Pilot Scale Fermentations of Sangiovese: An Overview on the Impact of Saccharomyces and Non-Saccharomyces Wine Yeasts
Abstract
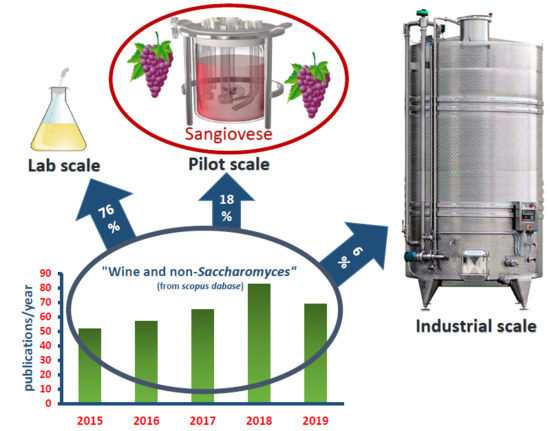
:1. Introduction
2. Material and Methods
2.1. Yeast Strains
2.2. Pilot Scale Fermentation
2.3. Analyses
2.3.1. Evaluation of Cell Growth
2.3.2. Analytical Determinations
2.3.3. Sensory Evaluation of Wines
2.4. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bisson, L.F.; Joseph, C.L.; Domizio, P.Y. Biology of Microorganisms on Grapes, in Must and in Wine; Springer: Cham, Switzerland, 2017; pp. 65–101. [Google Scholar]
- Bely, M.; Stoeckle, P.; Masneuf-Pomarède, I.; Dubourdieu, D. Impact of mixed Torulaspora delbrueckii-Saccharomyces cerevisiae culture on high-sugar fermentation. Int. J. Food Microbiol. 2008, 122, 312–320. [Google Scholar] [CrossRef]
- Renault, P.; Miot-Sertier, C.; Marullo, P.; Lagarrigue, L.; Lonvaud-Funel, A.; Bely, M. Genetic characterization and phenotypic variability in Torulaspora delbrueckii species: Potential applications in the wine industry. Int. J. Food Microbiol. 2009, 134, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Comitini, F.; Gobbi, M.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Selected non-Saccharomyces wine yeasts in controlled multistarter fermentations with Saccharomyces cerevisiae. Food Microbiol. 2011, 28, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Sadoudi, M.; Tourdot-Maréchal, R.; Rousseaux, S.; Steyer, D.; Gallardo-Chacón, J.-J.; Ballester, J.; Vichi, S.; Guérin-Schneider, R.; Caixach, J.; Alexandre, H. Yeast–yeast interactions revealed by aromatic profile analysis of Sauvignon Blanc wine fermented by single or co-culture of non-Saccharomyces and Saccharomyces yeasts. Food Microbiol. 2012, 32, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Van Breda, V.; Jolly, N.; Van Wyk, J. Characterisation of commercial and natural Torulaspora delbrueckii wine yeast strains. Int. J. Food Microbiol. 2013, 163, 80–88. [Google Scholar] [CrossRef]
- Benito, S. The impact of Torulaspora delbrueckii yeast in winemaking. Appl. Microbiol. Biotechnol. 2018, 102, 3081–3094. [Google Scholar] [CrossRef]
- Renault, P.; Coulon, J.; Moine, V.; Thibon, C.; Bely, M. Enhanced 3-sulfanylhexan-1-ol production in sequential mixed fermentation with Torulaspora delbrueckii/Saccharomyces cerevisiae reveals a situation of synergistic interaction between two industrial strains. Front. Microbiol. 2016, 7, 293–303. [Google Scholar] [CrossRef] [Green Version]
- Belda, I.; Ruiz, J.; Beisert, B.; Navascués, E.; Marquina, D.; Calderón, F.; Rauhut, D.; Benito, S.; Santos, A. Influence of Torulaspora delbrueckii in varietal thiol (3-SH and 4-MSP) release in wine sequential fermentations. Int. J. Food Microbiol. 2017, 257, 183–191. [Google Scholar] [CrossRef]
- Rantsiou, K.; Dolci, P.; Giacosa, S.; Torchio, F.; Tofalo, R.; Torriani, S.; Suzzi, G.; Rolle, L.; Cocolin, L. Candida zemplinina can reduce acetic acid produced by Saccharomyces cerevisiae in sweet wine fermentations. Appl. Environ. Microbiol. 2012, 78, 1987–1994. [Google Scholar] [CrossRef] [Green Version]
- Englezos, V.; Rantsiou, K.; Torchio, F.; Rolle, L.; Gerbi, V.; Cocolin, L. Exploitation of the non-Saccharomyces yeast Starmerella bacillaris (synonym Candida zemplinina) in wine fermentation: Physiological and molecular characterizations. Int. J. Food Microbiol. 2015, 199, 33–40. [Google Scholar] [CrossRef]
