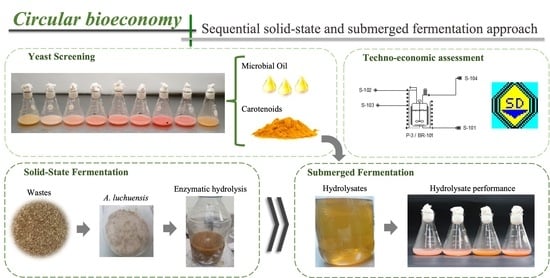
Coproduction of Microbial Oil and Carotenoids within the Circular Bioeconomy Concept: A Sequential Solid-State and Submerged Fermentation Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material Recollection
2.2. Microorganisms
2.3. Simultaneous Production of Microbial Oil and Carotenoids: Screening Study
2.4. Production of Agro-Industrial Waste Hydrolysates through Solid-State Fermentation
2.5. Analytical Methods
2.6. Data Analysis
2.7. Techno-Economic Assessment of Microbial Oil and Carotenoids
2.7.1. Simulation Description
2.7.2. Process Description
2.7.3. Economic Evaluation
2.7.4. Sensitivity Analysis
3. Results
3.1. Simultaneous Production of Microbial Oil and Carotenoids: Screening Study
3.2. Agro-Food Residues Valorization as Hydrolysates
3.3. Economic Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morone, P.; Koutinas, A.; Gathergood, N.; Arshadi, M.; Matharu, A. Food Waste: Challenges and Opportunities for Enhancing the Emerging Bio-Economy. J. Clean. Prod. 2019, 221, 10–16. [Google Scholar] [CrossRef]
- Lopes, H.J.S.; Bonturi, N.; Kerkhoven, E.J.; Miranda, E.A.; Lahtvee, P.J. C/N Ratio and Carbon Source-Dependent Lipid Production Profiling in Rhodotorula toruloides. Appl. Microbiol. Biotechnol. 2020, 104, 2639–2649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lakshmidevi, R.; Ramakrishnan, B.; Ratha, S.K.; Bhaskar, S.; Chinnasamy, S. Valorisation of Molasses by Oleaginous Yeasts for Single Cell Oil (SCO) and Carotenoids Production. Environ. Technol. Innov. 2021, 21, 101281. [Google Scholar] [CrossRef]
- Papadaki, A.; Kopsahelis, N.; Mallouchos, A.; Mandala, I.; Koutinas, A.A. Bioprocess Development for the Production of Novel Oleogels from Soybean and Microbial Oils. Food Res. Int. 2019, 126, 108684. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Tan, X.; Show, P.L.; Rambabu, K.; Banat, F.; Veeramuthu, A.; Lau, B.F.; Ng, E.P.; Ling, T.C. Incorporating Biowaste into Circular Bioeconomy: A Critical Review of Current Trend and Scaling up Feasibility. Environ. Technol. Innov. 2020, 19, 101034. [Google Scholar] [CrossRef]
- Danielson, N.; McKay, S.; Bloom, P.; Dunn, J.; Jakel, N.; Bauer, T.; Hannon, J.; Jewett, M.C.; Shanks, B. Industrial Biotechnology—An Industry at an Inflection Point. Ind. Biotechnol. 2020, 16, 321–332. [Google Scholar] [CrossRef]
- Tsakona, S.; Skiadaresis, A.G.; Kopsahelis, N.; Chatzifragkou, A.; Papanikolaou, S.; Kookos, I.K.; Koutinas, A.A. Valorisation of Side Streams from Wheat Milling and Confectionery Industries for Consolidated Production and Extraction of Microbial Lipids. Food Chem. 2016, 198, 85–92. [Google Scholar] [CrossRef]
- Boviatsi, E.; Papadaki, A.; Efthymiou, M.N.; Nychas, G.J.E.; Papanikolaou, S.; da Silva, J.A.C.; Freire, D.M.G.; Koutinas, A. Valorisation of Sugarcane Molasses for the Production of Microbial Lipids via Fermentation of Two Rhodosporidium Strains for Enzymatic Synthesis of Polyol Esters. J. Chem. Technol. Biotechnol. 2020, 95, 402–407. [Google Scholar] [CrossRef]
- Liu, Z.; Feist, A.M.; Dragone, G.; Mussatto, S.I. Lipid and Carotenoid Production from Wheat Straw Hydrolysates by Different Oleaginous Yeasts. J. Clean. Prod. 2020, 249, 119308. [Google Scholar] [CrossRef]
