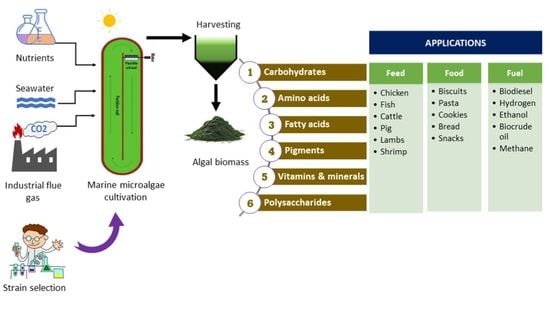
The Potential of Marine Microalgae for the Production of Food, Feed, and Fuel (3F)
Abstract
:1. Introduction
2. The Potential of Reducing Water Footprint by Marine Microalgae
3. Bioactive Compounds from Marine Microalgae
3.1. Amino Acids
3.2. Fatty Acids
3.3. Pigments
3.4. Vitamins and Minerals
3.5. Polysaccharides
4. Utilization of Marine Microalgae as Feed and Food Supplements
4.1. Marine Microalgae as Feed for Aquatic and Terrestrial Animals
4.2. Marine Microalgae as Human Food
5. Biofuels
5.1. Biodiesel
5.2. Bioethanol
5.3. Biomethane
5.4. Biocrude Oil
5.5. Biohydrogen
6. Challenges and Future Prospective of Producing 3F from Marine Microalgae
6.1. Selection of a Suitable Strain
6.2. Cultivation
6.3. Harvesting
6.4. Downstream Conversion Process
6.4.1. Biofuel Production
6.4.2. Algae-Based Feed and Food
6.5. Biorefinery Concept
6.5.1. Producing Microalgal Biomass with High-Value Metabolites
6.5.2. Exploring the Applications of the Left-Over Biomass
7. Cost Analysis of Marine Microalgal Biomass Production
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Yuan, H.-W.; Tan, L.; Kida, K.; Morimura, S.; Sun, Z.Y.; Tang, Y.Q. Potential for Reduced Water Consumption in Biorefining of Lignocellulosic Biomass to Bioethanol and Biogas. J. Biosci. Bioeng. 2021, 131, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Abomohra, A.E.F.; El-Naggar, A.H.; Baeshen, A.A. Potential of Macroalgae for Biodiesel Production: Screening and Evaluation Studies. J. Biosci. Bioeng. 2018, 125, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Lenferna, G.A. Can We Equitably Manage the End of the Fossil Fuel Era? Energy Res. Soc. Sci. 2018, 35, 217–223. [Google Scholar] [CrossRef]
- Hassan, M.H.; Kalam, M.A. An Overview of Biofuel as a Renewable Energy Source: Development and Challenges. Procedia Eng. 2013, 56, 39–53. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.G.; Long, S.P.; Ort, D.R. Improving Photosynthetic Efficiency for Greater Yield. Annu. Rev. Plant Biol. 2010, 61, 235–261. [Google Scholar] [CrossRef] [Green Version]
- Melis, A. Solar Energy Conversion Efficiencies in Photosynthesis: Minimizing the Chlorophyll Antennae to Maximize Efficiency. Plant Sci. 2009, 177, 272–280. [Google Scholar] [CrossRef]
- Kusmayadi, A.; Leong, Y.K.; Yen, H.W.; Huang, C.Y.; Chang, J.S. Microalgae as Sustainable Food and Feed Sources for Animals and Humans—Biotechnological and Environmental Aspects. Chemosphere 2021, 271, 129800. [Google Scholar] [CrossRef]
- Rzymski, P.; Budzulak, J.; Niedzielski, P.; Klimaszyk, P.; Proch, J.; Kozak, L.; Poniedziałek, B. Essential and Toxic Elements in Commercial Microalgal Food Supplements. J. Appl. Phycol. 2019, 31, 3567–3579. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.; Thomas-Hall, S.R.; Chua, E.T.; Schenk, P.M. Development of High-Level Omega-3 Eicosapentaenoic Acid (EPA) Production from Phaeodactylum tricornutum. J. Phycol. 2021, 57, 258–268. [Google Scholar] [CrossRef]
- Montes-González, O.; González-Silvera, A.; Valenzuela-Espinoza, E.; Santamaría-Del-Ángel, E.; López-Calderón, J. Effect of Light Intensity and Nutrient Concentration on Growth and Pigments of the Green Microalga Tetraselmis suecica. Lat. Am. J. Aquat. Res. 2021, 49, 431–441. [Google Scholar] [CrossRef]
- Dammak, M.; Haase, S.M.; Miladi, R.; Ben Amor, F.; Barkallah, M.; Gosset, D.; Pichon, C.; Huchzermeyer, B.; Fendri, I.; Denis, M.; et al. Enhanced Lipid and Biomass Production by a Newly Isolated and Identified Marine Microalga. Lipids Health Dis. 2016, 15, 209. [Google Scholar] [CrossRef] [Green Version]
- Das, P. Development of Microalgal Biomass for Biodiesel Production. Ph.D. Thesis, National University of Singapore, Singapore, 2010. [Google Scholar]
- Martínez-Roldán, A.J.; Perales-Vela, H.V.; Cañizares-Villanueva, R.O.; Torzillo, G. Physiological Response of Nannochloropsis Sp. to Saline Stress in Laboratory Batch Cultures. J. Appl. Phycol. 2014, 26, 115–121. [Google Scholar] [CrossRef]
- BenMoussa-Dahmen, I.; Chtourou, H.; Rezgui, F.; Sayadi, S.; Dhouib, A. Salinity Stress Increases Lipid, Secondary Metabolites and Enzyme Activity in Amphora subtropica and Dunaliella Sp. for Biodiesel Production. Bioresour. Technol. 2016, 218, 816–825. [Google Scholar] [CrossRef]
- Das, P.; Thaher, M.I.; Hakim, M.A.Q.M.A.; Al-Jabri, H.M.S.J.; Alghasal, G.S.H.S. A Comparative Study of the Growth of Tetraselmis Sp. in Large Scale Fixed Depth and Decreasing Depth Raceway Ponds. Bioresour. Technol. 2016, 216, 114–120. [Google Scholar] [CrossRef]
- Shalaby, M.M.; Nassar, I.N.; Abdallah, A.M. Evaporation Suppression from Open Water Surface Using Various Floating Covers with Consideration of Water Ecology. J. Hydrol. 2021, 598, 126482. [Google Scholar] [CrossRef]
- Nagappan, S.; Das, P.; AbdulQuadir, M.; Thaher, M.; Khan, S.; Mahata, C.; Al-Jabri, H.; Vatland, A.K.; Kumar, G. Potential of Microalgae as a Sustainable Feed Ingredient for Aquaculture. J. Biotechnol. 2021, 341, 1–20. [Google Scholar] [CrossRef]
- Ludwig, K.; Rihko-Struckmann, L.; Brinitzer, G.; Unkelbach, G.; Sundmacher, K. β-Carotene Extraction from Dunaliella salina by Supercritical CO2. J. Appl. Phycol. 2021, 33, 1435–1445. [Google Scholar] [CrossRef]
- Jiménez Callejón, M.J.; Robles Medina, A.; González Moreno, P.A.; Esteban Cerdán, L.; Orta Guillén, S.; Molina Grima, E. Simultaneous Extraction and Fractionation of Lipids from the Microalga Nannochloropsis Sp. for the Production of EPA-Rich Polar Lipid Concentrates. J. Appl. Phycol. 2020, 32, 1117–1128. [Google Scholar] [CrossRef] [Green Version]
- Chew, K.W.; Chia, S.R.; Lee, S.Y.; Zhu, L.; Show, P.L. Enhanced Microalgal Protein Extraction and Purification Using Sustainable Microwave-Assisted Multiphase Partitioning Technique. Chem. Eng. J. 2019, 367, 1–8. [Google Scholar] [CrossRef]
- Mota, R.; Guimarães, R.; Büttel, Z.; Rossi, F.; Colica, G.; Silva, C.J.; Santos, C.; Gales, L.; Zille, A.; De Philippis, R.; et al. Production and Characterization of Extracellular Carbohydrate Polymer from Cyanothece sp. CCY 0110. Carbohydr. Polym. 2013, 92, 1408–1415. [Google Scholar] [CrossRef]
- Bleakley, S.; Hayes, M. Algal Proteins: Extraction, Application, and Challenges Concerning Production. Foods 2017, 6, 33. [Google Scholar] [CrossRef] [Green Version]
- Becker, E.W. Micro Algae as a Source of Protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef]
- Tibbetts, S.; Melanson, R.; Park, K.C.; Banskota, A.; Stefanova, R.; Mcginn, P. Nutritional Evaluation of Whole and Lipid-Extracted Biomass of the Microalga Scenedesmus Sp. AMDD Isolated in Saskatchewan, Canada for Animal Feeds: Proximate, Amino Acid, Fatty Acid, Carotenoid and Elemental Composition. Curr. Biotechnol. 2015, 4, 530–546. [Google Scholar] [CrossRef]
- Schwenzfeier, A.; Wierenga, P.A.; Gruppen, H. Isolation and Characterization of Soluble Protein from the Green Microalgae Tetraselmis sp. Bioresour. Technol. 2011, 102, 9121–9127. [Google Scholar] [CrossRef]
