Commercially Available Non-Saccharomyces Yeasts for Winemaking: Current Market, Advantages over Saccharomyces, Biocompatibility, and Safety
Abstract
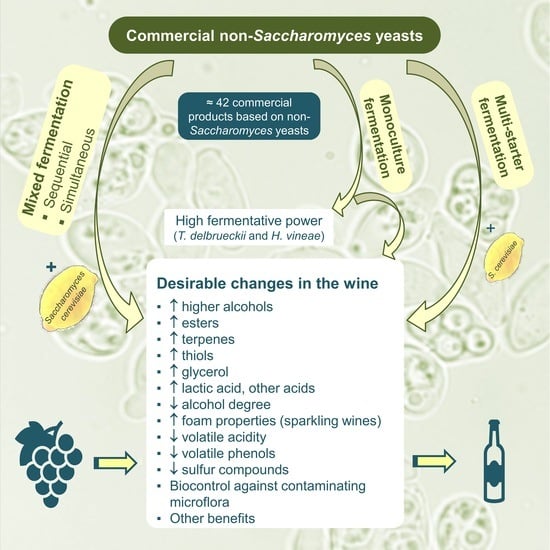
:1. Introduction
2. Non-Saccharomyces Yeasts Available on the Market
2.1. Torulaspora Delbrueckii
2.2. Lachancea Thermotolerans
| Commercial Yeast | Level | Fermentation | TYPE OF WINE | Changes with Respect to S. cerevisiae | Sensory Impact with Respect to S. cerevisiae | Reference |
|---|---|---|---|---|---|---|
| Torulaspora delbrueckii Biodiva TD291 (Lallemand, Montreal, QC, Canada) | Semi-industrial (Two wineries: 150 and 250 L) | Sequential and simultaneous + S. cerevisiae Lalvin EC1118 (Lallemand, Montreal, QC, Canada) | Amarone (Corvina, Rondinella, and Corvinone red grapes) | ↑ 2-phenylethanol; ethyl butyrate, ethyl lactate, isoamyl lactate; 4-carbethoxy-γ-butyrolactone, sherry lactones; α-terpineol, Ho-diendiol I, and endiol ↓ isoamyl acetate | Higher aroma intensity, fruitiness, sweetness, ripe red fruit (cherry) | [9] |
| Torulaspora delbrueckii Biodiva TD291 (Lallemand, Montreal, QC, Canada) | Laboratory | Sequential + S. cerevisiae 734 * | Gewürztraminer | ↑ linalool (OAV ≈ 1.0) ↓ citronellol and geraniol | Higher overall score (balance between terpenes) | [20] |
| Torulaspora delbrueckii Zymaflore Alpha (Laffort, Bordeaux, France) Torulaspora delbrueckii Biodiva TD291 (Lallemand, Montreal, QC, Canada) | Semi-industrial (150 L) | Sequential + S. cerevisiae Lalvin EC1118 (Lallemand, Montreal, QC, Canada) | Soave (Garganega white grape) and Chardonnay | ↑ 2-phenylethanol; diethyl succinate ↓ 4-vinylguaiacol and 4-vinylphenol: with Alpha in both wines (4-vinylguaiacol OAV < 1.0) ↓ isoamyl acetate: Soave wine with Alpha; Chardonnay wine with Alpha and Biodiva) | Both wines: higher aroma intensity and persistence, complexity, and body Better floral and tropical fruit attributes (especially in Soave wine) | [10] |
| Torulaspora delbrueckii Biodiva TD291 (Lallemand, Montreal, QC, Canada) | Laboratory (500 mL) | Sequential + S. cerevisiae Lalvin EC1118 (Lallemand, Montreal, QC, Canada) | Santo (Sweet white wine from Nosiola grape) | ↑ 2-phenylethanol; ethyl lactate; sherry lactones ↓ 4-vinylphenol and 4-vinylguaiacol ↓ isoamyl acetate ↑ 3-methylthio-1-propanol | Sensory analysis not performed | [10] |
| Torulaspora delbrueckii Zymaflore Alpha (Laffort, Bordeaux, France) | Laboratory (1.2 L) | Sequential and simultaneous + S. cerevisiae Zymaflore X5 (Laffort, Bordeaux, France) | Sauvignon Blanc | ↑ isoamyl acetate (OAV > 1.0), isobutyl acetate, 2-phenylethyl acetate, ethyl isobutyrate, ethyl propanoate, ethyl dihydroxycinnamate | Sensory analysis not performed | [11] |
| Torulaspora delbrueckii Zymaflore Alpha (Laffort, Bordeaux, France) | Semi-industrial (150 L) | Sequential + S. cerevisiae Zymaflore FX10 (Laffort, Bordeaux, France) | Merlot | ↑ isoamyl acetate (OAV > 1.0), ethyl isobutyrate (OAV > 1.0), isobutyl acetate, ethyl propanoate, ethyl dihydroxycinnamate | Higher complexity and fruity notes (interaction between esters) | [11] |
| Torulaspora delbrueckii Biodiva TD291 (Lallemand, Montreal, QC, Canada) Lachancea thermotolerans Concerto (CHR Hansen, Hørsholm, Denmark) Metschnikowia pulcherrima Flavia MP346 (Lallemand, Montreal, QC, Canada) | Laboratory (60 mL) | Monoculture Must/wine analyzed in the initial stages of the fermentation (2.0–3.0% v/v ethanol) | Sauvignon Blanc and Syrah | Wines produced with T. delbrueckii: ↑ phenethyl propanoate (>50 times in both wines); linalool (both wines), β-damascenone (Sauvignon Blanc wine) Wines produced with L. thermotolerans: ↑ in both wines: 2-phenylethanol; phenethyl propanoate, other esters; nerol, terpinen-4-ol ↑ in both wines: 3-methylthio-1-propanol Wines produced with M. pulcherrima: ↑ phenethyl propanoate, phenethyl butyrate, isoeugenil phenylacetate (Syrah wine); linalool (Syrah wine); β-damascenone (Sauvignon Blanc wine) ↑ 2-methoxy-4-vinylphenol (both wines), 3-methylthio-1-propanol (Syrah wine) | Sensory analysis not performed | [21] |