- Carmona-Cabello, M.; García, I.L.; Papadaki, A.; Tsouko, E.; Koutinas, A.; Dorado, M.P. Biodiesel Production Using Microbial Lipids Derived from Food Waste Discarded by Catering Services. Bioresour. Technol. 2021, 323, 124597. [Google Scholar] [CrossRef]
- Sen, T.; Barrow, C.J.; Deshmukh, S.K. Microbial Pigments in the Food Industry—Challenges and the Way Forward. Front. Nutr. 2019, 6, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parsons, S.; Allen, M.J.; Chuck, C.J. Coproducts of Algae and Yeast-Derived Single Cell Oils: A Critical Review of Their Role in Improving Biorefinery Sustainability. Bioresour. Technol. 2020, 303, 122862. [Google Scholar] [CrossRef] [PubMed]
- Papadaki, A.; Cipolatti, E.P.; Aguieiras, E.C.G.; Pinto, M.C.C.; Kopsahelis, N.; Freire, D.M.G.; Mandala, I.; Koutinas, A.A. Development of Microbial Oil Wax-Based Oleogel with Potential Application in Food Formulations. Food Bioprocess Technol. 2019, 12, 899–909. [Google Scholar] [CrossRef]
- Cipolatti, E.P.; Remedi, R.D.; Sá, C.d.S.; Rodrigues, A.B.; Ramos, J.M.G.; Burkert, C.A.V.; Furlong, E.B.; Burkert, J.F.d.M. Use of Agroindustrial Byproducts as Substrate for Production of Carotenoids with Antioxidant Potential by Wild Yeasts. Biocatal. Agric. Biotechnol. 2019, 20, 101208. [Google Scholar] [CrossRef]
- Chopra, J.; Sen, R. Process Optimization Involving Critical Evaluation of Oxygen Transfer, Oxygen Uptake and Nitrogen Limitation for Enhanced Biomass and Lipid Production by Oleaginous Yeast for Biofuel Application. Bioprocess Biosyst. Eng. 2018, 41, 1103–1113. [Google Scholar] [CrossRef]
- Dias, C.; Santos, J.; Reis, A.; da Silva, T.L. Yeast and Microalgal Symbiotic Cultures Using Low-Cost Substrates for Lipid Production. Bioresour. Technol. Rep. 2019, 7, 100261. [Google Scholar] [CrossRef]
- Wen, Z.; Zhang, S.; Odoh, C.K.; Jin, M.; Zhao, Z.K. Rhodosporidium toruloides—A Potential Red Yeast Chassis for Lipids and Beyond. FEMS Yeast Res. 2020, 20, foaa038. [Google Scholar] [CrossRef]
- Dias, C.; Reis, A.; Santos, J.A.L.; da Silva, T.L. Concomitant Wastewater Treatment with Lipid and Carotenoid Production by the Oleaginous Yeast Rhodosporidium toruloides Grown on Brewery Effluent Enriched with Sugarcane Molasses and Urea. Process. Biochem. 2020, 94, 1–14. [Google Scholar] [CrossRef]
- Kot, A.M.; Błażejak, S.; Kieliszek, M.; Gientka, I.; Bryś, J. Simultaneous Production of Lipids and Carotenoids by the Red Yeast Rhodotorula from Waste Glycerol Fraction and Potato Wastewater. Appl. Biochem. Biotechnol. 2019, 189, 589–607. [Google Scholar] [CrossRef]
- Pereira, R.N.; da Silveira, J.M.; de Burkert, J.F.M.; da Ores, J.C.; Burkert, C.A.V. Simultaneous Lipid and Carotenoid Production by Stepwise Fed-Batch Cultivation of Rhodotorula Mucilaginosa with Crude Glycerol. Braz. J. Chem. Eng. 2019, 36, 1099–1108. [Google Scholar] [CrossRef]
- Gong, G.; Zhang, X.; Tan, T. Simultaneously Enhanced Intracellular Lipogenesis and β-Carotene Biosynthesis of Rhodotorula Glutinis by Light Exposure with Sodium Acetate as the Substrate. Bioresour. Technol. 2020, 295, 122274. [Google Scholar] [CrossRef] [PubMed]
- Tkáčová, J.; Klempová, T.; Čertík, M. Kinetic Study of Growth, Lipid and Carotenoid Formation in β-Carotene Producing Rhodotorula Glutinis. Chem. Pap. 2018, 72, 1193–1203. [Google Scholar] [CrossRef]