- Guil-Guerrero, J.L.; Navarro-Juárez, R.; López-Martínez, J.C.; Campra-Madrid, P.; Rebolloso-Fuentes, M.M. Functional Properties of the Biomass of Three Microalgal Species. J. Food Eng. 2004, 65, 511–517. [Google Scholar] [CrossRef]
- Chai, X.J.; Chen, H.X.; Xu, W.Q.; Xu, Y.W. Expression of Soybean Kunitz Trypsin Inhibitor Gene SKTI in Dunaliella salina. J. Appl. Phycol. 2012, 25, 139–144. [Google Scholar] [CrossRef]
- Feng, S.; Feng, W.; Zhao, L.; Gu, H.; Li, Q.; Shi, K.; Guo, S.; Zhang, N. Preparation of Transgenic Dunaliella salina for Immunization against White Spot Syndrome Virus in Crayfish. Arch. Virol. 2014, 159, 519–525. [Google Scholar] [CrossRef]
- Feng, S.; Li, S.; Li, Q.; Shi, K.; Xue, L. Preparation of Recombinant Human Canstatin Using Transgenic Dunaliella salina. Acta Biochim. Biophys. Sin. 2014, 46, 428–430. [Google Scholar] [CrossRef] [Green Version]
- Van Vliet, S.; Burd, N.A.; van Loon, L.J.C. The Skeletal Muscle Anabolic Response to Plant-versus Animal-Based Protein Consumption. J. Nutr. 2015, 145, 1981–1991. [Google Scholar] [CrossRef] [Green Version]
- Fabregas, J.; Herrero, C. Marine Microalgae as a Potential Source of Single Cell Protein (SCP). Appl. Microbiol. Biotechnol. 1985, 23, 110–113. [Google Scholar] [CrossRef]
- Rahmanto, A.S.; Davies, M.J. Selenium-Containing Amino Acids as Direct and Indirect Antioxidants. IUBMB Life 2012, 64, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Hever, J.; Cronise, R.J. Plant-Based Nutrition for Healthcare Professionals: Implementing Diet as a Primary Modality in the Prevention and Treatment of Chronic Disease. J. Geriatr. Cardiol. 2017, 14, 355. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, B.O.; de Boer, K.; Gremmen, P.; Drinkwaard, A.; Wieggers, R.; Wijffels, R.H.; Barbosa, M.J.; D’Adamo, S. Selenium Enrichment in the Marine Microalga Nannochloropsis oceanica. Algal Res. 2021, 59, 102427. [Google Scholar] [CrossRef]
- Fan, T.W.-M.; Lane, A.N.; Higashi, R.M. Selenium Biotransformations by a Euryhaline Microalga Isolated from a Saline Evaporation Pond. Environ. Sci. Technol. 1997, 31, 569–576. [Google Scholar] [CrossRef]
- Hempel, F.; Maurer, M.; Brockmann, B.; Mayer, C.; Biedenkopf, N.; Kelterbaum, A.; Becker, S.; Maier, U.G. From Hybridomas to a Robust Microalgal-Based Production Platform: Molecular Design of a Diatom Secreting Monoclonal Antibodies Directed against the Marburg Virus Nucleoprotein. Microb. Cell Fact. 2017, 16, 131. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, C.; Xu, C. Nutritional Evaluation of Two Marine Microalgae as Feedstock for Aquafeed. Aquac. Res. 2020, 51, 946–956. [Google Scholar] [CrossRef]
- Kang, K.H.; Qian, Z.J.; Ryu, B.; Kim, D.; Kim, S.K. Protective Effects of Protein Hydrolysate from Marine Microalgae Navicula Incerta on Ethanol-Induced Toxicity in HepG2/CYP2E1 Cells. Food Chem. 2012, 132, 677–685. [Google Scholar] [CrossRef]
- Villar-Navarro, E.; Garrido-Pérez, C.; Perales, J.A. The Potential of Different Marine Microalgae Species to Recycle Nutrients from Recirculating Aquaculture Systems (RAS) Fish Farms and Produce Feed Additives. Algal Res. 2021, 58, 102389. [Google Scholar] [CrossRef]
- Caporgno, M.P.; Olkiewicz, M.; Torras, C.; Salvadó, J.; Clavero, E.; Bengoa, C. Effect of Pre-Treatments on the Production of Biofuels from Phaeodactylum tricornutum. J. Environ. Manag. 2016, 177, 240–246. [Google Scholar] [CrossRef]
- Kiron, V.; Phromkunthong, W.; Huntley, M.; Archibald, I.; De Scheemaker, G. Marine Microalgae from Biorefinery as a Potential Feed Protein Source for Atlantic Salmon, Common Carp and Whiteleg Shrimp. Aquac. Nutr. 2012, 18, 521–531. [Google Scholar] [CrossRef]
- Tibbetts, S.M.; Bjornsson, W.J.; McGinn, P.J. Biochemical Composition and Amino Acid Profiles of Nannochloropsis granulata Algal Biomass before and after Supercritical Fluid CO2 Extraction at Two Processing Temperatures. Anim. Feed Sci. Technol. 2015, 204, 62–71. [Google Scholar] [CrossRef]
- Tibbetts, S.M.; Patelakis, S.J.J.; Whitney-Lalonde, C.G.; Garrison, L.L.; Wall, C.L.; MacQuarrie, S.P. Nutrient Composition and Protein Quality of Microalgae Meals Produced from the Marine Prymnesiophyte Pavlova Sp. 459 Mass-Cultivated in Enclosed Photobioreactors for Potential Use in Salmonid Aquafeeds. J. Appl. Phycol. 2019, 32, 299–318. [Google Scholar] [CrossRef]
- Wang, X.; Fosse, H.K.; Li, K.; Chauton, M.S.; Vadstein, O.; Reitan, K.I. Influence of Nitrogen Limitation on Lipid Accumulation and EPA and DHA Content in Four Marine Microalgae for Possible Use in Aquafeed. Front. Mar. Sci. 2019, 63, 95. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The Importance of the Ratio of Omega-6/Omega-3 Essential Fatty Acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Mehariya, S.; Goswami, R.K.; Karthikeysan, O.P.; Verma, P. Microalgae for High-Value Products: A Way towards Green Nutraceutical and Pharmaceutical Compounds. Chemosphere 2021, 280, 130553. [Google Scholar] [CrossRef]
- Swanson, D.; Block, R.; Mousa, S.A. Omega-3 Fatty Acids EPA and DHA: Health Benefits throughout Life. Adv. Nutr. 2012, 3, 1–7. [Google Scholar] [CrossRef]
- Guo, D.S.; Ji, X.J.; Ren, L.J.; Li, G.L.; Sun, X.M.; Chen, K.Q.; Gao, S.; Huang, H. Development of a Scale-up Strategy for Fermentative Production of Docosahexaenoic Acid by Schizochytrium sp. Chem. Eng. Sci. 2018, 176, 600–608. [Google Scholar] [CrossRef]
- Haas, S.; Bauer, J.L.; Adakli, A.; Meyer, S.; Lippemeier, S.; Schwarz, K.; Schulz, C. Marine Microalgae Pavlova viridis and Nannochloropsis Sp. as n-3 PUFA Source in Diets for Juvenile European Sea Bass (Dicentrarchus labrax L.). J. Appl. Phycol. 2016, 28, 1011–1021. [Google Scholar] [CrossRef]
- Peng, X.; Meng, F.; Wang, Y.; Yi, X.; Cui, H. Effect of PH, Temperature, and CO2 Concentration on Growth and Lipid Accumulation of Nannochloropsis sp. MASCC. J. Ocean Univ. China 2020, 19, 1183–1192. [Google Scholar] [CrossRef]
- Gachelin, M.; Boutoute, M.; Carrier, G.; Talec, A.; Pruvost, E.; Guihéneuf, F.; Bernard, O.; Sciandra, A. Enhancing PUFA-Rich Polar Lipids in Tisochrysis lutea Using Adaptive Laboratory Evolution (ALE) with Oscillating Thermal Stress. Appl. Microbiol. Biotechnol. 2021, 105, 301–312. [Google Scholar] [CrossRef]
- Remize, M.; Planchon, F.; Garnier, M.; Loh, A.N.; Le Grand, F.; Bideau, A.; Lambert, C.; Corvaisier, R.; Volety, A.; Soudant, P. A 13CO2 Enrichment Experiment to Study the Synthesis Pathways of Polyunsaturated Fatty Acids of the Haptophyte Tisochrysis lutea. Mar. Drugs 2021, 20, 22. [Google Scholar] [CrossRef]
- Rosário Domingues, M.; Calado, R. Lipids of Marine Algae—Biomolecules with High Nutritional Value and Important Bioactive Properties. Biomolecules 2022, 12, 134. [Google Scholar] [CrossRef]
- Sahin, M.S.; Khazi, M.I.; Demirel, Z.; Dalay, M.C. Variation in Growth, Fucoxanthin, Fatty Acids Profile and Lipid Content of Marine Diatoms Nitzschia Sp. and Nanofrustulum shiloi in Response to Nitrogen and Iron. Biocatal. Agric. Biotechnol. 2019, 17, 390–398. [Google Scholar] [CrossRef]