| Torulaspora delbrueckii Biodiva TD291 (Lallemand, Montreal, QC, Canada) Metschnikowia pulcherrima Flavia MP346 (Lallemand, Montreal, QC, Canada) | Semi-industrial (100 L) | Sequential + S. cerevisiae QA23 (Lallemand, Montreal, QC, Canada) | Base wine for Cava (Macabeo grape) | Wine produced with T. delbrueckii: ↑ glycerol ↑ foamability: Hm > 17%, foam persistence: Hs > 20% ↓ volatile acidity ↑ 4-ethylguaiacol, 4-ethylphenol, 4-vinylphenol Wine produced with M. pulcherrima: ↑ foam persistence: Hs > 35% ↓ esters ↑ 4-ethylguaiacol, 4-vinylphenol, 2-methoxyphenol, 2,6-dimethoxyphenol (2,6-dimethoxyphenol: OAV > 1.0, smoky aroma) | Higher preference for wine produced with T. delbrueckii (more similar to the control) Higher smoky and floral notes in wine produced with M. pulcherrima | [12] |
| Lachancea thermotolerans Concerto (CHR Hansen, Hørsholm, Denmark) Metschnikowia pulcherrima Flavia MP346 (Lallemand, Montreal, QC, Canada) Pichia kluyveri FrootZen (CHR Hansen, Hørsholm, Denmark) | Laboratory (5 L) | Sequential + S. cerevisiae Lalvin EC1118 (Lallemand, Montreal, QC, Canada) | Riesling | Wine produced with L. thermotolerans: ↑ lactic acid; ethyl esters; terpenes ↓ 2-phenylethyl acetate; acetaldehyde Wine produced with M. pulcherrima: ↓ 2-phenylethanol, other higher alcohols; acetate esters; acetaldehyde Wine produced with P. kluyveri: ↑ 2-phenylethyl acetate ↓ isoamyl acetate; acetaldehyde | All wines: higher preference and Riesling typicity; lower oxidation, acetaldehyde, and ethyl acetate perception Higher perception peach/apricot (L. thermotolerans and P. kluyveri), citrus/grapefruit (M. pulcherrima) | [16] |
| Hanseniaspora vineae T02/5AF (from Uruguayan vineyards) | Semi-industrial (100 L) | Monoculture Control: S. cerevisiae QA23 (Lallemand, Montreal, QC, Canada) | Macabeo | ↑ 2-phenylethyl acetate (50 times higher than S. cerevisiae), isobutyl acetate, ethyl lactate; α-terpineol ↓ acetoin (73% lower than S. cerevisiae) ↓ higher alcohols Synthesis of N-acetiltiramine and 1H-indole-3-ethanol acetate (not synthesized by S. cerevisiae) | Higher preference, fruity, and floral scores | [22] |
| Torulaspora delbrueckii Zymaflore Alpha (Laffort, Bordeaux, France) | Laboratory (1.2 L) | Sequential and simultaneous + S. cerevisiae Zymaflore X5 (Laffort, Bordeaux, France) | Sauvignon Blanc | ↑ aromatic thiols: 3SH and 3SHA | Sensory analysis not performed | [23] |
| Lachancea thermotolerans Viniflora Concerto (CHR Hansen, Hørsholm, Denmark) | Laboratory (5 L) | Sequential + Schizosaccharomyces pombe V2 * or Sequential + S. cerevisiae 88 * | Tempranillo | ↑ lactic acid and pyruvic acid (>2.0 and >3.7, respectively, respect to AF + MLF) ↑ vitisin A and vitisin B (>1.5 and >2.6, respectively, respect to AF + MLF) ↑ total anthocyanins (>1.6 respect to AF + MLF) S. pombe: residual urea (97% lower than AF + MLF) | L. thermotolerans/S. pombe Higher acidity Higher aroma intensity and quality, sensory acceptability | [17] |
| Metschnikowia pulcherrima AWRI Obsession (AB Biotek, London, United Kingdom) | Semi-industrial (50 kg of grape) | Simultaneous + S. cerevisiae AWRI838 | Merlot | ↓ alcohol degree ( < 1.0% v/v) ↑ total esters; higher alcohols ↑ sulfur compounds: H2S (>22 times), dimethyl sulfide (>2.1 times), ethanethiol, methanethiol | High score: red fruits aroma and flavor and fruit in general Low score: vegetal, meat, and barnyard aromas | [24] |
| Torulaspora delbrueckii Zymaflore Alpha (Laffort, Bordeaux, France) Torulaspora delbrueckii Biodiva TD291 (Lallemand, Montreal, QC, Canada) Torulaspora delbrueckii Prelude (CHR Hansen, Hørsholm, Denmark) Lachancea thermotolerans Viniflora Concerto (CHR Hansen, Hørsholm, Denmark) Metschnikowia pulcherrima Flavia MP346 (Lallemand, Montreal, QC, Canada) Melody (Torulaspora delbrueckii/Lachancea thermotolerans/Saccharomyces cerevisiae) (CHR Hansen, Hørsholm, Denmark) | Laboratory (20 L) | Sequential + S. cerevisiae PDM (Maurivin, Australia) and Multi-starter Melody | Shiraz (Two different ripeness level: 24 and 29 °Brix) | Wine produced from must of 24 °Brix: ↓ alcohol degree: <0.6% v/v (multi-starter Melody) ↑ glycerol: >0.85 g/L (Concerto), >1.84 g/L (Flavia) ↑ isoamyl acetate (Prelude, Melody), 2-phenylethyl acetate and ethyl isobutyrate (Alpha, Biodiva, Prelude), isobutyl acetate (Prelude, Concerto, Melody) ↑ terpenes: Alpha, Biodiva, Prelude ↓ tannins: Alpha, Biodiva, Prelude, Concerto, Flavia Wine produced from must of 29 °Brix: ↑ 2-phenylethanol (mainly Alpha, Biodiva, Prelude; and to a lesser extent Concerto, Flavia, Melody) ↑ terpenes: Alpha, Biodiva, Prelude Residual sugars: >5 g/L (Alpha, Biodiva, Prelude) | Wine produced from must of 24 °Brix: Better aroma intensity, floral attribute, perception of red fruit (Melody, Biodiva, Alpha, Flavia) Wine produced from must of 29 °Brix: Sweetness (Alpha, Biodiva, Prelude) | [2] |