- Bonatsos, N.; Marazioti, C.; Moutousidi, E.; Anagnostou, A.; Koutinas, A.; Kookos, I.K. Techno-Economic Analysis and Life Cycle Assessment of Heterotrophic Yeast-Derived Single Cell Oil Production Process. Fuel 2020, 264, 116839. [Google Scholar] [CrossRef]
- Parsons, S.; Abeln, F.; McManus, M.C.; Chuck, C.J. Techno-Economic Analysis (TEA) of Microbial Oil Production from Waste Resources as Part of a Biorefinery Concept: Assessment at Multiple Scales under Uncertainty. J. Chem. Technol. Biotechnol. 2019, 94, 701–711. [Google Scholar] [CrossRef]
- Karamerou, E.E.; Parsons, S.; McManus, M.C.; Chuck, C.J. Using Techno-Economic Modelling to Determine the Minimum Cost Possible for a Microbial Palm Oil Substitute. Biotechnol. Biofuels 2021, 14, 57. [Google Scholar] [CrossRef]
- Liu, Z.; Natalizio, F.; Dragone, G.; Mussatto, S.I. Maximizing the Simultaneous Production of Lipids and Carotenoids by Rhodosporidium Toruloides from Wheat Straw Hydrolysate and Perspectives for Large-Scale Implementation. Bioresour. Technol. 2021, 340, 125598. [Google Scholar] [CrossRef]
- Mussagy, C.U.; Winterburn, J.; Santos-Ebinuma, V.C.; Pereira, J.F.B. Production and Extraction of Carotenoids Produced by Microorganisms. Appl. Microbiol. Biotechnol. 2019, 103, 1095–1114. [Google Scholar] [CrossRef]
- Igreja, W.S.; Maia, F.d.A.; Lopes, A.S.; Chisté, R.C. Biotechnological Production of Carotenoids Using Low Cost-Substrates Is Influenced by Cultivation Parameters: A Review. Int. J. Mol. Sci. 2021, 22, 8819. [Google Scholar] [CrossRef]
- Dursun, D.; Koulouris, A.; Dalgıç, A.C. Process Simulation and Techno Economic Analysis of Astaxanthin Production from Agro-Industrial Wastes. Waste Biomass Valorization 2020, 11, 943–954. [Google Scholar] [CrossRef]
- Vieira, J.P.F.; Ienczak, J.L.; Costa, P.S.; Rossell, C.E.V.; Franco, T.T.; Pradella, J.G.C. Single Cell Oil Production Integrated to a Sugarcane-Mill: Conceptual Design, Process Specifications and Economic Analysis Using Molasses as Raw Material. Ind. Crops Prod. 2016, 89, 478–485. [Google Scholar] [CrossRef]
- Maina, S.; Pateraki, C.; Kopsahelis, N.; Paramithiotis, S.; Drosinos, E.H.; Papanikolaou, S.; Koutinas, A. Microbial Oil Production from Various Carbon Sources by Newly Isolated Oleaginous Yeasts. Eng. Life Sci. 2017, 17, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Ayadi, I.; Belghith, H.; Gargouri, A.; Guerfali, M. Screening of New Oleaginous Yeasts for Single Cell Oil Production, Hydrolytic Potential Exploitation and Agro-Industrial by-Products Valorization. Process Saf. Environ. Prot. 2018, 119, 104–114. [Google Scholar] [CrossRef]
- Tsakona, S.; Papadaki, A.; Kopsahelis, N.; Kachrimanidou, V.; Papanikolaou, S.; Koutinas, A. Development of a Circular Oriented Bioprocess for Microbial Oil Production Using Diversified Mixed Confectionery Side-Streams. Foods 2019, 8, 300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AOAC. Official Methods of Analysis of AOAC International; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Megazyme Total Starch Assay Procedure (Amyloglucosidase/Alpha-Amylase Method). AOAC Method 996.11. 2020. Available online: https://www.megazyme.com/documents/Assay_Protocol/K-TSHK_DATA.pdf (accessed on 20 October 2020).