- Lencioni, L.; Taccari, M.; Ciani, M.; Domizio, P. Zygotorulaspora florentina and Starmerella bacillaris in multistarter fermentation with Saccharomyces cerevisiae to reduce volatile acidity of high sugar musts. Aust. J. Grape Wine R. 2018, 24, 368–372. [Google Scholar] [CrossRef]
- Ciani, M.; Ferraro, L. Combined use of immobilized Candida stellata cells and Saccharomyces cerevisiae to improve the quality of wines. J. Appl. Microbiol. 1998, 85, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Soden, A.; Francis, I.L.; Oakey, H.; Henschke, P.A. Effects of co-fermentation with Candida stellata and Saccharomyces cerevisiae on the aroma and composition of Chardonnay wine. Aust. J. Grape Wine Res. 2000, 6, 21–30. [Google Scholar] [CrossRef]
- Sipiczki, M. Candida zemplinina sp. nov., an osmotolerant and psychrotolerant yeast that ferments sweet botrytized wines. Int. J. Syst. Evol. Microbiol. 2003, 53, 2079–2083. [Google Scholar] [CrossRef] [Green Version]
- Magyar, I.; Tóth, T. Comparative evaluation of some oenological properties in wine strains of Candida stellata, Candida zemplinina, Saccharomyces uvarum and Saccharomyces cerevisiae. Food Microbiol. 2011, 28, 94–100. [Google Scholar] [CrossRef]
- Tofalo, R.; Schirone, M.; Torriani, S.; Rantsiou, K.; Cocolin, L.; Perpetuini, G.; Suzzi, G. Diversity of Candida zemplinina strains from grapes and Italian wines. Food Microbiol. 2012, 29, 18–26. [Google Scholar] [CrossRef]
- Di Maio, S.; Genna, G.; Gandolfo, V.; Amore, G.; Ciaccio, M.; Oliva, D. Presence of Candida zemplinina in Sicilian musts and selection of a strain for wine mixed fermentations. S. Afr. J. Enol. Vitic. 2012, 33, 80–87. [Google Scholar] [CrossRef]
- Canonico, L.; Comitini, F.; Oro, L.; Ciani, M. Sequential fermentation with selected immobilized non-Saccharomyces yeast for reduction of ethanol content in wine. Front. Microbiol. 2016, 7, 278. [Google Scholar] [CrossRef] [Green Version]
- Englezos, V.; Rantsiou, K.; Cravero, F.; Torchio, F.; Ortiz-Julien, A.; Gerbi, V.; Rolle, L.; Cocolin, L. Starmerella bacillaris and Saccharomyces cerevisiae mixed fermentations to reduce ethanol content in wine. Appl. Microbiol. Biot. 2016, 100, 5515–5526. [Google Scholar] [CrossRef]
- Kapsopoulou, K.; Mourtzini, A.; Anthoulas, M.; Nerantzis, E. Biological acidification during grape must fermentation using mixed cultures of Kluyveromyces thermotolerans and Saccharomyces cerevisiae. World J. Microbiol. Biotechnol. 2007, 23, 735–739. [Google Scholar] [CrossRef]
- Gobbi, M.; Comitini, F.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Lachancea thermotolerans and Saccharomyces cerevisiae in simultaneous and sequential co-fermentation: A strategy to enhance acidity and improve the overall quality of wine. Food Microbiol. 2013, 33, 271–281. [Google Scholar] [CrossRef]
- Benito, Á.; Calderón, F.; Palomero, F.; Benito, S. Quality and composition of airén wines fermented by sequential inoculation of Lachancea thermotolerans and Saccharomyces cerevisiae. Food Technol. Biotechnol. 2016, 54, 135–144. [Google Scholar] [CrossRef]
- Vilela, A. Lachancea thermotolerans, the non-Saccharomyces yeast that reduces the volatile acidity of wines. Fermentation 2018, 4, 56. [Google Scholar] [CrossRef] [Green Version]