| Metschnikowia pulcherrima NS-EM-34 (Reported as pre-commercial strain by authors) | Laboratory (5 L) | Sequential + S. cerevisiae Viniferm Diana (Agrovin, Alcázar de San Juan, Spain) or Sequential + S. cerevisiae Viniferm Revelacion (Agrovin, Alcázar de San Juan, Spain) | Verdejo | M. pulcherrima/S. cerevisiae Diana: ↓ alcohol degree: <0.62% v/v ↑ 4MSP (≈28 ng/L vs. ≈4 ng/L in S. cerevisiae control) ↑ glycerol (>0.72 g/L) ↓ higher alcohols M. pulcherrima/S. cerevisiae Revelacion: ↓ alcohol degree: <0.63% v/v ↑ 4MSP (≈28 ng/L vs. 0 ng/L in S. cerevisiae control) ↑ glycerol (>0.52 g/L) ↓ higher alcohols | Both wines: highest scores in Verdejo typicity, fruity, intensity, and aromatic quality | [25] |
| Hanseniaspora vineae (Currently under evaluation by Oenobrands, Montpellier, France) | Semi-industrial (120 L) | Monoculture Control: S. cerevisiae Fermivin 3C (Oenobrands, Montpellier, France) | Albillo | ↑ esters, especially 2-phenylethyl acetate (OAV = 31.84) | Sensory analysis not performed | [26] |
| Torulaspora delbrueckii Oenoferm Wild & Pure (Erbslöh, Geisenheim, Germany) Metschnikowia pulcherrima Flavia MP346 (Lallemand, Montreal, QC, Canada) | Laboratory (10 L) | Sequential + S. cerevisiae Oenoferm Bouquet (Erbslöh, Geisenheim, Germany) + S. bayanus LittoLevure CHA (Erbslöh, Geisenheim, Germany) | Sila | Decrease in alcohol degree M. pulcherrima/S. bayanus/S. cerevisiae: <0.91% v/v M. pulcherrima/S. bayanus: <0.62% v/v Glycerol production (S. cerevisiae control: 5.7 g/L) T. delbrueckii/S. bayanus: 7.0 g/L M. pulcherrima/S. bayanus/S. cerevisiae: 6.7 g/L | Higher score of aroma and overall flavor: M. pulcherrima/S. bayanus and T. delbrueckii/S. bayanus Higher score: citrus flavor (M. pulcherrima/S. bayanus), melon and banana flavor (M. pulcherrima/S. cerevisiae) | [13] |
| Torulaspora delbrueckii Biodiva TD291 (Lallemand, Montreal, QC, Canada) Metschnikowia pulcherrima Flavia MP346 (Lallemand, Montreal, QC, Canada) | Semi-industrial (50 L) | Monoculture Control: 3 commercial strains of S. cerevisiae | Base wine for Cava (Chardonnay and Xarel.lo) | Base wines with M. pulcherrima High content of proteins. High foamability (Hm) and foam persistence (Hs) Cava wines with T. delbrueckii Highest concentrations of esters, especially ethyl isovalerate (120–126 µg/L) in both wines | Cava wines Better fruity and fresh aromatic profiles, especially with T. delbrueckii | [8] |
| Torulaspora delbrueckii Biodiva TD291 (Lallemand, Montreal, QC, Canada) Metschnikowia pulcherrima Flavia MP346 (Lallemand, Montreal, QC, Canada) Hanseniaspora vineae (Currently under evaluation by Oenobrands, Montpellier, France) Lachancea thermotolerans L31 * | Laboratory (1 L) | Simultaneous at the beginning of fermentation L. thermotolerans + T. delbrueckii; L. thermotolerans + M. pulcherrima; L. thermotolerans + H. vineae + Addition on day 8: S. cerevisiae 7VA * | Airén | L. thermotolerans/M. pulcherrima + S. cerevisiae ↑ lactic acid: up to 3.27 g/L ↓ pH: reduction to 3.42 (grape must 3.84) ↓ alcohol degree: <0.66% v/v (residual sugars = 0) ↑ higher alcohols; esters | L. thermotolerans/M. pulcherrima + S. cerevisiae Higher overall score Higher acidity | [27] |
2.3. Metschnikowia Pulcherrima
2.4. Pichia Kluyveri
2.5. Schizosaccharomyces Pombe
2.6. Hanseniaspora Vineae
3. Improvement in Fermentative Aromatic Profile Regarding Saccharomyces
3.1. Torulaspora Delbrueckii
3.2. Lachancea Thermotolerans
3.3. Metschnikowia Pulcherrima
3.4. Pichia Kluyveri
3.5. Hanseniaspora Vineae
3.6. Commercial Non-Saccharomyces Yeasts in Sparkling Wines
4. Commercial Non-Saccharomyces to Improve Varietal Aromatic Profile