- Van Wagenen, J.; Miller, T.W.; Hobbs, S.; Hook, P.; Crowe, B.; Huesemann, M. Effects of Light and Temperature on Fatty Acid Production in Nannochloropsis salina. Energies 2012, 5, 731–740. [Google Scholar] [CrossRef] [Green Version]
- Shah, S.M.U.; Che Radziah, C.; Ibrahim, S.; Latiff, F.; Othman, M.F.; Abdullah, M.A. Effects of Photoperiod, Salinity and PH on Cell Growth and Lipid Content of Pavlova lutheri. Ann. Microbiol. 2013, 64, 157–164. [Google Scholar] [CrossRef]
- Makri, A.; Bellou, S.; Birkou, M.; Papatrehas, K.; Dolapsakis, N.P.; Bokas, D.; Papanikolaou, S.; Aggelis, G. Lipid Synthesized by Micro-Algae Grown in Laboratory- and Industrial-Scale Bioreactors. Eng. Life Sci. 2011, 11, 52–58. [Google Scholar] [CrossRef]
- Lian, M.; Huang, H.; Ren, L.; Ji, X.; Zhu, J.; Jin, L. Increase of Docosahexaenoic Acid Production by Schizochytrium Sp. through Mutagenesis and Enzyme Assay. Appl. Biochem. Biotechnol. 2010, 162, 935–941. [Google Scholar] [CrossRef]
- Ren, L.J.; Ji, X.J.; Huang, H.; Qu, L.; Feng, Y.; Tong, Q.Q.; Ouyang, P.K. Development of a Stepwise Aeration Control Strategy for Efficient Docosahexaenoic Acid Production by Schizochytrium sp. Appl. Microbiol. Biotechnol. 2010, 87, 1649–1656. [Google Scholar] [CrossRef]
- Couto, D.; Conde, T.A.; Melo, T.; Neves, B.; Costa, M.; Cunha, P.; Guerra, I.; Correia, N.; Silva, J.T.; Pereira, H.; et al. Effects of Outdoor and Indoor Cultivation on the Polar Lipid Composition and Antioxidant Activity of Nannochloropsis oceanica and Nannochloropsis limnetica: A Lipidomics Perspective. Algal Res. 2022, 64, 102718. [Google Scholar] [CrossRef]
- Shiels, K.; Tsoupras, A.; Lordan, R.; Nasopoulou, C.; Zabetakis, I.; Murray, P.; Saha, S.K. Bioactive Lipids of Marine Microalga Chlorococcum Sp. SABC 012504 with Anti-Inflammatory and Anti-Thrombotic Activities. Mar. Drugs 2021, 19, 28. [Google Scholar] [CrossRef]
- Geider, R.J.; La Roche, J. Redfield Revisited: Variability of C:N:P in Marine Microalgae and Its Biochemical Basis. Eur. J. Phycol. 2002, 37, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Manisali, A.Y.; Sunol, A.K.; Philippidis, G.P. Effect of Macronutrients on Phospholipid Production by the Microalga Nannochloropsis oculata in a Photobioreactor. Algal Res. 2019, 41, 101514. [Google Scholar] [CrossRef]
- Guschina, I.A.; Harwood, J.L. Lipids and Lipid Metabolism in Eukaryotic Algae. Prog. Lipid Res. 2006, 45, 160–186. [Google Scholar] [CrossRef] [PubMed]
- Parmar, R.S.; Singh, C. A Comprehensive Study of Eco-Friendly Natural Pigment and Its Applications. Biochem. Biophys. Rep. 2018, 13, 22–26. [Google Scholar] [CrossRef]
- Favas, R.; Morone, J.; Martins, R.; Vasconcelos, V.; Lopes, G. Cyanobacteria and Microalgae Bioactive Compounds in Skin-Ageing: Potential to Restore Extracellular Matrix Filling and Overcome Hyperpigmentation. J. Enzym. Inhib. Med. Chem. 2021, 36, 1829–1838. [Google Scholar] [CrossRef]
- De Jesus Raposo, M.F.; De Morais, R.M.S.C.; De Morais, A.M.M.B. Health Applications of Bioactive Compounds from Marine Microalgae. Life Sci. 2013, 93, 479–486. [Google Scholar] [CrossRef]
- Pagels, F.; Guedes, A.C.; Amaro, H.M.; Kijjoa, A.; Vasconcelos, V. Phycobiliproteins from Cyanobacteria: Chemistry and Biotechnological Applications. Biotechnol. Adv. 2019, 37, 422–443. [Google Scholar] [CrossRef]
- Hsieh-Lo, M.; Castillo, G.; Ochoa-Becerra, M.A.; Mojica, L. Phycocyanin and Phycoerythrin: Strategies to Improve Production Yield and Chemical Stability. Algal Res. 2019, 42, 101600. [Google Scholar] [CrossRef]
- González-Ramírez, E.; Andújar-Sánchez, M.; Ortiz-Salmerón, E.; Bacarizo, J.; Cuadri, C.; Mazzuca-Sobczuk, T.; Ibáñez, M.J.; Cámara-Artigas, A.; Martínez-Rodríguez, S. Thermal and PH Stability of the B-Phycoerythrin from the Red Algae Porphyridium Cruentum. Food Biophys. 2014, 9, 184–192. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, C.; Hou, Y.; Chen, S.; Xiao, D.; Zhang, J.; Chen, F. Isolation and Characterization of a Marine Microalga for Biofuel Production with Astaxanthin as a Co-Product. Energies 2013, 6, 2759–2772. [Google Scholar] [CrossRef] [Green Version]
- Gayathri, S.; Rajasree Radhika, S.R.; Suman, T.Y.; Aranganathan, L. Ultrasound-Assisted Microextraction of β, ε-Carotene-3, 3′-Diol (Lutein) from Marine Microalgae Chlorella salina: Effect of Different Extraction Parameters. Biomass Convers. Biorefin. 2018, 8, 791–797. [Google Scholar] [CrossRef]
- Rammuni, M.N.; Ariyadasa, T.U.; Nimarshana, P.H.V.; Attalage, R.A. Comparative Assessment on the Extraction of Carotenoids from Microalgal Sources: Astaxanthin from H. Pluvialis and β-Carotene from D. Salina. Food Chem. 2019, 277, 128–134. [Google Scholar] [CrossRef]
- Maadane, A.; Merghoub, N.; Ainane, T.; El Arroussi, H.; Benhima, R.; Amzazi, S.; Bakri, Y.; Wahby, I. Antioxidant Activity of Some Moroccan Marine Microalgae: Pufa Profiles, Carotenoids and Phenolic Content. J. Biotechnol. 2015, 215, 13–19. [Google Scholar] [CrossRef]
- González-Vega, R.I.; Cárdenas-López, J.L.; López-Elías, J.A.; Ruiz-Cruz, S.; Reyes-Díaz, A.; Perez-Perez, L.M.; Cinco-Moroyoqui, F.J.; Robles-Zepeda, R.E.; Borboa-Flores, J.; Del-Toro-Sánchez, C.L. Optimization of Growing Conditions for Pigments Production from Microalga Navicula Incerta Using Response Surface Methodology and Its Antioxidant Capacity. Saudi J. Biol. Sci. 2021, 28, 1401–1416. [Google Scholar] [CrossRef]
- Binti Ibnu Rasid, E.N.; Mohamad, S.E.; Jamaluddin, H.; Salleh, M.M. Screening Factors Influencing the Production of Astaxanthin from Freshwater and Marine Microalgae. Appl. Biochem. Biotechnol. 2013, 172, 2160–2174. [Google Scholar] [CrossRef]
- Akalya, K.; Kumar, S.D.; Manigandan, G.; Santhanam, P.; Perumal, P.; Krishnaveni, N.; Arthikha, R.; Begum, A.; Dhanalakshmi, B.; Kim, M.K. The Influence of the Macroalgae Liquid Extracts on the Pigments and Fatty Acids Profile of the Marine Microalga, Picochlorum Maculatum (PSDK01). Thalassas 2021, 38, 553–564. [Google Scholar] [CrossRef]
- Xie, J.; Chen, S.; Wen, Z. Effects of Light Intensity on the Production of Phycoerythrin and Polyunsaturated Fatty Acid by Microalga Rhodomonas salina. Algal Res. 2021, 58, 102397. [Google Scholar] [CrossRef]
- Latsos, C.; van Houcke, J.; Blommaert, L.; Verbeeke, G.P.; Kromkamp, J.; Timmermans, K.R. Effect of Light Quality and Quantity on Productivity and Phycoerythrin Concentration in the Cryptophyte Rhodomonas sp. J. Appl. Phycol. 2021, 33, 729–741. [Google Scholar] [CrossRef]
- Latsos, C.; Bakratsas, G.; Moerdijk, T.; van Houcke, J.; Timmermans, K.R. Effect of Salinity and PH on Growth, Phycoerythrin, and Non-Volatile Umami Taste Active Compound Concentration of Rhodomonas salina Using a D-Optimal Design Approach. J. Appl. Phycol. 2021, 33, 3591–3602. [Google Scholar] [CrossRef]
- Prabakaran, G.; Sampathkumar, P.; Kavisri, M.; Moovendhan, M. Extraction and Characterization of Phycocyanin from Spirulina platensis and Evaluation of Its Anticancer, Antidiabetic and Antiinflammatory Effect. Int. J. Biol. Macromol. 2020, 153, 256–263. [Google Scholar] [CrossRef]