- Ciani, M.; Comitini, F.; Mannazzu, I.; Domizio, P. Controlled mixed culture fermentation: A new perspective on the use of non-Saccharomyces yeasts in winemaking. FEMS Yeast Res. 2010, 10, 123–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosi, I.; Vinella, M.; Domizio, P. Characterization of β-glucosidase activity in yeasts of oenological origin. J. Appl. Microbiol. 1994, 77, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.; Ubeda, J.F.; Briones, A.I. Typing of non-Saccharomyces yeasts with enzymatic activities of interest in wine-making. Int. J. Food Microbiol. 2000, 59, 29–36. [Google Scholar] [CrossRef]
- Barbosa, C.; Lage, P.; Esteves, M.; Chambel, L.; Mendes-Faia, A.; Mendes-Ferreira, A. Molecular and Phenotypic Characterization of Metschnikowia pulcherrima Strains from Douro Wine Region. Fermentation 2018, 4, 8. [Google Scholar] [CrossRef] [Green Version]
- Zott, K.; Thibon, C.; Bely, M.; Lonvaud-Funel, A.; Dubourdieu, D.; Masneuf-Pomarede, I. The grape must non-Saccharomyces microbial community: Impact on volatile thiol release. Int. J. Food Microbiol. 2011, 151, 210–215. [Google Scholar] [CrossRef]
- Belda, I.; Ruiz, J.; Navascue’s, E.; Marquina, D.; Santos, A. Improvement of aromatic thiol release through the selection of yeasts with increased β-lyase activity. Int. J. Food Microbiol. 2016, 225, 1–8. [Google Scholar] [CrossRef]
- Benito, S.; Palomero, F.; Morata, A.; Calderón, F.; Suárez-Lepe, J.A. New applications for Schizosaccharomyces pombe in the alcoholic fermentation of red wines. Int. J. Food Sci. Technol. 2012, 47, 2101–2108. [Google Scholar] [CrossRef]
- Benito, S.; Palomero, F.; Calderón, F.; Palmero, D.; Suárez-Lepe, J.A. Selection of appropriate Schizosaccharomyces strains for winemaking. Food Microbiol. 2014, 42, 218–224. [Google Scholar] [CrossRef]
- Romani, C.; Domizio, P.; Lencioni, L.; Gobbi, M.; Comitini, F.; Ciani, M.; Mannazzu, I. Polysaccharides and glycerol production by non-Saccharomyces wine yeasts in mixed fermentation. Quad. Vitic. Enol. Univ. Torino 2010, 31, 185–189. [Google Scholar]
- Domizio, P.; Liu, Y.; Bisson, L.F.; Barile, D. Cell wall polysaccharides released during the alcoholic fermentation by Schizosaccharomyces pombe and S. japonicus: Quantification and characterization. Food Microbiol. 2017, 61, 136–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domizio, P.; Lencioni, L.; Calamai, L.; Portaro, L.; Bisson, L.F. Evaluation of the yeast Schizosaccharomyces japonicus for use in wine production. Am. J. Enol. Vitic. 2018, 69, 266–277. [Google Scholar] [CrossRef] [Green Version]
- Romani, C.; Lencioni, L.; Gobbi, M.; Mannazzu, I.; Ciani, M.; Domizio, P. Schizosaccharomyces japonicus: A polysaccharide-overproducing yeast to be used in winemaking. Fermentation 2018, 4, 14. [Google Scholar] [CrossRef] [Green Version]
- Domizio, P.; Romani, C.; Comitini, F.; Gobbi, M.; Ciani, M.; Lencioni, L.; Mannazzu, I. Potential spoilage non-Saccharomyces yeasts in mixed cultures with Saccharomyces cerevisiae. Ann. Microbiol. 2011, 61, 137–144. [Google Scholar] [CrossRef]