4.1. Improvement in Terpenes Content
4.2. Improvement in the Content of Aromatic Thiols
5. Biocompatibility between Commercial Non-Saccharomyces and Saccharomyces
5.1. Multi-Starter Inoculations with Commercial Non-Saccharomyces
5.2. Non-Saccharomyces as Biocontrollers: Compatibility with Saccharomyces
6. Safety of Commercial Non-Saccharomyces Yeasts: Production of Toxic Metabolites
7. Critical Appreciation and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jolly, N.P.; Varela, C.; Pretorius, I.S. Not your ordinary yeast: Non-Saccharomyces yeasts in wine production uncovered. FEMS Yeast Res. 2014, 14, 215–237. [Google Scholar] [CrossRef] [Green Version]
- Hranilovic, A.; Li, S.; Boss, P.K.; Bindon, K.; Ristic, R.; Grbin, P.R.; Van der Westhuizen, T.; Jiranek, V. Chemical and sensory profiling of Shiraz wines co-fermented with commercial non-Saccharomyces inocula. Aust. J. Grape Wine Res. 2018, 24, 166–180. [Google Scholar] [CrossRef]
- Petruzzi, L.; Capozzi, V.; Berbegal, C.; Corbo, M.R.; Bevilacqua, A.; Spano, G.; Sinigaglia, M. Microbial resources and enological significance: Opportunities and benefits. Front. Microbiol. 2017, 8, 995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morata, A.; Escott, C.; Bañuelos, M.A.; Loira, I.; Del Fresno, J.M.; González, C.; Suárez-lepe, J.A. Contribution of non-Saccharomyces yeasts to wine freshness. A review. Biomolecules 2020, 10, 34. [Google Scholar] [CrossRef] [Green Version]
- Roudil, L.; Russo, P.; Berbegal, C.; Albertin, W.; Spano, G.; Capozzi, V. Non-Saccharomyces commercial starter cultures: Scientific trends, recent patents and innovation in the wine sector. Recent Pat. Food. Nutr. Agric. 2020, 11, 27–39. [Google Scholar] [CrossRef]
- Sütterlin, K.A. Fructophilic Yeasts to Cure Stuck Fermentations in Alcoholic Beverages. Ph.D. Thesis, University of Stellenbosch, Stellenbosch, South Africa, 2010. [Google Scholar]
- van Breda, V.; Jolly, N.; van Wyk, J. Characterisation of commercial and natural Torulaspora delbrueckii wine yeast strains. Int. J. Food Microbiol. 2013, 163, 80–88. [Google Scholar] [CrossRef]
- Mislata, A.M.; Puxeu, M.; Andorrà, I.; Espligares, N.; de Lamo, S.; Mestres, M.; Ferrer-Gallego, R. Effect of the addition of non-Saccharomyces at first alcoholic fermentation on the enological characteristics of Cava wines. Fermentation 2021, 7, 64. [Google Scholar] [CrossRef]
- Azzolini, M.; Fedrizzi, B.; Tosi, E.; Finato, F.; Vagnoli, P.; Scrinzi, C.; Zapparoli, G. Effects of Torulaspora delbrueckii and Saccharomyces cerevisiae mixed cultures on fermentation and aroma of Amarone wine. Eur. Food Res. Technol. 2012, 235, 303–313. [Google Scholar] [CrossRef]
- Azzolini, M.; Tosi, E.; Lorenzini, M.; Finato, F.; Zapparoli, G. Contribution to the aroma of white wines by controlled Torulaspora delbrueckii cultures in association with Saccharomyces cerevisiae. World J. Microbiol. Biotechnol. 2015, 31, 277–293. [Google Scholar] [CrossRef] [PubMed]
- Renault, P.; Coulon, J.; de Revel, G.; Barbe, J.C.; Bely, M. Increase of fruity aroma during mixed T. delbrueckii/S. cerevisiae wine fermentation is linked to specific esters enhancement. Int. J. Food Microbiol. 2015, 207, 40–48. [Google Scholar] [CrossRef]
- González-Royo, E.; Pascual, O.; Kontoudakis, N.; Esteruelas, M.; Esteve-Zarzoso, B.; Mas, A.; Canals, J.M.; Zamora, F. Oenological consequences of sequential inoculation with non-Saccharomyces yeasts (Torulaspora delbrueckii or Metschnikowia pulcherrima) and Saccharomyces cerevisiae in base wine for sparkling wine production. Eur. Food Res. Technol. 2015, 240, 999–1012. [Google Scholar] [CrossRef]
- Puškaš, V.S.; Miljić, U.D.; Djuran, J.J.; Vučurović, V.M. The aptitude of commercial yeast strains for lowering the ethanol content of wine. Food Sci. Nutr. 2020, 8, 1489–1498. [Google Scholar] [CrossRef] [PubMed]
- Beckner Whitener, M.E.; Stanstrup, J.; Panzeri, V.; Carlin, S.; Divol, B.; Du Toit, M.; Vrhovsek, U. Untangling the wine metabolome by combining untargeted SPME-GCxGC-TOF-MS and sensory analysis to profile Sauvignon blanc co-fermented with seven different yeasts. Metabolomics 2016, 12, 53. [Google Scholar] [CrossRef]