- Ribeiro, J.E.S.; Sant’Ana, A.M.d.S.; Martini, M.; Sorce, C.; Andreucci, A.; de Melo, D.J.N.D.; da Silva, F.L.H. Rhodotorula glutinis Cultivation on Cassava Wastewater for Carotenoids and Fatty Acids Generation. Biocatal. Agric. Biotechnol. 2019, 22, 101419. [Google Scholar] [CrossRef]
- Kot, A.M.; Błażejak, S.; Kieliszek, M.; Gientka, I.; Piwowarek, K.; Brzezińska, R. Production of Lipids and Carotenoids by Rhodotorula gracilis ATCC 10788 Yeast in a Bioreactor Using Low-Cost Wastes. Biocatal. Agric. Biotechnol. 2020, 26, 101634. [Google Scholar] [CrossRef]
- Mishra, S.K.; Suh, W.I.; Farooq, W.; Moon, M.; Shrivastav, A.; Park, M.S.; Yang, J.W. Rapid Quantification of Microalgal Lipids in Aqueous Medium by a Simple Colorimetric Method. Bioresour. Technol. 2014, 155, 330–333. [Google Scholar] [CrossRef]
- Elfeky, N.; Elmahmoudy, M.; Zhang, Y.; Guo, J.L.; Bao, Y. Lipid and Carotenoid Production by Rhodotorula Glutinis with a Combined Cultivation Mode of Nitrogen, Sulfur, and Aluminium Stress. Appl. Sci. 2019, 9, 2444. [Google Scholar] [CrossRef] [Green Version]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Lie, S. The Ebc-Ninhydrin Method for Determination of Free Alpha Amino Nitrogen. J. Inst. Brew. 1973, 79, 37–41. [Google Scholar] [CrossRef]
- Candioti, L.V.; De Zan, M.M.; Cámara, M.S.; Goicoechea, H.C. Experimental Design and Multiple Response Optimization. Using the Desirability Function in Analytical Methods Development. Talanta 2014, 124, 123–138. [Google Scholar] [CrossRef]
- Leyton, A.; Flores, L.; Mäki-Arvela, P.; Lienqueo, M.E.; Shene, C. Macrocystis pyrifera Source of Nutrients for the Production of Carotenoids by a Marine Yeast Rhodotorula mucilaginosa. J. Appl. Microbiol. 2019, 127, 1069–1079. [Google Scholar] [CrossRef] [PubMed]
- Kot, A.M.; Błażejak, S.; Kieliszek, M.; Gientka, I.; Bryś, J.; Reczek, L.; Pobiega, K. Effect of Exogenous Stress Factors on the Biosynthesis of Carotenoids and Lipids by Rhodotorula Yeast Strains in Media Containing Agro-Industrial Waste. World J. Microbiol. Biotechnol. 2019, 35, 157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaturvedi, S.; Tiwari, R.; Bhattacharya, A.; Nain, L.; Khare, S.K. Production of Single Cell Oil by Using Cassava Peel Substrate from Oleaginous Yeast Rhodotorula glutinis. Biocatal. Agric. Biotechnol. 2019, 21, 101308. [Google Scholar] [CrossRef]
- Li, J.; Ma, C.; Ma, Y.; Li, Y.; Zhou, W.; Xu, P. Medium Optimization by Combination of Response Surface Methodology and Desirability Function: An Application in Glutamine Production. Appl. Microbiol. Biotechnol. 2007, 74, 563–571. [Google Scholar] [CrossRef]
- Liu, Z.; van den Berg, C.; Weusthuis, R.A.; Dragone, G.; Mussatto, S.I. Strategies for an Improved Extraction and Separation of Lipids and Carotenoids from Oleaginous Yeast. Sep. Purif. Technol. 2021, 257, 117946. [Google Scholar] [CrossRef]
- Passarinho, P.C.; Oliveira, B.; Dias, C.; Teles, M.; Reis, A.; da Silva, T.L. Sequential Carotenoids Extraction and Biodiesel Production from Rhodosporidium toruloides NCYC 921 Biomass. Waste Biomass Valorization 2020, 11, 2075–2086. [Google Scholar] [CrossRef] [Green Version]