- Hotos, G.N. Culture Growth of the Cyanobacterium Phormidium Sp. in Various Salinity and Light Regimes and Their Influence on Its Phycocyanin and Other Pigments Content. J. Mar. Sci. Eng. 2021, 9, 798. [Google Scholar] [CrossRef]
- Bae, M.; Kim, M.B.; Park, Y.K.; Lee, J.Y. Health Benefits of Fucoxanthin in the Prevention of Chronic Diseases. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2020, 1865, 158618. [Google Scholar] [CrossRef]
- Wang, S.; Wu, S.; Yang, G.; Pan, K.; Wang, L.; Hu, Z. A Review on the Progress, Challenges and Prospects in Commercializing Microalgal Fucoxanthin. Biotechnol. Adv. 2021, 53, 107865. [Google Scholar] [CrossRef]
- Wang, W.; Yu, L.J.; Xu, C.; Tomizaki, T.; Zhao, S.; Umena, Y.; Chen, X.; Qin, X.; Xin, Y.; Suga, M.; et al. Structural Basis for Blue-Green Light Harvesting and Energy Dissipation in Diatoms. Science 2019, 363, eaav0365. [Google Scholar] [CrossRef]
- Wang, S.; Verma, S.K.; Hakeem Said, I.; Thomsen, L.; Ullrich, M.S.; Kuhnert, N. Changes in the Fucoxanthin Production and Protein Profiles in Cylindrotheca Closterium in Response to Blue Light-Emitting Diode Light. Microb. Cell Fact. 2018, 17, 110. [Google Scholar] [CrossRef]
- Marella, T.K.; Tiwari, A. Marine Diatom Thalassiosira Weissflogii Based Biorefinery for Co-Production of Eicosapentaenoic Acid and Fucoxanthin. Bioresour. Technol. 2020, 307, 123245. [Google Scholar] [CrossRef]
- Gao, F.; Woolschot, S.; Cabanelas, I.T.D.; Wijffels, R.H.; Barbosa, M.J. Light Spectra as Triggers for Sorting Improved Strains of Tisochrysis lutea. Bioresour. Technol. 2021, 321, 124434. [Google Scholar] [CrossRef]
- Xia, S.; Wang, K.; Wan, L.; Li, A.; Hu, Q.; Zhang, C. Production, Characterization, and Antioxidant Activity of Fucoxanthin from the Marine Diatom Odontella Aurita. Mar. Drugs 2013, 11, 2667–2681. [Google Scholar] [CrossRef]
- McClure, D.D.; Luiz, A.; Gerber, B.; Barton, G.W.; Kavanagh, J.M. An Investigation into the Effect of Culture Conditions on Fucoxanthin Production Using the Marine Microalgae Phaeodactylum tricornutum. Algal Res. 2018, 29, 41–48. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, X.; Liu, J. Screening of Isochrysis Strains for Simultaneous Production of Docosahexaenoic Acid and Fucoxanthin. Algal Res. 2019, 41, 101545. [Google Scholar] [CrossRef]
- Li, Y.; Sun, H.; Wu, T.; Fu, Y.; He, Y.; Mao, X.; Chen, F. Storage Carbon Metabolism of Isochrysis zhangjiangensis under Different Light Intensities and Its Application for Co-Production of Fucoxanthin and Stearidonic Acid. Bioresour. Technol. 2019, 282, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Koyande, A.K.; Chew, K.W.; Rambabu, K.; Tao, Y.; Chu, D.T.; Show, P.L. Microalgae: A Potential Alternative to Health Supplementation for Humans. Food Sci. Hum. Wellness 2019, 8, 16–24. [Google Scholar] [CrossRef]
- Hernández-Carmona, G.; Carrillo-Domínguez, S.; Arvizu-Higuera, D.L.; Rodríguez-Montesinos, Y.E.; Murillo-Álvarez, J.I.; Muñoz-Ochoa, M.; Castillo-Domínguez, R.M. Monthly Variation in the Chemical Composition of Eisenia arborea J.E. Areschoug. J. Appl. Phycol. 2009, 21, 607–616. [Google Scholar] [CrossRef]
- Rengaraj, D.; Hong, Y.H. Effects of Dietary Vitamin E on Fertility Functions in Poultry Species. Int. J. Mol. Sci. 2015, 16, 9910. [Google Scholar] [CrossRef] [Green Version]
- Durmaz, Y. Vitamin E (α-Tocopherol) Production by the Marine Microalgae Nannochloropsis oculata (Eustigmatophyceae) in Nitrogen Limitation. Aquaculture 2007, 272, 717–722. [Google Scholar] [CrossRef]
- Carballo-Cárdenas, E.C.; Tuan, P.M.; Janssen, M.; Wijffels, R.H. Vitamin E (α-Tocopherol) Production by the Marine Microalgae Dunaliella tertiolecta and Tetraselmis suecica in Batch Cultivation. Biomol. Eng. 2003, 20, 139–147. [Google Scholar] [CrossRef]
- Fabregas, J.; Herrero, C. Marine Microalgae as a Potential Source of Minerals in Fish Diets. Aquaculture 1986, 51, 237–243. [Google Scholar] [CrossRef] [Green Version]
- Alagawany, M.; Taha, A.E.; Noreldin, A.; El-Tarabily, K.A.; Abd El-Hack, M.E. Nutritional Applications of Species of Spirulina and Chlorella in Farmed Fish: A Review. Aquaculture 2021, 542, 736841. [Google Scholar] [CrossRef]
- Hwang, J.; Yadav, D.; Lee, P.C.; Jin, J. Immunomodulatory Effects of Polysaccharides from Marine Algae for Treating Cancer, Infectious Disease, and Inflammation. Phytother. Res. 2021, 36, 761–777. [Google Scholar] [CrossRef]
- Matsui, M.S.; Muizzuddin, N.; Arad, S.; Marenus, K. Sulfated Polysaccharides from Red Microalgae Have Antiinflammatory Properties in Vitro and in Vivo. Appl. Biochem. Biotechnol. 2003, 104, 13–22. [Google Scholar] [CrossRef]
- Hasui, M.; Matsuda, M.; Okutani, K.; Shigeta, S. In Vitro Antiviral Activities of Sulfated Polysaccharides from a Marine Microalga (Cochlodinium Polykrikoides) against Human Immunodeficiency Virus and Other Enveloped Viruses. Int. J. Biol. Macromol. 1995, 17, 293–297. [Google Scholar] [CrossRef]
- Ngo, D.H.; Kim, S.K. Sulfated Polysaccharides as Bioactive Agents from Marine Algae. Int. J. Biol. Macromol. 2013, 62, 70–75. [Google Scholar] [CrossRef]
- Gaignard, C.; Gargouch, N.; Dubessay, P.; Delattre, C.; Pierre, G.; Laroche, C.; Fendri, I.; Abdelkafi, S.; Michaud, P. New Horizons in Culture and Valorization of Red Microalgae. Biotechnol. Adv. 2019, 37, 193–222. [Google Scholar] [CrossRef]
- Ge, H.; Zhang, J.; Zhou, X.; Xia, L.; Hu, C. Effects of Light Intensity on Components and Topographical Structures of Extracellular Polymeric Substances from Microcoleus Vaginatus (Cyanophyceae). Phycologia 2019, 53, 167–173. [Google Scholar] [CrossRef]
- Delattre, C.; Pierre, G.; Laroche, C.; Michaud, P. Production, Extraction and Characterization of Microalgal and Cyanobacterial Exopolysaccharides. Biotechnol. Adv. 2016, 34, 1159–1179. [Google Scholar] [CrossRef]
- Gaignard, C.; Laroche, C.; Pierre, G.; Dubessay, P.; Delattre, C.; Gardarin, C.; Gourvil, P.; Probert, I.; Dubuffet, A.; Michaud, P. Screening of Marine Microalgae: Investigation of New Exopolysaccharide Producers. Algal Res. 2019, 44, 101711. [Google Scholar] [CrossRef]
- De Jesus Raposo, M.F.; De Morais, R.M.S.C.; De Morais, A.M.M.B. Bioactivity and Applications of Sulphated Polysaccharides from Marine Microalgae. Mar. Drugs 2013, 11, 233. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Wang, H.; Guo, G.; Pu, Y.; Yan, B. The Isolation and Antioxidant Activity of Polysaccharides from the Marine Microalgae Isochrysis galbana. Carbohydr. Polym. 2014, 113, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Pletikapić, G.; Berquand, A.; Radić, T.; Svetlicic, V. Quantitative Nanomechanical Mapping of Marine Diatom in Seawater Using Peak Force Tapping AFM. J. Phycol. 2011, 48, 174–185. [Google Scholar] [CrossRef]
- Gasljevic, K.; Hall, K.; Chapman, D.; Matthys, E.F. Drag-Reducing Polysaccharides from Marine Microalgae: Species Productivity and Drag Reduction Effectiveness. J. Appl. Phycol. 2008, 20, 299–310. [Google Scholar] [CrossRef]