- Benito, Á.; Calderón, F.; Palomero, F.; Benito, S. Quality and composition of Airén wines fermented by sequential inoculation of Lachancea thermotolerans and Saccharomyces cerevisiae. Food Technol. Biotechnol. 2016, 54, 135–144. [Google Scholar] [CrossRef]
- Benito, S.; Hofmann, T.; Laier, M.; Lochbühler, B.; Schüttler, A.; Ebert, K.; Fritsch, S.; Röcker, J.; Rauhut, D. Effect on quality and composition of Riesling wines fermented by sequential inoculation with non-Saccharomyces and Saccharomyces cerevisiae. Eur. Food Res. Technol. 2015, 241, 707–717. [Google Scholar] [CrossRef]
- Benito, Á.; Calderón, F.; Benito, S. The combined use of Schizosaccharomyces pombe and Lachancea thermotolerans—Effect on the anthocyanin wine composition. Molecules 2017, 22, 739. [Google Scholar] [CrossRef] [Green Version]
- Morata, A.; Gómez-Cordovés, M.C.; Calderón, F.; Suárez-Lepe, J.A. Effects of pH, temperature and SO2 on the formation of pyranoanthocyanins during red wine fermentation with two species of Saccharomyces. Int. J. Food Microbiol. 2006, 106, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Vejarano, R.; Morata, A.; Loira, I.; González, M.C.; Suárez-Lepe, J.A. Theoretical considerations about usage of metabolic inhibitors as possible alternative to reduce alcohol content of wines from hot areas. Eur. Food Res. Technol. 2013, 237, 281–290. [Google Scholar] [CrossRef]
- Čuš, F.; Jenko, M. The influence of yeast strains on the composition and sensory quality of Gewürztraminer wine. Food Technol. Biotechnol. 2013, 51, 547–553. [Google Scholar]
- Beckner Whitener, M.E.; Carlin, S.; Jacobson, D.; Weighill, D.; Divol, B.; Conterno, L.; Du Toit, M.; Vrhovsek, U. Early fermentation volatile metabolite profile of non-Saccharomyces yeasts in red and white grape must: A targeted approach. LWT Food Sci. Technol. 2015, 64, 412–422. [Google Scholar] [CrossRef]
- Lleixà, J.; Martín, V.; del C. Portillo, M.; Carrau, F.; Beltran, G.; Mas, A. Comparison of fermentation and wines produced by inoculation of Hanseniaspora vineae and Saccharomyces cerevisiae. Front. Microbiol. 2016, 7, 338. [Google Scholar] [CrossRef] [Green Version]
- Renault, P.; Coulon, J.; Moine, V.; Thibon, C.; Bely, M. Enhanced 3-sulfanylhexan-1-ol production in sequential mixed fermentation with Torulaspora delbrueckii/Saccharomyces cerevisiae reveals a situation of synergistic interaction between two industrial strains. Front. Microbiol. 2016, 7, 293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varela, C.; Barker, A.; Tran, T.; Borneman, A.; Curtin, C. Sensory profile and volatile aroma composition of reduced alcohol Merlot wines fermented with Metschnikowia pulcherrima and Saccharomyces uvarum. Int. J. Food Microbiol. 2017, 252, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.; Belda, I.; Beisert, B.; Navascués, E.; Marquina, D.; Calderón, F.; Rauhut, D.; Santos, A.; Benito, S. Analytical impact of Metschnikowia pulcherrima in the volatile profile of Verdejo white wines. Appl. Microbiol. Biotechnol. 2018, 102, 8501–8509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Fresno, J.M.; Escott, C.; Loira, I.; Herbert-Pucheta, J.E.; Schneider, R.; Carrau, F.; Cuerda, R.; Morata, A. Impact of Hanseniaspora vineae in alcoholic fermentation and ageing on lees of high-quality white wine. Fermentation 2020, 6, 66. [Google Scholar] [CrossRef]
- Vaquero, C.; Loira, I.; Heras, J.M.; Carrau, F.; González, C.; Morata, A. Biocompatibility in ternary fermentations with Lachancea thermotolerans, other non-Saccharomyces and Saccharomyces cerevisiae to control pH and improve the sensory profile of wines from warm areas. Front. Microbiol. 2021, 12, 656262. [Google Scholar] [CrossRef]
- Vicente, J.; Calderón, F.; Santos, A.; Marquina, D.; Benito, S. High potential of Pichia kluyveri and other Pichia species in wine technology. Int. J. Mol. Sci. 2021, 22, 1196. [Google Scholar] [CrossRef]
- Mylona, A.E.; Del Fresno, J.M.; Palomero, F.; Loira, I.; Bañuelos, M.A.; Morata, A.; Calderón, F.; Benito, S.; Suárez-Lepe, J.A. Use of Schizosaccharomyces strains for wine fermentation⁻Effect on the wine composition and food safety. Int. J. Food Microbiol. 2016, 232, 63–72. [Google Scholar] [CrossRef] [Green Version]
