y.R. Microwave assisted green synthesis of fluorescent N-doped carbon dots: Cytotoxicity and bio-imaging applications. J. Photochem. Photobiol. B Biol. 2016, 161, 154–161. [Google Scholar] [CrossRef]
- Wang, H.; Lu, Q.; Hou, Y.; Liu, Y.; Zhang, Y. High fluorescence S, N co-doped carbon dots as an ultra-sensitive fluorescent probe for the determination of uric acid. Talanta 2016, 155, 62–69. [Google Scholar] [CrossRef]
- Pudza, M.Y.; Abidin, Z.Z.; Rashid, S.A.; Yasin, F.M.; Noor, A.S.M.; Issa, M.A. Eco-friendly sustainable fluorescent carbon dots for the adsorption of heavy metal ions in aqueous environment. Nanomaterials 2020, 10, 315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, W.; Fan, H.; Ai, S.; Zhu, L. Preparation of fluorescent graphene quantum dots from humic acid for bioimaging application. New J. Chem. 2015, 39, 7054–7059. [Google Scholar] [CrossRef]
- Shokry, A.; Khalil, M.M.A.; Ibrahim, H.; Soliman, M.; Ebrahim, S. Highly Luminescent Ternary Nanocomposite of Polyaniline, Silver Nanoparticles and Graphene Oxide Quantum Dots. Sci. Rep. 2019, 9, 16984. [Google Scholar] [CrossRef] [Green Version]
- Hu, G.; Lei, B.; Jiao, X.; Wu, S.; Zhang, X.; Zhuang, J.; Liu, X.; Hu, C.; Liu, Y. Synthesis of modified carbon dots with performance of ultraviolet absorption used in sunscreen. Opt. Express 2019, 27, 7629. [Google Scholar] [CrossRef]
- Zuo, D.; Liang, N.; Xu, J.; Chen, D.; Zhang, H. UV protection from cotton fabrics finished with boron and nitrogen co-doped carbon dots. Cellulose 2019, 26, 4205–4212. [Google Scholar] [CrossRef]
- Khalil, C.; Shebaby, W. UVB damage onset and progression 24 h post exposure in human-derived skin cells. Toxicol. Rep. 2017, 4, 441–449. [Google Scholar] [CrossRef]
- Smolinska, E.; Moskot, M.; Jakóbkiewicz-Banecka, J.; Wegrzyn, G.; Banecki, B.; Szczerkowska-Dobosz, A.; Purzycka-Bohdan, D.; Gabig-Ciminska, M. Molecular action of isoflavone genistein in the human epithelial cell line HaCaT. PLoS ONE 2018, 13, e0192297. [Google Scholar] [CrossRef] [PubMed]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatzimitakos, T.G.; Kasouni, A.; Troganis, A.; Leonardos, I.; Tzovenis, I.; Ntzouvaras, A.; Stalikas, C. Carbon Nanodots Synthesized from Dunaliella salina as Sun Protection Filters. C 2020, 6, 69. https://doi.org/10.3390/c6040069
Chatzimitakos TG, Kasouni A, Troganis A, Leonardos I, Tzovenis I, Ntzouvaras A, Stalikas C. Carbon Nanodots Synthesized from Dunaliella salina as Sun Protection Filters. C. 2020; 6(4):69. https://doi.org/10.3390/c6040069
Chicago/Turabian StyleChatzimitakos, Theodoros G., Athanasia Kasouni, Anastassios Troganis, Ioannis Leonardos, Ioannis Tzovenis, Alexandros Ntzouvaras, and Constantine Stalikas. 2020. "Carbon Nanodots Synthesized from Dunaliella salina as Sun Protection Filters" C 6, no. 4: 69. https://doi.org/10.3390/c6040069
APA StyleChatzimitakos, T. G., Kasouni, A., Troganis, A., Leonardos, I., Tzovenis, I., Ntzouvaras, A., & Stalikas, C. (2020). Carbon Nanodots Synthesized from Dunaliella salina as Sun Protection Filters. C, 6(4), 69. https://doi.org/10.3390/c6040069