- Hayashi, T.; Hayashi, K.; Maeda, M.; Kojima, I. Calcium Spirulan, an Inhibitor of Enveloped Virus Replication, from a Blue-Green Alga Spirulina platensis. J. Nat. Prod. 1996, 59, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Zhao, X.Q.; Yen, H.W.; Ho, S.H.; Cheng, C.L.; Lee, D.J.; Bai, F.W.; Chang, J.S. Microalgae-Based Carbohydrates for Biofuel Production. Biochem. Eng. J. 2013, 78, 1–10. [Google Scholar] [CrossRef]
- Fernandes, T.; Fernandes, I.; Andrade, C.A.P.; Ferreira, A.; Cordeiro, N. Marine Microalgae Monosaccharide Fluctuations as a Stress Response to Nutrients Inputs. Algal Res. 2017, 24, 340–346. [Google Scholar] [CrossRef]
- Annamalai, S.N.; Das, P.; Thaher, M.I.A.; Abdul Quadir, M.; Khan, S.; Mahata, C.; Al Jabri, H. Nutrients and Energy Digestibility of Microalgal Biomass for Fish Feed Applications. Sustainability 2021, 13, 3211. [Google Scholar] [CrossRef]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as Nutritional and Functional Food Sources: Revisiting Our Understanding. J. Appl. Phycol. 2017, 29, 949. [Google Scholar] [CrossRef]
- He, M.L.; Hollwich, W.; Rambeck, W.A. Supplementation of Algae to the Diet of Pigs: A New Possibility to Improve the Iodine Content in the Meat. J. Anim. Physiol. Anim. Nutr. 2002, 86, 97–104. [Google Scholar] [CrossRef]
- Samarakoon, K.; Jeon, Y.J. Bio-Functionalities of Proteins Derived from Marine Algae—A Review. Food Res. Int. 2012, 48, 948–960. [Google Scholar] [CrossRef]
- Urrutia, O.; Mendizabal, J.A.; Insausti, K.; Soret, B.; Purroy, A.; Arana, A. Effects of Addition of Linseed and Marine Algae to the Diet on Adipose Tissue Development, Fatty Acid Profile, Lipogenic Gene Expression, and Meat Quality in Lambs. PLoS ONE 2016, 11, e0156765. [Google Scholar] [CrossRef] [Green Version]
- Ginzberg, A.; Cohen, M.; Sod-Moriah, U.A.; Shany, S.; Rosenshtrauch, A.; Arad, S. (Malis) Chickens Fed with Biomass of the Red Microalga Porphyridium Sp. Have Reduced Blood Cholesterol Level and Modified Fatty Acid Composition in Egg Yolk. J. Appl. Phycol. 2000, 12, 325–330. [Google Scholar] [CrossRef]
- Toyomizu, M.; Sato, K.; Taroda, H.; Kato, T.; Akiba, Y. Effects of Dietary Spirulina on Meat Colour in Muscle of Broiler Chickens. Br. Poult. Sci. 2010, 42, 197–202. [Google Scholar] [CrossRef]
- Zahroojian, N.; Moravej, H.; Shivazad, M. Comparison of Marine Algae (Spirulina platensis) and Synthetic Pigment in Enhancing Egg Yolk Colour of Laying Hens. Br. Poult. Sci. 2011, 52, 584–588. [Google Scholar] [CrossRef]
- Park, J.H.; Upadhaya, S.D.; Kim, I.H. Effect of Dietary Marine Microalgae (Schizochytrium) Powder on Egg Production, Blood Lipid Profiles, Egg Quality, and Fatty Acid Composition of Egg Yolk in Layers. Asian-Australas. J. Anim. Sci. 2015, 28, 391. [Google Scholar] [CrossRef] [Green Version]
- Tam, L.T.; Van Cong, N.; Thom, L.T.; Ha, N.C.; Hang, N.T.M.; Van Minh, C.; Vien, D.T.H.; Hong, D.D. Cultivation and Biomass Production of the Diatom Thalassiosira Weissflogii as a Live Feed for White-Leg Shrimp in Hatcheries and Commercial Farms in Vietnam. J. Appl. Phycol. 2021, 33, 1559–1577. [Google Scholar] [CrossRef]
- Pascon, G.; Messina, M.; Petit, L.; Valente, L.M.P.; Oliveira, B.; Przybyla, C.; Dutto, G.; Tulli, F. Potential Application and Beneficial Effects of a Marine Microalgal Biomass Produced in a High-Rate Algal Pond (HRAP) in Diets of European Sea Bass, Dicentrarchus Labrax. Environ. Sci. Pollut. Res. 2021, 28, 62185–62199. [Google Scholar] [CrossRef]
- Lamminen, M.; Halmemies-Beauchet-Filleau, A.; Kokkonen, T.; Jaakkola, S.; Vanhatalo, A. Different Microalgae Species as a Substitutive Protein Feed for Soya Bean Meal in Grass Silage Based Dairy Cow Diets. Anim. Feed Sci. Technol. 2019, 247, 112–126. [Google Scholar] [CrossRef]
- Rizwan, M.; Mujtaba, G.; Memon, S.A.; Lee, K.; Rashid, N. Exploring the Potential of Microalgae for New Biotechnology Applications and beyond: A Review. Renew. Sustain. Energy Rev. 2018, 92, 394–404. [Google Scholar] [CrossRef]
- Rasdi, N.W.; Qin, J.G.; Li, Y. Effects of Dietary Microalgae on Fatty Acids and Digestive Enzymes in Copepod Cyclopina Kasignete, a Potential Live Food for Fish Larvae. Aquac. Res. 2016, 47, 3254–3264. [Google Scholar] [CrossRef]
- Brown, M.R.; Blackburn, S.I. Live Microalgae as Feeds in Aquaculture Hatcheries. In Advances in Aquaculture Hatchery Technology; Woodhead Publishing: Sawston, UK, 2013; pp. 117–158. [Google Scholar] [CrossRef]
- Dhert, P.; Rombaut, G.; Suantika, G.; Sorgeloos, P. Advancement of Rotifer Culture and Manipulation Techniques in Europe. Aquaculture 2001, 200, 129–146. [Google Scholar] [CrossRef] [Green Version]
- Basford, A.J.; Mos, B.; Francis, D.S.; Turchini, G.M.; White, C.A.; Dworjanyn, S. A Microalga Is Better than a Commercial Lipid Emulsion at Enhancing Live Feeds for an Ornamental Marine Fish Larva. Aquaculture 2020, 523, 735203. [Google Scholar] [CrossRef]
- Valente, L.M.P.; Custódio, M.; Batista, S.; Fernandes, H.; Kiron, V. Defatted Microalgae (Nannochloropsis Sp.) from Biorefinery as a Potential Feed Protein Source to Replace Fishmeal in European Sea Bass Diets. Fish Physiol. Biochem. 2019, 45, 1067–1081. [Google Scholar] [CrossRef]
- Sørensen, M.; Gong, Y.; Bjarnason, F.; Vasanth, G.K.; Dahle, D.; Huntley, M.; Kiron, V. Nannochloropsis oceania-Derived Defatted Meal as an Alternative to Fishmeal in Atlantic Salmon Feeds. PLoS ONE 2017, 12, e0179907. [Google Scholar] [CrossRef]
- Austic, R.E.; Mustafa, A.; Jung, B.; Gatrell, S.; Lei, X.G. Potential and Limitation of a New Defatted Diatom Microalgal Biomass in Replacing Soybean Meal and Corn in Diets for Broiler Chickens. J. Agric. Food Chem. 2013, 61, 7341–7348. [Google Scholar] [CrossRef]
- Pereira, H.; Sardinha, M.; Santos, T.; Gouveia, L.; Barreira, L.; Dias, J.; Varela, J. Incorporation of Defatted Microalgal Biomass (Tetraselmis Sp. CTP4) at the Expense of Soybean Meal as a Feed Ingredient for Juvenile Gilthead Seabream (Sparus Aurata). Algal Res. 2020, 47, 101869. [Google Scholar] [CrossRef]
- Vaz, B.d.; Moreira, J.B.; de Morais, M.G.; Costa, J.A.V. Microalgae as a New Source of Bioactive Compounds in Food Supplements. Curr. Opin. Food Sci. 2016, 7, 73–77. [Google Scholar] [CrossRef]
- El-Baz, F.K.; Abdo, S.M.; Hussein, A.M.S. Microalgae Dunaliella salina for Use as Food Supplement to Improve Pasta Quality. Int. J. Pharm. Sci. Rev. 2017, 46, 45–51. [Google Scholar]
- Qazi, W.M.; Ballance, S.; Uhlen, A.K.; Kousoulaki, K.; Haugen, J.E.; Rieder, A. Protein Enrichment of Wheat Bread with the Marine Green Microalgae Tetraselmis chuii—Impact on Dough Rheology and Bread Quality. LWT 2021, 143, 111115. [Google Scholar] [CrossRef]
- Khemiri, S.; Khelifi, N.; Nunes, M.C.; Ferreira, A.; Gouveia, L.; Smaali, I.; Raymundo, A. Microalgae Biomass as an Additional Ingredient of Gluten-Free Bread: Dough Rheology, Texture Quality and Nutritional Properties. Algal Res. 2020, 50, 101998. [Google Scholar] [CrossRef]