- Domizio, P.; Romani, C.; Lencioni, L.; Comitini, F.; Gobbi, M.; Mannazzu, I.; Ciani, M. Outlining a future for non-Saccharomyces yeasts: Selection of putative spoilage wine strains to be used in association with Saccharomyces cerevisiae for grape juice fermentation. Int. J. Food Microbiol. 2011, 147, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Lencioni, L.; Romani, C.; Gobbi, M.; Comitini, F.; Ciani, M.; Domizio, P. Controlled mixed fermentation at winery scale using Zygotorulaspora florentina and Saccharomyces cerevisiae. Int. J. Food Microbiol. 2016, 234, 36–44. [Google Scholar] [CrossRef]
- Pallmann, C.L.; Brown, J.A.; Olineka, T.L.; Cocolin, L.; Mills, D.A.; Bisson, L.F. Use of WL medium to profile native flora fermentations. Am. J. Enol. Vitic. 2001, 52, 198–203. [Google Scholar]
- Di Stefano, R.; Guidoni, S. La determinazione dei polifenoli totali nei mosti e nei vini. Vignevini 1989, 1, 47–52. [Google Scholar]
- Di Stefano, R.; Cravero, M.C.; Gentilini, N. Metodi per lo studio dei polifenoli dei vini. L’Enotecnico 1989, 5, 43–89. [Google Scholar]
- Glories, Y. Recherches Sur la Matiere Colorante des Vins Rouges; l’Université de Bordeaux II: Bordeaux, France, 1978. [Google Scholar]
- Delteil, D. Evaluation sensorielle du profil gustatif des vins. Rev. Oenol. 2000, 94, 21–23. [Google Scholar]
- Granes, D.; Pic-Blateyron, L.; Negrel, J.; Bonnefond, C. L’analyse sensorielle descriptive quantifiée (ASDQ): Une methode pour un langage commun. Rev. Franc. Oenol. 2009, 238, 16–21. [Google Scholar]
- Izquierdo-Cañas, P.M.; Palacios-Garcia, A.T.; Garcia-Romero, E. Enhancement of flavour properties in wines using sequential inoculations of non-Saccharomyces (Hansenula and Torulaspora) and Saccharomyces yeast starter. Vitis 2011, 50, 177–182. [Google Scholar]
- Tronchoni, J.; Curiel, J.A.; Sáenz-Navajas, M.P.; Morales, P.; De-La-Fuente-Blanco, A.; Fernández-Zurbano, P.; Ferreira, V.; Gonzalez, R. Aroma profiling of an aerated fermentation of natural grape must with selected yeast strains at pilot scale. Food Microbiol. 2018, 70, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Contreras, A.; Hidalgo, C.; Henschke, P.A.; Chambers, P.J.; Curtin, C.; Varela, C. Evaluation of non-Saccharomyces yeasts for the reduction of alcohol content in wine. Appl. Environ. Microbiol. 2014, 80, 1670–1678. [Google Scholar] [CrossRef] [Green Version]
- Gobbi, M.; de Vero, L.; Solieri, L.; Comitini, F.; Oro, L.; Giudici, P. Fermentative aptitude of non-Saccharomyces wine yeast for reduction in the ethanol content in wine. Eur. Food Res. Technol. 2014, 239, 41–48. [Google Scholar] [CrossRef]
- Quirós, M.; Rojas, V.; Gonzalez, R.; Morales, P. Selection of non-Saccharomyces yeast strains for reducing alcohol levels in wine by sugar respiration. Int. J. Food microbiol. 2014, 181, 85–91. [Google Scholar] [CrossRef] [Green Version]
- Contreras, A.; Curtin, C.; Varela, C. Yeast population dynamics reveal a potential collaboration between Metschnikowia pulcherrima and Saccharomyces uvarum for the production of reduced alcohol wines during Shiraz fermentation. Appl. Microbiol. Biotechnol. 2015, 99, 1885–1895. [Google Scholar] [CrossRef]
- Contreras, A.; Hidalgo, C.; Schmidt, S.; Henschke, P.A.; Curtin, C.; Varela, C. The application of non-Saccharomyces yeast in fermentations with limited aeration as a strategy for the production of wine with reduced alcohol content. Int. J. Food Microbiol. 2015, 205, 7–15. [Google Scholar] [CrossRef]