- Benito, S.; Palomero, F.; Calderón, F.; Palmero, D.; Suárez-Lepe, J.A. Selection of appropriate Schizosaccharomyces strains for winemaking. Food Microbiol. 2014, 42, 218–224. [Google Scholar] [CrossRef]
- Vejarano, R. Non-Saccharomyces in winemaking: Source of mannoproteins, nitrogen, enzymes, and antimicrobial compounds. Fermentation 2020, 6, 76. [Google Scholar] [CrossRef]
- Slaghenaufi, D.; Boscaini, A.; Prandi, A.; Cin, A.D.; Zandonà, V.; Luzzini, G.; Ugliano, M. Influence of different modalities of grape withering on volatile compounds of young and aged Corvina wines. Molecules 2020, 25, 2141. [Google Scholar] [CrossRef]
- Mecca, D.; Benito, S.; Beisert, B.; Brezina, S.; Fritsch, S.; Semmler, H.; Rauhut, D. Influence of nutrient supplementation on Torulaspora delbrueckii wine fermentation aroma. Fermentation 2020, 6, 35. [Google Scholar] [CrossRef] [Green Version]
- Guth, H. Quantitation and sensory studies of character impact odorants of different white wine varieties. J. Agric. Food Chem. 1997, 45, 3027–3032. [Google Scholar] [CrossRef]
- Moreira, N.; Mendes, F.; Pereira, O.; Guedes De Pinho, P.; Hogg, T.; Vasconcelos, I. Volatile sulphur compounds in wines related to yeast metabolism and nitrogen composition of grape musts. Anal. Chim. Acta 2002, 458, 157–167. [Google Scholar] [CrossRef]
- Harbertson, J.F.; Mireles, M.S.; Harwood, E.D.; Weller, K.M.; Ross, C.F. Chemical and sensory effects of saignée, water addition, and extended maceration on high brix must. Am. J. Enol. Vitic. 2009, 60, 450–460. [Google Scholar]
- Lleixà, J.; Manzano, M.; Mas, A.; Portillo, M.C. Saccharomyces and non-Saccharomyces competition during microvinification under different sugar and nitrogen conditions. Front. Microbiol. 2016, 7, 1959. [Google Scholar] [CrossRef] [PubMed]
- Loscos, N.; Hernandez-Orte, P.; Cacho, J.; Ferreira, V. Release and formation of varietal aroma compounds during alcoholic fermentation from nonfloral grape odorless flavor precursors fractions. J. Agric. Food Chem. 2007, 55, 6674–6684. [Google Scholar] [CrossRef] [PubMed]
- Lambrechts, M.G.; Pretorius, I.S. Yeast and its importance to wine aroma—A review. South. Afri. J. Enol. Vitic. 2000, 21, 97–129. [Google Scholar] [CrossRef] [Green Version]
- Boido, E.; Lloret, A.; Medina, K.; Farñia, L.; Carrau, F.; Versini, G.; Dellacassa, E. Aroma composition of Vitis vinifera cv. Tannat: The typical red wine from Uruguay. J. Agric. Food Chem. 2003, 51, 5408–5413. [Google Scholar] [CrossRef]
- King, A.; Dickinson, J.R. Biotransformation of monoterpene alcohols by Saccharomyces cerevisiae, Torulaspora delbrueckii and Kluyveromyces lactis. Yeast 2000, 16, 499–506. [Google Scholar] [CrossRef]
- Cordero-Bueso, G.; Esteve-Zarzoso, B.; Cabellos, J.M.; Gil-Díaz, M.; Arroyo, T. Biotechnological potential of non-Saccharomyces yeasts isolated during spontaneous fermentations of Malvar (Vitis vinifera cv. L.). Eur. Food Res. Technol. 2013, 236, 193–207. [Google Scholar] [CrossRef]
- Bisotto, A.; Julien, A.; Rigou, P.; Schneider, R.; Salmon, J.M. Evaluation of the inherent capacity of commercial yeast strains to release glycosidic aroma precursors from Muscat grape must. Aust. J. Grape Wine Res. 2015, 21, 194–199. [Google Scholar] [CrossRef]
- Morata, A.; Loira, I.; Vejarano, R.; González, C.; Callejo, M.J.; Suárez-Lepe, J.A. Emerging preservation technologies in grapes for winemaking. Trends Food Sci. Technol. 2017, 67, 36–43. [Google Scholar] [CrossRef]
- Vejarano, R. Saccharomycodes ludwigii, control and potential uses in winemaking processes. Fermentation 2018, 4, 71. [Google Scholar] [CrossRef] [Green Version]
- Musumeci, L.E.; Ryona, I.; Pan, B.S.; Loscos, N.; Feng, H.; Cleary, M.T.; Sacks, G.L. Quantification of polyfunctional thiols in wine by HS-SPME-GC-MS following extractive alkylation. Molecules 2015, 20, 12280–12299. [Google Scholar] [CrossRef] [Green Version]
- Belda, I.; Ruiz, J.; Navascués, E.; Marquina, D.; Santos, A. Improvement of aromatic thiol release through the selection of yeasts with increased β-lyase activity. Int. J. Food Microbiol. 2016, 225, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chasseriaud, L.; Coulon, J.; Marullo, P.; Albertin, W.; Bely, M. New oenological practice to promote non-Saccharomyces species of interest: Saturating grape juice with carbon dioxide. Appl. Microbiol. Biotechnol. 2018, 102, 3779–3791. [Google Scholar] [CrossRef] [PubMed]