- Nunes, M.C.; Fernandes, I.; Vasco, I.; Sousa, I.; Raymundo, A. Tetraselmis chuii as a Sustainable and Healthy Ingredient to Produce Gluten-Free Bread: Impact on Structure, Colour and Bioactivity. Foods 2020, 9, 579. [Google Scholar] [CrossRef] [PubMed]
- Lucas, B.F.; de Morais, M.G.; Santos, T.D.; Costa, J.A.V. Spirulina for Snack Enrichment: Nutritional, Physical and Sensory Evaluations. LWT 2018, 90, 270–276. [Google Scholar] [CrossRef]
- Batista, A.P.; Niccolai, A.; Fradinho, P.; Fragoso, S.; Bursic, I.; Rodolfi, L.; Biondi, N.; Tredici, M.R.; Sousa, I.; Raymundo, A. Microalgae Biomass as an Alternative Ingredient in Cookies: Sensory, Physical and Chemical Properties, Antioxidant Activity and in Vitro Digestibility. Algal Res. 2017, 26, 161–171. [Google Scholar] [CrossRef]
- Janssen, J.H.; Wijffels, R.H.; Barbosa, M.J. Lipid Production in Nannochloropsis gaditana during Nitrogen Starvation. Biology 2019, 8, 5. [Google Scholar] [CrossRef] [Green Version]
- Nagappan, S.; Devendran, S.; Tsai, P.-C.; Dinakaran, S.; Dahms, H.-U.; Ponnusamy, V.K. Passive Cell Disruption Lipid Extraction Methods of Microalgae for Biofuel Production—A Review. Fuel 2019, 252, 699–709. [Google Scholar] [CrossRef]
- Rodrigues, A.; Bordado, J.C.; Dos Santos, R.G. Upgrading the Glycerol from Biodiesel Production as a Source of Energy Carriers and Chemicals—A Technological Review for Three Chemical Pathways. Energies 2017, 10, 1817. [Google Scholar] [CrossRef] [Green Version]
- Teo, S.H.; Islam, A.; Taufiq-Yap, Y.H. Algae Derived Biodiesel Using Nanocatalytic Transesterification Process. Chem. Eng. Res. Des. 2016, 111, 362–370. [Google Scholar] [CrossRef]
- Cao, H.; Zhang, Z.; Wu, X.; Miao, X. Direct Biodiesel Production from Wet Microalgae Biomass of Chlorella pyrenoidosa through In Situ Transesterification. BioMed Res. Int. 2013, 2013, 930686. [Google Scholar] [CrossRef] [Green Version]
- Kasim, F.; Harvey, A.; Zakaria, R. Biodiesel Production by in Situ Transesterification. Biofuels 2010, 1, 355–365. [Google Scholar] [CrossRef] [Green Version]
- Wahlen, B.D.; Willis, R.M.; Seefeldt, L.C. Biodiesel Production by Simultaneous Extraction and Conversion of Total Lipids from Microalgae, Cyanobacteria, and Wild Mixed-Cultures. Bioresour. Technol. 2011, 102, 2724–2730. [Google Scholar] [CrossRef]
- Arumugam, M.; Sivaramasamy, E.; Ajithkumar, T.; Kumaresan, S.; Thangavel, B. Biodiesel Production from Marine Microalgae Chlorella marina and Nannochloropsis salina. J. Pet. Technol Altern. Fuels 2012, 3, 58–62. [Google Scholar] [CrossRef]
- Saranya, G.; Ramachandra, T. V Novel Biocatalyst for Optimal Biodiesel Production from Diatoms. Renew. Energy 2020, 153, 919–934. [Google Scholar] [CrossRef]
- Elarroussi, H.; Benhima, R.; Bennis, I.; Elmernissi, N.; Wahby, I. Improvement of the Potential of Dunaliella tertiolecta as a Source of Biodiesel by Auxin Treatment Coupled to Salt Stress. Renew. Energy 2015, 77, 15–19. [Google Scholar] [CrossRef]
- Kumar, K.; Ghosh, S.; Angelidaki, I.; Holdt, S.L.; Karakashev, D.B.; Morales, M.A.; Das, D. Recent Developments on Biofuels Production from Microalgae and Macroalgae. Renew. Sustain. Energy Rev. 2016, 65, 235–249. [Google Scholar] [CrossRef]
- Lakatos, G.E.; Ranglová, K.; Manoel, J.C.; Grivalský, T.; Kopecký, J.; Masojídek, J. Bioethanol Production from Microalgae Polysaccharides. Folia Microbiol. 2019, 64, 627–644. [Google Scholar] [CrossRef]
- Ho, S.-H.; Huang, S.-W.; Chen, C.-Y.; Hasunuma, T.; Kondo, A.; Chang, J.-S. Bioethanol Production Using Carbohydrate-Rich Microalgae Biomass as Feedstock. Bioresour. Technol. 2013, 135, 191–198. [Google Scholar] [CrossRef]
- Yao, C.; Ai, J.; Cao, X.; Xue, S.; Zhang, W. Enhancing Starch Production of a Marine Green Microalga Tetraselmis subcordiformis through Nutrient Limitation. Bioresour. Technol. 2012, 118, 438–444. [Google Scholar] [CrossRef]
- Reyimu, Z.; Özçimen, D. Batch Cultivation of Marine Microalgae Nannochloropsis oculata and Tetraselmis suecica in Treated Municipal Wastewater toward Bioethanol Production. J. Clean. Prod. 2017, 150, 40–46. [Google Scholar] [CrossRef]
- Möllers, K.B.; Cannella, D.; Jørgensen, H.; Frigaard, N.-U. Cyanobacterial Biomass as Carbohydrate and Nutrient Feedstock for Bioethanol Production by Yeast Fermentation. Biotechnol. Biofuels 2014, 7, 64. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Oh, Y.; Kim, D.; Kwon, D.; Lee, C.; Lee, J. Converting Carbohydrates Extracted from Marine Algae into Ethanol Using Various Ethanolic Escherichia Coli Strains. Appl. Biochem. Biotechnol. 2011, 164, 878–888. [Google Scholar] [CrossRef]
- Harun, R.; Jason, W.S.Y.; Cherrington, T.; Danquah, M.K. Exploring Alkaline Pre-Treatment of Microalgal Biomass for Bioethanol Production. Appl. Energy 2011, 88, 3464–3467. [Google Scholar] [CrossRef]
- Lee, O.K.; Kim, A.L.; Seong, D.H.; Lee, C.G.; Jung, Y.T.; Lee, J.W.; Lee, E.Y. Chemo-Enzymatic Saccharification and Bioethanol Fermentation of Lipid-Extracted Residual Biomass of the Microalga, Dunaliella tertiolecta. Bioresour. Technol. 2013, 132, 197–201. [Google Scholar] [CrossRef]
- Deng, M.-D.; Coleman, J.R. Ethanol Synthesis by Genetic Engineering in Cyanobacteria. Appl. Environ. Microbiol. 1999, 65, 523–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueno, Y.; Kurano, N.; Miyachi, S. Ethanol Production by Dark Fermentation in the Marine Green Alga, Chlorococcum littorale. J. Ferment. Bioeng. 1998, 86, 38–43. [Google Scholar] [CrossRef]
- Gao, Z.; Zhao, H.; Li, Z.; Tan, X.; Lu, X. Photosynthetic Production of Ethanol from Carbon Dioxide in Genetically Engineered Cyanobacteria. Energy Environ. Sci. 2012, 5, 9857–9865. [Google Scholar] [CrossRef]
- Zhao, B.; Ma, J.; Zhao, Q.; Laurens, L.; Jarvis, E.; Chen, S.; Frear, C. Efficient Anaerobic Digestion of Whole Microalgae and Lipid-Extracted Microalgae Residues for Methane Energy Production. Bioresour. Technol. 2014, 161, 423–430. [Google Scholar] [CrossRef]
- Zabed, H.M.; Qi, X.; Yun, J.; Zhang, H. Anaerobic Digestion of Microalgae Biomass for Methane Production. In Microalgae Biotechnology for Development of Biofuel and Wastewater Treatment; Alam, M.A., Wang, Z., Eds.; Springer: Singapore, 2019; pp. 397–421. ISBN 978-981-13-2264-8. [Google Scholar]
- Kendir, E.; Ugurlu, A. A Comprehensive Review on Pretreatment of Microalgae for Biogas Production. Int. J. Energy Res. 2018, 42, 3711–3731. [Google Scholar] [CrossRef]
- Mendez, L.; Mahdy, A.; Ballesteros, M.; González-Fernández, C. Chlorella vulgaris vs. Cyanobacterial Biomasses: Comparison in Terms of Biomass Productivity and Biogas Yield. Energy Convers. Manag. 2015, 92, 137–142. [Google Scholar] [CrossRef]
- Alzate, M.E.; Muñoz, R.; Rogalla, F.; Fdz-Polanco, F.; Pérez-Elvira, S.I. Biochemical Methane Potential of Microalgae Biomass after Lipid Extraction. Chem. Eng. J. 2014, 243, 405–410. [Google Scholar] [CrossRef]