- Canonico, L.; Solomon, M.; Comitini, F.; Ciani, M.; Varela, C. Volatile profile of reduced alcohol wines fermented with selected non-Saccharomyces yeasts under different aeration conditions. Food Microbiol. 2019, 84, 103247. [Google Scholar] [CrossRef] [PubMed]
- Varela, J.; Varela, C. Microbiological strategies to produce beer and wine with reduced ethanol concentration. Curr. Opin. Biotechnol. 2019, 56, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Whitener, M.E.B.; Stanstrup, J.; Panzeri, V.; Carlin, S.; Divol, B.; Du Toit, M.; Vrhovsek, U. Untangling the wine metabolome by combining untargeted SPME–GCxGC-TOF-MS and sensory analysis to profile Sauvignon blanc co-fermented with seven different yeasts. Metabolomics 2016, 12, 53. [Google Scholar] [CrossRef]
- Nisiotou, A.; Sgouros, G.; Mallouchos, A.; Nisiotis, C.S.; Michaelidis, C.; Tassou, C.; Banilas, G. The use of indigenous Saccharomyces cerevisiae and Starmerella bacillaris strains as a tool to create chemical complexity in local wines. Food Res. Int. 2018, 111, 498–508. [Google Scholar] [CrossRef]
- Benito, S. The impacts of Lachancea thermotolerans yeast strains on winemaking. Appl. Microbiol. Biotechnol. 2018, 102, 6775–6790. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Escott, C.; Loira, I.; del Fresno, J.M.; Morata, A.; Tesfaye, W.; Calderon, F.; Suárez-Lepe, J.A.; Han, S.; Benito, S. Use of non-Saccharomyces yeasts and oenological tannin in red winemaking: Influence on colour, aroma and sensorial properties of young wines. Food Microbiol. 2018, 69, 51–63. [Google Scholar] [CrossRef]
- Medina, K.; Boido, E.; Dellacassa, E.; Carrau, F. Effects of non-Saccharomyces yeasts on color, anthocyanin and anthocyanin-derived pigments of Tannat grapes during fermentation. Am. J. Enol. Vitic. 2018, 69, 148–156. [Google Scholar] [CrossRef]
- Medina, K.; Boido, E.; Fariña, L.; Dellacassa, E.; Carrau, F. Non-Saccharomyces and Saccharomyces strains co-fermentation increases acetaldehyde accumulation: Effect on anthocyanin-derived pigments in Tannat red wines. Yeast 2016, 33, 339–343. [Google Scholar] [CrossRef] [Green Version]
- Vidal, S.; Francis, L.; Williams, P.; Kwiatkowski, M.; Gawel, R.; Cheynier, V.; Waters, E. The mouth-feel properties of polysaccharides and anthocyanins in a wine like medium. Food. Chem. 2004, 85, 519–525. [Google Scholar] [CrossRef]
- Carvalho, E.; Mateus, N.; Plet, B.; Pianet, I.; Dufourc, E.; De Freitas, V. Influence of wine pectic polysaccharides on the interactions between condensed tannins and salivary proteins. J. Food. Chem. 2006, 54, 8936–8944. [Google Scholar] [CrossRef]
- Chalier, P.; Angot, B.; Delteil, D.; Doco, T.; Gunata, Z. Interactions between aroma compounds and whole mannoprotein isolated from Saccharomyces cerevisiae strains. Food Chem. 2007, 100, 22–30. [Google Scholar] [CrossRef]
- Juega, M.; Nunez, Y.P.; Carrascosa, A.V.; Martinez-Rodriguez, A.J. Influence of yeast mannoproteins in the aroma improvement of white wines. J. Food Sci. 2012, 77, M499–M504. [Google Scholar] [CrossRef] [Green Version]
- Noble, A.C.; Bursick, G.F. The contribution of glycerol to perceived viscosity and sweetness in white wine. Am. J. Enol. Vitic. 1984, 35, 110–112. [Google Scholar]