- Belda, I.; Ruiz, J.; Alastruey-Izquierdo, A.; Navascués, E.; Marquina, D.; Santos, A. Unraveling the enzymatic basis of wine “Flavorome”: A phylo-functional study of wine related yeast species. Front. Microbiol. 2016, 7, 12. [Google Scholar] [CrossRef] [Green Version]
- Coetzee, C.; du Toit, W.J. A comprehensive review on Sauvignon blanc aroma with a focus on certain positive volatile thiols. Food Res. Int. 2012, 45, 287–298. [Google Scholar] [CrossRef]
- Cordente, A.G.; Capone, D.L.; Curtin, C.D. Unravelling glutathione conjugate catabolism in Saccharomyces cerevisiae: The role of glutathione/dipeptide transporters and vacuolar function in the release of volatile sulfur compounds 3-mercaptohexan-1-ol and 4-mercapto-4-methylpentan-2-one. Appl. Microbiol. Biotechnol. 2015, 99, 9709–9722. [Google Scholar] [CrossRef]
- Sadoudi, M.; Tourdot-Maréchal, R.; Rousseaux, S.; Steyer, D.; Gallardo-Chacón, J.J.; Ballester, J.; Vichi, S.; Guérin-Schneider, R.; Caixach, J.; Alexandre, H. Yeast-yeast interactions revealed by aromatic profile analysis of Sauvignon blanc wine fermented by single or co-culture of non-Saccharomyces and Saccharomyces yeasts. Food Microbiol. 2012, 32, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Anfang, N.; Brajkovich, M.; Goddard, M.R. Co-fermentation with Pichia kluyveri increases varietal thiol concentrations in Sauvignon blanc. Aust. J. Grape Wine Res. 2009, 15, 1–8. [Google Scholar] [CrossRef]
- Zott, K.; Thibon, C.; Bely, M.; Lonvaud-Funel, A.; Dubourdieu, D.; Masneuf-Pomarede, I. The grape must non-Saccharomyces microbial community: Impact on volatile thiol release. Int. J. Food Microbiol. 2011, 151, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Lopes, C.A.; Sangorrín, M.P. Optimization of killer assays for yeast selection protocols. Rev. Argent. Microbiol. 2010, 42, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Türkel, S.; Ener, B. Isolation and characterization of new Metschnikowia pulcherrima strains as producers of the antimicrobial pigment pulcherrimin. Zeitschrift fur Naturforsch. Sect. C J. Biosci. 2009, 64, 405–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simonin, S.; Alexandre, H.; Nikolantonaki, M.; Coelho, C.; Tourdot-Maréchal, R. Inoculation of Torulaspora delbrueckii as a bio-protection agent in winemaking. Food Res. Int. 2018, 107, 451–461. [Google Scholar] [CrossRef]
- Villalba, M.L.; Sáez, J.S.; del Monaco, S.; Lopes, C.A.; Sangorrín, M.P. TdKT, a new killer toxin produced by Torulaspora delbrueckii effective against wine spoilage yeasts. Int. J. Food Microbiol. 2016, 217, 94–100. [Google Scholar] [CrossRef]
- Middelbeek, E.J.; Stumm, C.; Vogels, G.D. Effects of Pichia kluyveri killer toxin on sensitive cells. Antonie Van Leeuwenhoek 1980, 46, 205–220. [Google Scholar] [CrossRef]
- Labbani, F.Z.K.; Turchetti, B.; Bennamoun, L.; Dakhmouche, S.; Roberti, R.; Corazzi, L.; Meraihi, Z.; Buzzini, P. A novel killer protein from Pichia kluyveri isolated from an Algerian soil: Purification and characterization of its in vitro activity against food and beverage spoilage yeasts. Antonie Van Leeuwenhoek 2015, 107, 961–970. [Google Scholar] [CrossRef]
- Cravero, F.; Englezos, V.; Torchio, F.; Giacosa, S.; Río Segade, S.; Gerbi, V.; Rantsiou, K.; Rolle, L.; Cocolin, L. Post-harvest control of wine-grape mycobiota using electrolyzed water. Innov. Food Sci. Emerg. Technol. 2016, 35, 21–28. [Google Scholar] [CrossRef]
- Ivit, N.N.; Kemp, B. The impact of non-Saccharomyces yeast on traditional method sparkling wine. Fermentation 2018, 4, 73. [Google Scholar] [CrossRef] [Green Version]
- Benito, Á.; Calderón, F.; Palomero, F.; Benito, S. Combine use of selected Schizosaccharomyces pombe and Lachancea thermotolerans yeast strains as an alternative to the traditional malolactic fermentation in red wine production. Molecules 2015, 20, 9510–9523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uthurry, C.A.; Suárez-Lepe, J.A.; Lombardero, J.; García Del Hierro, J.R. Ethyl carbamate production by selected yeasts and lactic acid bacteria in red wine. Food Chem. 2006, 94, 262–270. [Google Scholar] [CrossRef]