- Sinha, S.; Singh, G.; Arora, A.; Paul, D. Carotenoid Production by Red Yeast Isolates Grown in Agricultural and “Mandi” Waste. Waste Biomass Valorization 2021, 12, 3939–3949. [Google Scholar] [CrossRef]
- Elfeky, N.; Elmahmoudy, M.; Bao, Y. Manipulation of Culture Conditions: Tool for Correlating/Improving Lipid and Carotenoid Production by Rhodotorula glutinis. Processes 2020, 8, 140. [Google Scholar] [CrossRef] [Green Version]
- Ibarruri, J.; Cebrián, M.; Hernández, I. Solid State Fermentation of Brewer’s Spent Grain Using Rhizopus Sp. to Enhance Nutritional Value. Waste Biomass Valorization 2019, 10, 3687–3700. [Google Scholar] [CrossRef]
- Paz, A.; Sabo, S.d.S.; Vallejo, M.; Marguet, E.; Oliveira, R.P.d.S.; Domínguez, J.M. Using Brewer’s Spent Grain to Formulate Culture Media for the Production of Bacteriocins Using Patagonian Strains. LWT 2018, 96, 166–174. [Google Scholar] [CrossRef]
- Haque, M.A.; Kachrimanidou, V.; Koutinas, A.; Lin, C.S.K. Valorization of Bakery Waste for Biocolorant and Enzyme Production by Monascus purpureus. J. Biotechnol. 2016, 231, 55–64. [Google Scholar] [CrossRef] [PubMed]
- López-Gómez, J.P.; Venus, J. Potential Role of Sequential Solid-State and Submerged-Liquid Fermentations in a Circular Bioeconomy. Fermentation 2021, 7, 76. [Google Scholar] [CrossRef]
- Tsakona, S.; Kopsahelis, N.; Chatzifragkou, A.; Papanikolaou, S.; Kookos, I.K.; Koutinas, A.A. Formulation of Fermentation Media from Flour-Rich Waste Streams for Microbial Lipid Production by Lipomyces starkeyi. J. Biotechnol. 2014, 189, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Mettu, S.; Martin, G.J.O.; Ashokkumar, M.; Lin, C.S.K. Ultrasonic Pretreatment of Food Waste to Accelerate Enzymatic Hydrolysis for Glucose Production. Ultrason. Sonochem. 2019, 53, 77–82. [Google Scholar] [CrossRef]
- Han, W.; Liu, Y.; Xu, X.; He, H.; Chen, L.; Tian, X.; Hou, P.; Tang, J. A Novel Combination of Enzymatic Hydrolysis and Microbial Fuel Cell for Electricity Production from Bakery Waste. Bioresour. Technol. 2020, 297, 122387. [Google Scholar] [CrossRef] [PubMed]
- Papadaki, A.; Fernandes, K.V.; Chatzifragkou, A.; Aguieiras, E.C.G.; da Silva, J.A.C.; Fernandez-Lafuente, R.; Papanikolaou, S.; Koutinas, A.; Freire, D.M.G. Bioprocess Development for Biolubricant Production Using Microbial Oil Derived via Fermentation from Confectionery Industry Wastes. Bioresour. Technol. 2018, 267, 311–318. [Google Scholar] [CrossRef]
- Petrides, D.; Carmichael, D.; Siletti, C.; Koulouris, A. Biopharmaceutical Process Optimization with Simulation and Scheduling Tools. Bioengineering 2014, 1, 154. [Google Scholar] [CrossRef]
- Kwan, T.H.; Pleissner, D.; Lau, K.Y.; Venus, J.; Pommeret, A.; Lin, C.S.K. Techno-Economic Analysis of a Food Waste Valorization Process via Microalgae Cultivation and Co-Production of Plasticizer, Lactic Acid and Animal Feed from Algal Biomass and Food Waste. Bioresour. Technol. 2015, 198, 292–299. [Google Scholar] [CrossRef]