- De, C.; Neves, V.T.; Sales, E.A.; Perelo, L.W. Influence of Lipid Extraction Methods as Pre-Treatment of Microalgal Biomass for Biogas Production. Renew. Sustain. Energy Rev. 2016, 59, 160–165. [Google Scholar] [CrossRef]
- Santos, N.O.; Oliveira, S.M.; Alves, L.C.; Cammarota, M.C. Methane Production from Marine Microalgae Isochrysis galbana. Bioresour. Technol. 2014, 157, 60–67. [Google Scholar] [CrossRef]
- Hernández, D.; Solana, M.; Riaño, B.; García-González, M.C.; Bertucco, A. Biofuels from Microalgae: Lipid Extraction and Methane Production from the Residual Biomass in a Biorefinery Approach. Bioresour. Technol. 2014, 170, 370–378. [Google Scholar] [CrossRef]
- Al-Jabri, H.; Das, P.; Khan, S.; AbdulQuadir, M.; Thaher, M.I.; Hoekman, K.; Hawari, A.H. A Comparison of Bio-Crude Oil Production from Five Marine Microalgae—Using Life Cycle Analysis. Energy 2022, 251, 123954. [Google Scholar] [CrossRef]
- Das, P.; Thaher, M.I.; Khan, S.; AbdulQuadir, M.; Chaudhary, A.K.; Alghasal, G.; Al-Jabri, H. Comparison of Biocrude Oil Production from Self-Settling and Non-Settling Microalgae Biomass Produced in the Qatari Desert Environment. Int. J. Environ. Sci. Technol. 2019, 16, 7443–7454. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Chen, W.-T. 5-Hydrothermal Liquefaction of Protein-Containing Feedstocks. In Direct Thermochemical Liquefaction for Energy Applications; Rosendahl, L., Ed.; Woodhead Publishing: Sawston, UK, 2018; pp. 127–168. ISBN 978-0-08-101029-7. [Google Scholar]
- Valdez, P.J.; Tocco, V.J.; Savage, P.E. A General Kinetic Model for the Hydrothermal Liquefaction of Microalgae. Bioresour. Technol. 2014, 163, 123–127. [Google Scholar] [CrossRef]
- Aljabri, H.; Das, P.; Khan, S.; AbdulQuadir, M.; Thaher, M.; Hawari, A.H.; Al-Shamary, N.M. A Study to Investigate the Energy Recovery Potential from Different Macromolecules of a Low-Lipid Marine Tetraselmis sp. Biomass through HTL Process. Renew. Energy 2022, 189, 78–89. [Google Scholar] [CrossRef]
- Das, P.; Khan, S.; Thaher, M.; AbdulQuadir, M.; Hoekman, S.K.; Al-Jabri, H. Effect of Harvesting Methods on the Energy Requirement of Tetraselmis Sp. Biomass Production and Biocrude Yield and Quality. Bioresour. Technol. 2019, 284, 9–15. [Google Scholar] [CrossRef]
- Valdez, P.J.; Dickinson, J.G.; Savage, P.E. Characterization of Product Fractions from Hydrothermal Liquefaction of Nannochloropsis Sp. and the Influence of Solvents. Energy Fuels 2011, 25, 3235–3243. [Google Scholar] [CrossRef]
- Haider, M.S.; Castello, D.; Michalski, K.M.; Pedersen, T.H.; Rosendahl, L.A. Catalytic Hydrotreatment of Microalgae Biocrude from Continuous Hydrothermal Liquefaction: Heteroatom Removal and Their Distribution in Distillation Cuts. Energies 2018, 11, 3360. [Google Scholar] [CrossRef] [Green Version]
- Elliott, D.C.; Hart, T.R.; Schmidt, A.J.; Neuenschwander, G.G.; Rotness, L.J.; Olarte, M.V.; Zacher, A.H.; Albrecht, K.O.; Hallen, R.T.; Holladay, J.E. Process Development for Hydrothermal Liquefaction of Algae Feedstocks in a Continuous-Flow Reactor. Algal Res. 2013, 2, 445–454. [Google Scholar] [CrossRef] [Green Version]
- Vo, T.K.; Kim, S.S.; Ly, H.V.; Lee, E.Y.; Lee, C.G.; Kim, J. A General Reaction Network and Kinetic Model of the Hydrothermal Liquefaction of Microalgae Tetraselmis sp. Bioresour. Technol. 2017, 241, 610–619. [Google Scholar] [CrossRef]
- Biller, P.; Ross, A.B. Potential Yields and Properties of Oil from the Hydrothermal Liquefaction of Microalgae with Different Biochemical Content. Bioresour. Technol. 2011, 102, 215–225. [Google Scholar] [CrossRef]
- Yang, C.; Jia, L.; Chen, C.; Liu, G.; Fang, W. Bio-Oil from Hydro-Liquefaction of Dunaliella salina over Ni/REHY Catalyst. Bioresour. Technol. 2011, 102, 4580–4584. [Google Scholar] [CrossRef]
- Toor, S.S.; Reddy, H.; Deng, S.; Hoffmann, J.; Spangsmark, D.; Madsen, L.B.; Holm-Nielsen, J.B.; Rosendahl, L.A. Hydrothermal Liquefaction of Spirulina and Nannochloropsis salina under Subcritical and Supercritical Water Conditions. Bioresour. Technol. 2013, 131, 413–419. [Google Scholar] [CrossRef]
- Shakya, R.; Whelen, J.; Adhikari, S.; Mahadevan, R.; Neupane, S. Effect of Temperature and Na2CO3 Catalyst on Hydrothermal Liquefaction of Algae. Algal Res. 2015, 12, 80–90. [Google Scholar] [CrossRef] [Green Version]
- Mahata, C.; Ray, S.; Das, D. Optimization of Dark Fermentative Hydrogen Production from Organic Wastes Using Acidogenic Mixed Consortia. Energy Convers. Manag. 2020, 219, 113047. [Google Scholar] [CrossRef]
- Mahata, C.; Das, D. Current Status and Prospects of Biohydrogen Production Process. In Microbial Biotechnology for Renewable and Sustainable Energy; Springer: Singapore, 2022; pp. 99–133. [Google Scholar] [CrossRef]
- Nagarajan, D.; Chang, J.S.; Lee, D.J. Pretreatment of Microalgal Biomass for Efficient Biohydrogen Production—Recent Insights and Future Perspectives. Bioresour. Technol. 2020, 302, 122871. [Google Scholar] [CrossRef]
- Mahata, C.; Dhar, S.; Ray, S.; Das, D. Effect of Thermal Pretreated Organic Wastes on the Dark Fermentative Hydrogen Production Using Mixed Microbial Consortia. Fuel 2021, 284, 119062. [Google Scholar] [CrossRef]
- Das, D.; Veziroglu, T.N. Advances in Biological Hydrogen Production Processes. Int. J. Hydrogen Energy 2008, 33, 6046–6057. [Google Scholar] [CrossRef]
- Xia, A.; Cheng, J.; Song, W.; Su, H.; Ding, L.; Lin, R.; Lu, H.; Liu, J.; Zhou, J.; Cen, K. Fermentative Hydrogen Production Using Algal Biomass as Feedstock. Renew. Sustain. Energy Rev. 2015, 51, 209–230. [Google Scholar] [CrossRef]
- Das, D. Advances in Biohydrogen Production Processes: An Approach towards Commercialization. Int. J. Hydrogen Energy 2009, 34, 7349–7357. [Google Scholar] [CrossRef]
- Kapdan, I.K.; Kargi, F. Bio-Hydrogen Production from Waste Materials. Enzyme Microb. Technol. 2006, 38, 569–582. [Google Scholar] [CrossRef]
- Kumari, S.; Nasr, M.; Kumar, S. Technological Advances in Biohydrogen Production from Microalgae. In Algal Biofuels; Springer: Cham, Switzerland, 2017; pp. 347–360. [Google Scholar] [CrossRef]
- Johnson, M.C.; Palou-Rivera, I.; Frank, E.D. Energy Consumption during the Manufacture of Nutrients for Algae Cultivation. Algal Res. 2013, 2, 426–436. [Google Scholar] [CrossRef]
- Mahata, C.; Dhar, S.; Ray, S.; Das, D. Flocculation Characteristics of Anaerobic Sludge Driven-Extracellular Polymeric Substance (EPS) Extracted by Different Methods on Microalgae Harvesting for Lipid Utilization. Biochem. Eng. J. 2021, 167, 107898. [Google Scholar] [CrossRef]
- Chauton, M.S.; Reitan, K.I.; Norsker, N.H.; Tveterås, R.; Kleivdal, H.T. A Techno-Economic Analysis of Industrial Production of Marine Microalgae as a Source of EPA and DHA-Rich Raw Material for Aquafeed: Research Challenges and Possibilities. Aquaculture 2015, 436, 95–103. [Google Scholar] [CrossRef]
- Das, P.; Obbard, J.P. Incremental Energy Supply for Microalgae Culture in a Photobioreactor. Bioresour. Technol. 2011, 102, 2973–2978. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Mishra, S.K.; Shrivastav, A.; Park, M.S.; Yang, J.-W. Recent Trends in the Mass Cultivation of Algae in Raceway Ponds. Renew. Sustain. Energy Rev. 2015, 51, 875–885. [Google Scholar] [CrossRef]