| Yeast Species | Commercial Brand | Providing Company (Country) | Format 1 |
|---|---|---|---|
| Torulaspora delbrueckii | Biodiva TD291 | Lallemand (Canada) | ADY |
| Prelude | CHR Hansen (Denmark) | ADY | |
| Zymaflore Alpha | Laffort (France) | ADY | |
| Viniferm NSTD | Agrovin (Spain) | ADY | |
| EnartisFerm Qτ | Enartis (Italy) | ADY | |
| EnartisFerm Qτ Liquido | Enartis (Italy) | CRY | |
| Oenovin Torulaspora BIO | Oeno (Italy) | ADY | |
| Torulaspora delbrueckii | Probiotec (Italy) | FLY | |
| Torulaspora delbrueckii 12.2 | Probiotec (Italy) | FLY | |
| Lachancea thermotolerans | Laktia | Lallemand (Canada) | ADY |
| Concerto | CHR Hansen (Denmark) | ADY | |
| Octave | CHR Hansen (Denmark) | ADY | |
| EnartisFerm Qƙ | Enartis (Italy) | CRY | |
| Excellence X’Fresh | Lamothe-Abiet (France) | ADY | |
| LEVULIA Alcomeno | AEB Group (Italy) | ADY | |
| Kluyveromyces thermotolerans | Probiotec (Italy) | FLY | |
| Metschnikowia pulcherrima | Flavia MP346 | Lallemand (Canada) | ADY |
| Oenoferm MProtect | Erbslöeh (Germany) | ADY | |
| AWRI Obsession | AB Biotek (United Kingdom) | ADY | |
| LEVULIA Pulcherrima | AEB Group (Italy) | ADY | |
| Primaflora VB BIO | AEB Group (Italy) | ADY | |
| Excellence B-Nature | Lamothe-Abiet (France) | ADY | |
| Metschnikowia fructicola | Levia Nature | Oeno (Italy) | ADY |
| Gaïa | Lallemand (Canada) | ADY | |
| Schizosaccharomyces pombe | Atecrem 12H | BioEnologia (Italy) | CRY |
| Promalic | Proenol (Portugal) | ENCY | |
| Wicheranomyces anomalus | Anti Brett 1 | Probiotec (Italy) | FLY |
| Kluyveromyces wickerhamii | Anti Brett 2 | Probiotec (Italy) | FLY |
| Starmerella bacillaris | Atecrem 11H | BioEnologia (Italy) | CRY |
| Zygosaccharomyces bailii | Fructoferm W3 2 | Lallemand (Canada) | ADY |
| Zygosaccharomyces parabailii | Hardened Spaniard | Mainiacal Yeast (United States) | FLY |
| Pichia kluyveri | Frootzen | CHR Hansen (Denmark) | AFY |
| Pichia kluveri MIP-001 | Propagate Lab (United States) | FLY | |
| Pichia kluyveri + Kazachastania servazzii | Trillyeast | BioEnologia (Italy) | CRY |
| Torulaspora delbrueckii + Saccharomyces cerevisiae | Oenoferm Wild & Pure | Erbslöeh (Germany) | ADY |
| Torulaspora delbrueckii + Saccharomyces cerevisiae | New Nordic Ale Yeast | White Labs (United States) | FLY |
| Torulaspora delbrueckii + Metschnikowia pulcherrima | Zymaflore Égide | Laffort (France) | ADY |
| Metschnikowia pulcherrima + Saccharomyces cerevisiae | Primaflora VR BIO | AEB Group (Italy) | ADY |
| Lachancea thermotolerans + Saccharomyces cerevisiae | Symphony | CHR Hansen (Denmark) | ADY |
| Lachancea thermotolerans + Saccharomyces cerevisiae | Rhythm | CHR Hansen (Denmark) | ADY |
| Lachancea thermotolerans + Torulaspora delbrueckii + Saccharomyces cerevisiae | Harmony | CHR Hansen (Denmark) | ADY |
| Lachancea thermotolerans + Torulaspora delbrueckii + Saccharomyces cerevisiae | Melody | CHR Hansen (Denmark) | ADY |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vejarano, R.; Gil-Calderón, A. Commercially Available Non-Saccharomyces Yeasts for Winemaking: Current Market, Advantages over Saccharomyces, Biocompatibility, and Safety. Fermentation 2021, 7, 171. https://doi.org/10.3390/fermentation7030171
Vejarano R, Gil-Calderón A. Commercially Available Non-Saccharomyces Yeasts for Winemaking: Current Market, Advantages over Saccharomyces, Biocompatibility, and Safety. Fermentation. 2021; 7(3):171. https://doi.org/10.3390/fermentation7030171
Chicago/Turabian StyleVejarano, Ricardo, and Angie Gil-Calderón. 2021. "Commercially Available Non-Saccharomyces Yeasts for Winemaking: Current Market, Advantages over Saccharomyces, Biocompatibility, and Safety" Fermentation 7, no. 3: 171. https://doi.org/10.3390/fermentation7030171
APA StyleVejarano, R., & Gil-Calderón, A. (2021). Commercially Available Non-Saccharomyces Yeasts for Winemaking: Current Market, Advantages over Saccharomyces, Biocompatibility, and Safety. Fermentation, 7(3), 171. https://doi.org/10.3390/fermentation7030171