- Carberry, J.J.; Walker, W.H.; White, A.H.; Jackson, D.D.; James, J.H.; Lewis, W.K.; Curtis, H.A.; Parmelee, H.C. Plant Design and Economics for Chemical Engineers; McGraw-Hill: New York, NY, USA, 2003. [Google Scholar]
- El-Halwagi, M.M. Overview of Process Economics. In Sustainable Design through Process Integration; El-Halwagi, M.M., Ed.; Butterworth-Heinemann: Oxford, UK, 2012; pp. 15–61. ISBN 978-1-85617-744-3. [Google Scholar]
- Koutinas, A.A.; Chatzifragkou, A.; Kopsahelis, N.; Papanikolaou, S.; Kookos, I.K. Design and Techno-Economic Evaluation of Microbial Oil Production as a Renewable Resource for Biodiesel and Oleochemical Production. Fuel 2014, 116, 566–577. [Google Scholar] [CrossRef]
- Masri, M.A.; Garbe, D.; Mehlmer, N.; Brück, T.B. A Sustainable, High-Performance Process for the Economic Production of Waste-Free Microbial Oils That Can Replace Plant-Based Equivalents. Energy Environ. Sci. 2019, 12, 2717–2732. [Google Scholar] [CrossRef]
- Cheng, C.; Zhou, Y.; Lin, M.; Wei, P.; Yang, S.T. Polymalic Acid Fermentation by Aureobasidium Pullulans for Malic Acid Production from Soybean Hull and Soy Molasses: Fermentation Kinetics and Economic Analysis. Bioresour. Technol. 2017, 223, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Fang, J.; Liu, Z.; Tang, J. Techno-Economic Evaluation of a Combined Bioprocess for Fermentative Hydrogen Production from Food Waste. Bioresour. Technol. 2016, 202, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Kumar, L.R.; Yellapu, S.K.; Tyagi, R.D.; Drogui, P. Cost, Energy and GHG Emission Assessment for Microbial Biodiesel Production through Valorization of Municipal Sludge and Crude Glycerol. Bioresour. Technol. 2020, 297, 122404. [Google Scholar] [CrossRef]
- Braunwald, T.; French, W.T.; Claupein, W.; Graeff-Hönninger, S. Economic Assessment of Microbial Biodiesel Production Using Heterotrophic Yeasts. Int. J. Green Energy 2016, 13, 274–282. [Google Scholar] [CrossRef]
- Freitas, C.; Parreira, T.M.; Roseiro, J.; Reis, A.; Da Silva, T.L. Selecting Low-Cost Carbon Sources for Carotenoid and Lipid Production by the Pink Yeast Rhodosporidium toruloides NCYC 921 Using Flow Cytometry. Bioresour. Technol. 2014, 158, 355–359. [Google Scholar] [CrossRef] [Green Version]







| Strain | Incubation Time, h | Origin | Code |
|---|---|---|---|
| Xanthophyllomyces dendrorhous ATCC 24202 | 96 | Purchased ATCC, USA | Xd |
| Rhodotorula mucilaginosa L4 | 72 | Isolated from aguamiel. Saltillo, Mexico | L4 |
| Sporobolomyces johnsonii ATCC 20490 | 96 | Purchased. ATCC, USA | Sj |
| Rhodotorula mucilaginosa L8 | 72 | Isolated from aguamiel. Saltillo, Mexico | L8 |
| Cystobasidium minutum CFGU-S-022 | 96 | Isolated from Lake of Salinas. Arequipa, Perú | P22 |
| Rhodotorula glutinis PM422 | 72 | Isolated from sotol. Saltillo, Mexico | Rg |
| Sakaguchia lamellibrachiae CFGU-S-010 | 96 | Isolated from Lake of Salinas. Arequipa, Perú | P10 |