- Hufnagel, J.C.; Hofmann, T. Quantitative reconstruction of the nonvolatile sensometabolome of a red wine. J. Agric. Food Chem. 2008, 56, 9190–9199. [Google Scholar] [CrossRef] [PubMed]
- Giaramida, P.; Ponticello, G.; Di Maio, S.; Squadrito, M.; Genna, G.; Barone, E.; Scacco, A.; Corona, O.; Amore, G.; Di Stefano, R.; et al. Candida zemplinina for production of wines with less alcohol and more glycerol. S. Afr. J. Enol. Vitic. 2013, 34, 204–211. [Google Scholar] [CrossRef] [Green Version]
- Zara, G.; Mannazzu, I.; Del Caro, A.; Budroni, M.; Pinna, M.B.; Murru, M.; Farris, G.A.; Zara, S. Wine quality improvement through the combined utilisation of yeast hulls and Candida zemplinina/Saccharomyces cerevisiae mixed starter cultures. Aust. J. Grape Wine Res. 2014, 20, 199–207. [Google Scholar] [CrossRef]
- Romano, P.; Suzzi, G. Higher alcohol and acetoin production by Zygosaccharomyces wine yeasts. J. Appl. Bacteriol. 1993, 75, 541–545. [Google Scholar] [CrossRef]
- Rojas, V.; Gil, J.V.; Pinaga, F.; Manzanares, P. Acetate ester formation in wine by mixed cultures in laboratory fermentations. Int. J. Food. Microbiol. 2003, 86, 181–188. [Google Scholar] [CrossRef]
- Moreira, N.; Mendes, F.; Guedes de Pinho, P.; Hogg, T.; Vasconcelos, I. Heavy sulphur compounds, higher alcohols and esters production profile of Hanseniaspora uvarum and Hanseniaspora guilliermondii grown as pure and mixed cultures in grape must. Int. J. Food Microbiol. 2008, 124, 231–238. [Google Scholar] [CrossRef]
- Viana, F.; Gil, J.V.; Genove’s, S.; Valle’s, S.; Manzanares, P. Rational selection of non-Saccharomyces wine yeasts for mixed starters based on ester formation and enological traits. Food Microbiol. 2008, 25, 778–785. [Google Scholar] [CrossRef]
- Herraiz, T.; Reglero, G.; Herraiz, M.; Martin-Alvarez, P.J.; Cabezudo, M.D. The influence of the yeast and type of culture on the volatile composition of wines fermented without sulfur dioxide. Am. J. Enol. Vitic. 1990, 41, 313–318. [Google Scholar]
- Clemente-Jimenez, J.M.; Mingorance-Carzola, L.; Martinez-Rodriguez, S.; Las Heras-Vazquez, F.J.; Rodriguez-Vico, F. Molecular characterization and oenological properties of wine yeasts isolated during spontaneous fermentation of six varieties of grape must. Food Microbiol. 2004, 21, 149–155. [Google Scholar] [CrossRef]
- Andorra, I.; Berradre, M.; Roze’s, N.; Mas, A.; Guillamon, J.M.; Esteve-Zarzoso, B. Effect of pure and mixed cultures of the main wine yeast species on grape must fermentations. Eur. Food Res. Technol. 2010, 231, 215–224. [Google Scholar] [CrossRef] [Green Version]
- Rojas, V.; Gil, J.V.; Pinaga, F.; Manzanares, P. Studies on acetate ester production by non-Saccharomyces wine yeasts. Int. J. Food Microbiol. 2001, 70, 283–289. [Google Scholar] [CrossRef]
- Benito, S.; Hofmann, T.; Laier, M.; Lochbühler, B.; Schüttler, A.; Ebert, K.; Fritsch, S.; Röcker, J.; Rauhut, D. Effect on quality and composition of Riesling wines fermented by sequential inoculation with non-Saccharomyces and Saccharomyces cerevisiae. Eur. Food Res. Technol. 2015, 241, 707–717. [Google Scholar] [CrossRef]
- Hranilovic, A.; Gambetta, J.M.; Schmidtke, L.; Boss, P.K.; Grbin, P.R.; Masneuf-Pomarede, I.; Bely, M.; Albertin, W.; Jiranek, V. Oenological traits of Lachancea thermotolerans show signs of domestication and allopatric di_erentiation. Sci. Rep. 2018, 8, 14812–14825. [Google Scholar] [CrossRef] [PubMed]