| Rhodotorula sp. JR1 | 72 | Isolated from aguamiel. Saltillo, Mexico | JR1 |
| Sporobolomyces roseus CFGU-S005 | 96 | Isolated from Lake of Salinas. Arequipa, Perú | P5 |
| Component | Brewer´s Spent Grain | Pasta Processing Waste | Bread Waste |
|---|---|---|---|
| Moisture | 4.91 ± 0.19 | 12.17 ± 0.03 | 8.77 ± 0.68 |
| Ash | 3.06 ± 0.03 | 2.33 ± 0.04 | 2.77 ± 0.19 |
| Protein | 19.68 ± 0.27 | 9.83 ± 0.16 | 9.48 ± 0.33 |
| Lipids | 6.08 ± 0.02 | 6.40 ± 0.12 | 12.29 ± 0.02 |
| Starch | 19.67 ± 2.04 | 33.25 ± 0.67 | 20.10 ± 2.04 |
| Hemicellulose | 32.51 ± 6.11 | 8.65 ± 0.61 | 7.28 ± 1.77 |
| Lignin | 7.32 ± 0.84 | 3.48 ± 0.47 | 4.93 ± 0.898 |
| Cellulose | 13.03 ± 5.08 | 0.1 ± 0 | 4.42 ± 0.90 |
| Component | Hydrolysate | ||
|---|---|---|---|
| Brewer´s Spent Grain | Pasta Processing Waste | Bread Waste | |
| Total sugar, g/L | 18.43 ± 1.2 | 30.57 ± 1.03 | 12.89 ± 0.79 |
| FAN 1, mg/L | 127.85 ± 1.84 | 106.97 ± 0.36 | 97.66 ± 0.67 |
| IP 2, mg/L | 111.16 ± 4.98 | 66.70 ± 2.79 | 50.30 ± 1.21 |
| Total sugar/Starch conversion yield, % | 33.33 | 80.85 | 28.90 |
| FAN/TKN 3 conversion yield, % | 12.07 | 32.64 | 21.55 |
| Yeast | Media | YP/X, µg/g | P, µg/L | L, g/L | YL/X, % w/w |
|---|---|---|---|---|---|
| P5 | NL 1 | 210.76 ± 13.44 c | 1559.69 ± 99.47 bc | 2.65 ± 0.07 a | 35.84 ± 1.79 a |
| PPWH 2 | 216.05 ± 26.37 c | 1339.53 ± 163.53 cd | 0.74 ± 0.12 cde | 12.11 ± 2.65 def | |
| BSGH 3 | 269.58 ± 19.38 b | 1761.24 ± 126.64 b | 0.93 ± 0.09 bcde | 13.79 ± 1.18 cdef | |
| PPWH + TE 4 | 317.83 ± 2.85 a | 2161.24 ± 19.36 a | 1.22 ± 0.02 bc | 18.01 ± 0.29 bcd | |
| BSGH + TE | 266.85 ± 8.04 b | 1618.60 ± 33.4 b | 0.53 ± 0.08 e | 8.79 ± 1.48 ef | |
| JR1 | NL | 131.00 ± 11.44 e | 951.94 ± 80.20 e | 1.45 ± 0.22 b | 23.08 ± 3.15 b |
| PPWH | 171.67 ± 3.88 d | 1258.91 ± 28.42 d | 0.99 ± 0.13 bcde | 13.60 ± 1.77 cdef | |
| BSGH | 128.87 ± 6.57 e | 936.43 ± 47.74 e | 1.16 ± 0.47 bcd | 15.65 ± 5.75 cde | |
| PPWH + TE | 140.55 ± 5.04 de | 958.14 ± 73.83 e | 1.38 ± 0.27 b | 19.97 ± 1.82 bc | |
| BSGH + TE | 105.07 ± 3.68 e | 812.40 ± 23.41 e | 0.59 ± 0.06 de | 7.68 ± 0.75 f |
| Project Indices | Scenario 1 | Scenario 2 | Scenario 3 | Scenario 4 |
|---|---|---|---|---|
| Extra bioreactor | 0 | 1 | 2 | 3 |
| Investment, USD | 76,174.00 | 1,081,494.00 | 1,401,240.00 | 1,715,270.00 |
| Annual Operating cost, USD/year | 222,946.00 | 366,596.00 | 508,855.00 | 589,877.00 |
| Annual Revenues, USD/year | 263,284.00 | 472,568.00 | 705,102.00 | 772,612.00 |
| Gross Margin, % | 5.65 | 22.42 | 27.83 | 23.65 |
| ROI, % | 10.00 | 14.79 | 17.30 | 15.30 |
| Payback Time, years | 10 | 6.76 | 5.78 | 6.54 |
| NPV at 7.00%, USD | −247,745.00 | 14,993.00 | 271,697.00 | 86,518.00 |
| Batches/year | 63 | 126 | 188 | 206 |
| Economic Indices | |
|---|---|
| Total Investment, USD | 2,268,000 |
| Total Revenues, USD | 2,032,000 |
| Operating Cost, USD/year | 820,000 |
| Batch Size, kg MP | 3.70 |
| Net Unit Production Cost, USD kg MP | 1210.76 |
| Unit Production Revenue, USD kg MP | 3000.43 |
| Gross Margin, % | 59.65 |
| Return On Investment, % | 40.95 |
| Payback Time, years | 2.44 |
| IRR After Taxes, % | 29.45 |
| NPV at (7.00%), USD | 4,235,000.00 |
| Units | Name | Description | Size | Unit Cost (USD) | Total Cost (USD) |
|---|---|---|---|---|---|
| 1 | PPF-101 | Plate & Frame Filter | 2 m2 | 2000 | 2000 |
| 3 | G-101 | Centrifugal compressor | 4 kW | 1000 | 3000 |
| 3 | AF-101 | Air Filter | <0.01 m3/s | 1000 | 3000 |
| 3 | AF-102 | Air Filter | 0.01 m3/s | 1000 | 3000 |
| 1 | HG-101 | Homogenizer | 12 L/h | 3000 | 3000 |
| 1 | DC-101 | Decanter Centrifuge | 0.1 m3/h | 3000 | 3000 |
| 1 | MSX-101 | Mixer-Settler Extractor | 75 L/h | 2000 | 2000 |
| 1 | V-102 | Blending Tank | 300 L | 29,000 | 29,000 |
| 4 | V-103 | Batch Distillation Vessel | 180 L | 6000 | 24,000 |
| 1 | V-104 | Blending Tank | 700 L | 8000 | 8000 |
| 3 | FR-102 | Bioreactor | 1500 L | 60,000 | 180,000 |
| 1 | R-101 | Fermentor (148.98 L) | 150 L | 23,000 | 23,000 |
| 1 | V-101 | Batch Distillation Vessel (155.64 L) | 160 | 6000 | 6000 |
| 1 | V-105 | Flat Bottom Tank (171.03 L) | 180 | 1000 | 1000 |
| 1 | HX-101 | Heat Exchanger (0.01 m2) | 0.01 m2 | 1000 | 1000 |
| Unlisted equipment | 72,000 | ||||
| TOTAL | 358,000 |
| Bulk Material | Unit Cost (USD) | Annual Amount (kg) | Annual Cost (USD) | % |
|---|---|---|---|---|
| HCl (37% w/w) | 0.037 | 91,100 | 3371 | 3.02 |
| Hexane | 2.370 | 1689 | 4003 | 3.58 |
| Ethanolic KOH 1.1 M | 1.008 | 37,326 | 37,619 | 33.67 |
| Production Medium | 0.316 | 197,185 | 62,404 | 55.85 |
| YM medium | 0.201 | 21,614 | 4336 | 3.88 |
| Total | 111,733 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villegas-Méndez, M.Á.; Montañez, J.; Contreras-Esquivel, J.C.; Salmerón, I.; Koutinas, A.; Morales-Oyervides, L. Coproduction of Microbial Oil and Carotenoids within the Circular Bioeconomy Concept: A Sequential Solid-State and Submerged Fermentation Approach. Fermentation 2022, 8, 258. https://doi.org/10.3390/fermentation8060258
Villegas-Méndez MÁ, Montañez J, Contreras-Esquivel JC, Salmerón I, Koutinas A, Morales-Oyervides L. Coproduction of Microbial Oil and Carotenoids within the Circular Bioeconomy Concept: A Sequential Solid-State and Submerged Fermentation Approach. Fermentation. 2022; 8(6):258. https://doi.org/10.3390/fermentation8060258
Chicago/Turabian StyleVillegas-Méndez, Miguel Ángel, Julio Montañez, Juan Carlos Contreras-Esquivel, Iván Salmerón, Apostolis Koutinas, and Lourdes Morales-Oyervides. 2022. "Coproduction of Microbial Oil and Carotenoids within the Circular Bioeconomy Concept: A Sequential Solid-State and Submerged Fermentation Approach" Fermentation 8, no. 6: 258. https://doi.org/10.3390/fermentation8060258
APA StyleVillegas-Méndez, M. Á., Montañez, J., Contreras-Esquivel, J. C., Salmerón, I., Koutinas, A., & Morales-Oyervides, L. (2022). Coproduction of Microbial Oil and Carotenoids within the Circular Bioeconomy Concept: A Sequential Solid-State and Submerged Fermentation Approach. Fermentation, 8(6), 258. https://doi.org/10.3390/fermentation8060258