- Escott, C.; Morata, A.; Loira, I.; Tesfaye, W.; Suarez-Lepe, J.A. Characterization of polymeric pigments and pyranoanthocyanins formed in microfermentations of non-Saccharomyces yeasts. J. Appl. Microbiol. 2016, 121, 1346–1356. [Google Scholar] [CrossRef]
- Escott, C.; del Fresno, J.M.; Loira, I.; Morata, A.; Tesfaye, W.; González, M.C.; Suárez-Lepe, J.A. Formation of polymeric pigments in red wines through sequential fermentation of flavanol-enriched musts with non-Saccharomyces yeasts. Food Chem. 2018, 239, 975–983. [Google Scholar] [CrossRef]
- Morata, A.; Loira, I.; Suárez Lepe, J.A. Influence of yeasts in wine colour. In Grape and Wine Biotechnology; Morata, A., Loira, I., Eds.; IntechOpen: London, UK, 2016; pp. 285–305. [Google Scholar]
- Morata, A.; Loira, I.; Tesfaye, W.; Bañuelos, M.A.; González, C.; Suárez Lepe, J.A. Lachancea thermotolerans applications in wine technology. Fermentation 2018, 4, 53. [Google Scholar] [CrossRef] [Green Version]
- Canuti, V.; Puccioni, S.; Storchi, P.; Zanoni, B.; Picchi, M.; Bertuccioli, M. Enological eligibility of grape clones based on the SIMCA method: The case of the Sangiovese cultivar from Tuscany. Ital. J. Food Sci. 2018, 30, 184–199. [Google Scholar]






| Strain | Species | Origin |
|---|---|---|
| # 4 | Pichia fermentans | DiSVA a |
| # 22 | Starmerella bacillaris | DAGRI b |
| # 32 | Hanseniaspora osmophila | DAGRI b |
| # 42 | Zygotorulaspora florentina | DAGRI b |
| # 46 | Metschnikowia pulcherrima | DiSVA a |
| # 92 | Torulaspora delbrueckii | DAGRI b |
| # 103 | Lachancea thermotolerans | DiSVA a |
| # EC1118 | Saccharomyces cerevisiae | Lallemand c |
| pH | Ethanol% (v/v) | Volatile Acidity (g/L) | Total Acidity (g/L) | Malic Acid (g/L) | Lactic Acid (g/L) | Free SO2 (mg/L) | Total Polyphenols (mg catechin/L) | Total Anthocyanins (mg malvidin/L) | |
|---|---|---|---|---|---|---|---|---|---|
| P. fermentans | 3.37 ± 0.03 ab | 12.80 ± 0.01 abc | 0.34 ± 0.02 bc | 5.50 ± 0.05 bc | 1.08 ± 0.02 abc | 0.11 ± 0.03 cd | 18 ± 0.8 bc | 1536 ± 27.32 a | 194 ± 3.55 ab |
| S. bacillaris | 3.34 ± 0.02 ab | 12.85 ± 0.00 bc | 0.37 ± 0.02 b | 5.80 ± 0.06 ab | 1.06 ± 0.02 bc | 0.11 ± 0.00 d | 13 ± 2.50 c | 1556 ± 30.50 a | 204 ± 0.50 a |
| H. osmophila | 3.41 ± 0.05 a | 12.88 ± 0.13 ab | 0.53 ± 0.02 a | 5.50 ± 0.33 bc | 0.96 ± 0.23 cd | 0.24 ± 0.09 b | 16 ± 1.00 bc | 1381 ± 66.00 ab | 173 ± 12.50 bc |
| Z. florentina | 3.34 ± 0.01 ab | 12.63 ± 0.03 bc | 0.26 ± 0.01 c | 6.15 ± 0.00 a | 1.34 ± 0.01 a | 0.22 ± 0.01 bc | 14 ± 0.00 bc | 1394 ± 44.50 ab | 172 ± 2.00 bc |
| M. pulcherrima | 3.34 ± 0.02 ab | 12.78 ± 0.03 abc | 0.25 ± 0.04 c | 5.87 ± 0.06 ab | 1.24 ± 0.01 ab | 0.15 ± 0.00 bcd | 12 ± 3.00 c | 1518 ± 53.50 ab | 187 ± 0.50 abc |
| T. delbrueckii | 3.32 ± 0.00 b | 12.70 ± 0.15 c | 0.30 ± 0.03 bc | 6.07 ± 0.02 a | 1.13 ± 0.02 bc | 0.17 ± 0.02 bcd | 14 ± 0.50 bc | 1350 ± 42.50 ab | 181 ± 11.50 abc |
| L. thermotolerans | 3.40 ± 0.02 a | 12.98 ± 0.03 a | 0.37 ± 0.02 b | 5.58 ± 0.07 bc | 1.04 ± 0.02 d | 0.78 ± 0.01 a | 19 ± 1.00 ab | 1421 ± 7.50 ab | 188 ± 2.00 ab |
| S. cerevisiae | 3.40 ± 0.02 ab | 13.00 ± 0.00 a | 0.28 ± 0.06 bc | 5.36 ± 0.10 c | 1.15 ± 0.09 abc | 0.16 ± 0.01 bcd | 24 ± 3.00 a | 1300 ± 92.00 b | 163 ± 12.50 c |
| P. fermentans | S. bacillaris | H. osmophila | Z. florentina | M. pulcherrima | T. delbrueckii | L. thermotolerans | S. cerevisiae | |
|---|---|---|---|---|---|---|---|---|
| Acetaldehyde | 71 ± 2 a | 66 ± 0 a | 61 ± 3 a | 62 ± 1 a | 72 ± 8 a | 61 ± 3 a | 62 ± 5 a | 64 ± 7 a |
| 1-propanol | 34 ± 2 b | 38 ± 1 ab | 33 ± 3 b | 41 ± 2 a | 35 ± 2 b | 33 ± 1 b | 41 ± 2 a | 36 ± 2 ab |
| 2-Methyl-1-propanol | 72 ± 2 d | 123 ± 8 a | 66 ± 3 d | 66 ± 1 d | 92 ± 1 b | 74 ± 3 cd | 84 ± 1 bc | 47 ± 2 e |
| 2-Methyl-1-butanol | 54 ± 0 bc | 42 ± 2 d | 45 ± 5 cd | 53 ± 1 bcd | 68 ± 7 a | 58 ± 1 ab | 63 ± 2 ab | 61 ± 3 ab |
| 3-Methyl-1-butanol | 212 ± 9 bcd | 184 ± 5 d | 213 ± 6 bcd | 187 ± 1 d | 260 ± 14 a | 241 ± 7 ab | 231 ± 5 abc | 205 ± 3 cd |
| Hexanol | 0.108 ± 0.012 bc | 0.096 ± 0.001 c | 0.109 ± 0.001 bc | 0.113 ± 0.003 b | 0.111 ± 0.007 bc | 0.115 ± 0.002 ab | 0.129 ± 0.002 a | 0.117 ± 0.001 ab |
| 2-Phenylethanol | 7.355 ± 0.140 ab | 7.995 ± 0.015 ab | 8.920 ± 0.5 a | 6.995 ± 0.045 ab | 9.240 ± 0.250 a | 9.455 ± 0.355 a | 8.440 ± 0.430 | 6.125 ± 0.095 b |
| Ethyl acetate | 35 ± 0 bc | 39 ± 2 b | 54 ± 1 a | 31 ± 0 cd | 51 ± 3 a | 38 ± 2 b | 39 ± 1 b | 28 ± 2 d |
| Isoamyl acetate | 0.027 ± 0.005 b | 0.020 ± 0.001 b | 0.042 ± 0.003 a | 0.028 ± 0.001 ab | 0.034 ± 0.002 ab | 0.034 ± 0.003 ab | 0.030 ± 0.001 ab | 0.040 ± 0.001 a |
| Phenylethyl acetate | 0.003 ± 0.001 b | 0.003 ± 0.001 b | 0.016 ± 0.005 a | 0.004 ± 0 b | 0.005 ± 0.001 b | 0.006 ± 0.001 b | 0.004 ± 0 b | 0.003 ± 0 b |
| Ethyl lactate | 3.4 ± 0.6 b | 3.7 ± 0 b | 5.5 ± 2.1 b | 5.6 ± 0.4 b | 5.5 ± 0.2 b | 4.4 ± 0.2 b | 16.5 ± 1 a | 4.7 ± 0.6 b |
| Ethyl butyrate | 0.181 ± 0.01 abc | 0.192 ± 0.006 ab | 0.149 ± 0.009 c | 0.205 ± 0.009 a | 0.166 ± 0.01 bc | 0.171 ± 0.021 bc | 0.194 ± 0.001 ab | 0.172 ± 0.001 abc |
| Ethyl hexanoate | 0.013 ± 0.002 bc | 0.008 ± 0.001 d | 0.007 ± 0.001 d | 0.010 ± 0.001 cd | 0.014 ± 0.001 b | 0.014 ± 0.001 b | 0.014 ± 0.001 b | 0.023 ± 0.002 a |
| Ethyl octanoate | 0.013 ± 0.003 b | 0.008 ± 0.001 cd | 0.003 ± 0 e | 0.011 ± 0.001 bc | 0.013 ± 0 b | 0.010 ± 0.001 bcd | 0.007 ± 0.001 de | 0.024 ± 0.001 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romani, C.; Lencioni, L.; Biondi Bartolini, A.; Ciani, M.; Mannazzu, I.; Domizio, P. Pilot Scale Fermentations of Sangiovese: An Overview on the Impact of Saccharomyces and Non-Saccharomyces Wine Yeasts. Fermentation 2020, 6, 63. https://doi.org/10.3390/fermentation6030063
Romani C, Lencioni L, Biondi Bartolini A, Ciani M, Mannazzu I, Domizio P. Pilot Scale Fermentations of Sangiovese: An Overview on the Impact of Saccharomyces and Non-Saccharomyces Wine Yeasts. Fermentation. 2020; 6(3):63. https://doi.org/10.3390/fermentation6030063
Chicago/Turabian StyleRomani, Cristina, Livio Lencioni, Alessandra Biondi Bartolini, Maurizio Ciani, Ilaria Mannazzu, and Paola Domizio. 2020. "Pilot Scale Fermentations of Sangiovese: An Overview on the Impact of Saccharomyces and Non-Saccharomyces Wine Yeasts" Fermentation 6, no. 3: 63. https://doi.org/10.3390/fermentation6030063
APA StyleRomani, C., Lencioni, L., Biondi Bartolini, A., Ciani, M., Mannazzu, I., & Domizio, P. (2020). Pilot Scale Fermentations of Sangiovese: An Overview on the Impact of Saccharomyces and Non-Saccharomyces Wine Yeasts. Fermentation, 6(3), 63. https://doi.org/10.3390/fermentation6030063