- Rogers, J.N.; Rosenberg, J.N.; Guzman, B.J.; Oh, V.H.; Mimbela, L.E.; Ghassemi, A.; Betenbaugh, M.J.; Oyler, G.A.; Donohue, M.D. A Critical Analysis of Paddlewheel-Driven Raceway Ponds for Algal Biofuel Production at Commercial Scales. Algal Res. 2014, 4, 76–88. [Google Scholar] [CrossRef] [Green Version]
- Caporgno, M.; Pruvost, J.; Legrand, J.; Lépine, O.; Tazerout, M.; Bengoa, C. Hydrothermal Liquefaction of Nannochloropsis oceanica in Different Solvents. Bioresour. Technol. 2016, 214, 404–410. [Google Scholar] [CrossRef]
- Moheimani, N. Tetraselmis suecica Culture for CO2 Bioremediation of Untreated Flue Gas from a Coal-Fired Power Station. J. Appl. Phycol. 2015, 28, 2139–2146. [Google Scholar] [CrossRef]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The Promising Future of Microalgae: Current Status, Challenges, and Optimization of a Sustainable and Renewable Industry for Biofuels, Feed, and Other Products. Microb. Cell Fact. 2018, 17, 36. [Google Scholar] [CrossRef]
- De Godos Crespo, I.; Martin, J.L.; Acien, G.; Molina-Grima, E.; Banks, C.; Heaven, S.; Rogalla, F. Evaluation of Carbon Dioxide Mass Transfer in Raceway Reactors for Microalgae Culture Using Flue Gases. Bioresour. Technol. 2013, 153C, 307–314. [Google Scholar] [CrossRef]
- Morais, K.C.C.; Ribeiro, R.; Santos, K.R.; Taher, D.M.; Mariano, A.; Vargas, J.V.C. Phaeodactylum tricornutum Microalgae Growth Rate in Heterotrophic and Mixotrophic Conditions. Therm. Eng. 2009, 8, 84–89. [Google Scholar] [CrossRef]
- Das, P.; Aziz, S.S.; Obbard, J.P. Two Phase Microalgae Growth in the Open System for Enhanced Lipid Productivity. Renew. Energy 2011, 36, 2524–2528. [Google Scholar] [CrossRef]
- Al-Jabri, H.; Das, P.; Thaher, M.; Khan, S.; AbdulQuadir, M. Potential Utilization of Waste Nitrogen Fertilizer from a Fertilizer Industry Using Marine Microalgae. Sci. Total Environ. 2020, 755, 142532. [Google Scholar] [CrossRef]
- Campos, J.L.; Crutchik, D.; Franchi, Ó.; Pavissich, J.P.; Belmonte, M.; Pedrouso, A.; Mosquera-Corral, A.; del Río, Á. Nitrogen and Phosphorus Recovery From Anaerobically Pretreated Agro-Food Wastes: A Review. Front. Sustain. Food Syst. 2019, 2, 91. [Google Scholar] [CrossRef] [Green Version]
- Vandamme, D.; Foubert, I.; Muylaert, K. Flocculation as a Low-Cost Method for Harvesting Microalgae for Bulk Biomass Production. Trends Biotechnol. 2013, 31, 233–239. [Google Scholar] [CrossRef] [Green Version]
- Branyikova, I.; Prochazkova, G.; Potocar, T.; Jezkova, Z.; Branyik, T. Harvesting of Microalgae by Flocculation. Fermentation 2018, 4, 93. [Google Scholar] [CrossRef] [Green Version]
- Conradi, F.D.; Zhou, R.-Q.; Oeser, S.; Schuergers, N.; Wilde, A.; Mullineaux, C.W. Factors Controlling Floc Formation and Structure in the Cyanobacterium Synechocystis Sp. Strain PCC 6803. J. Bacteriol. 2019, 201, e00344-19. [Google Scholar] [CrossRef] [Green Version]
- Das, P.; Quadir, M.A.; Chaudhary, A.K.; Thaher, M.I.; Khan, S.; Alghazal, G.; Al-Jabri, H. Outdoor Continuous Cultivation of Self-Settling Marine Cyanobacterium Chroococcidiopsis sp. Ind. Biotechnol. 2018, 14, 45–53. [Google Scholar] [CrossRef]
- Talukder, M.M.R.; Das, P.; Wu, J.C. Immobilization of Microalgae on Exogenous Fungal Mycelium: A Promising Separation Method to Harvest Both Marine and Freshwater Microalgae. Biochem. Eng. J. 2014, 91, 53–57. [Google Scholar] [CrossRef]
- Chatsungnoen, T.; Chisti, Y. Harvesting Microalgae by Flocculation–Sedimentation. Algal Res. 2016, 13, 271–283. [Google Scholar] [CrossRef]
- Chu, F.J.; Wan, T.J.; Chen, H.; Wu, C.H.; Kao, P.M. Magnetophoretic Harvesting of Nannochloropsis oculata Using Iron Oxide Immobilized Beads. Water 2020, 12, 236. [Google Scholar] [CrossRef] [Green Version]
- Salim, S.; Bosma, R.; Vermuë, M.H.; Wijffels, R.H. Harvesting of Microalgae by Bio-Flocculation. J. Appl. Phycol. 2011, 23, 849–855. [Google Scholar] [CrossRef] [Green Version]
- Eldridge, R.J.; Hill, D.R.A.; Gladman, B.R. A Comparative Study of the Coagulation Behaviour of Marine Microalgae. J. Appl. Phycol. 2012, 24, 1667–1679. [Google Scholar] [CrossRef]
- Lee, A.K.; Lewis, D.M.; Ashman, P.J. Harvesting of Marine Microalgae by Electroflocculation: The Energetics, Plant Design, and Economics. Appl. Energy 2013, 108, 45–53. [Google Scholar] [CrossRef]
- Vandamme, D.; Pontes, S.C.V.; Goiris, K.; Foubert, I.; Pinoy, L.J.J.; Muylaert, K. Evaluation of Electro-Coagulation-Flocculation for Harvesting Marine and Freshwater Microalgae. Biotechnol. Bioeng. 2011, 108, 2320–2329. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.; Kim, S.; Lee, J. Optimization of Cross Flow Filtration System for Dunaliella tertiolecta and Tetraselmis sp. Microalgae Harvest. Korean J. Chem. Eng. 2015, 32, 1377–1380. [Google Scholar] [CrossRef]
- Akhondi, E.; Zamani, F.; Law, A.W.K.; Krantz, W.B.; Fane, A.G.; Chew, J.W. Influence of Backwashing on the Pore Size of Hollow Fiber Ultrafiltration Membranes. J. Memb. Sci. 2017, 521, 33–42. [Google Scholar] [CrossRef]
- Das, P.; Thaher, M.; Khan, S.; AbdulQuadir, M.; Al-Jabri, H. The Effect of Culture Salinity on the Harvesting of Microalgae Biomass Using Pilot-Scale Tangential-Flow-Filter Membrane. Bioresour. Technol. 2019, 293, 122057. [Google Scholar] [CrossRef]
- Hosseinizand, H.; Sokhansanj, S.; Lim, C.J. Studying the Drying Mechanism of Microalgae Chlorella vulgaris and the Optimum Drying Temperature to Preserve Quality Characteristics. Dry. Technol. 2017, 36, 1049–1060. [Google Scholar] [CrossRef]
- Fasaei, F.; Bitter, J.H.; Slegers, P.M.; van Boxtel, A.J.B. Techno-Economic Evaluation of Microalgae Harvesting and Dewatering Systems. Algal Res. 2018, 31, 347–362. [Google Scholar] [CrossRef]
- Show, K.-Y.; Yan, Y.-G.; Lee, D.-J. Algal Biomass Harvesting and Drying. In Biofuels from Algae; Elsevier: Amsterdam, The Netherlands, 2019; pp. 135–166. [Google Scholar] [CrossRef]
- Kim, J.; Ryu, B.G.; Lee, Y.J.; Han, J.I.; Kim, W.; Yang, J.W. Continuous Harvest of Marine Microalgae Using Electrolysis: Effect of Pulse Waveform of Polarity Exchange. Bioprocess Biosyst. Eng. 2014, 37, 1249–1259. [Google Scholar] [CrossRef] [PubMed]
- Halim, R.; Gladman, B.; Danquah, M.K.; Webley, P.A. Oil Extraction from Microalgae for Biodiesel Production. Bioresour. Technol. 2011, 102, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Wahidin, S.; Idris, A.; Shaleh, S.R.M. Ionic Liquid as a Promising Biobased Green Solvent in Combination with Microwave Irradiation for Direct Biodiesel Production. Bioresour. Technol. 2016, 206, 150–154. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, G.W.; Knothe, G.; Williams, J.R.; Burlow, N.P.; Reddy, C.M. Decolorization Improves the Fuel Properties of Algal Biodiesel from Isochrysis sp. Fuel 2016, 179, 229–234. [Google Scholar] [CrossRef] [Green Version]
- Das, D.; Veziroǧlu, T.N.; Das, T.; Nejat, D.V.; Das, D. Hydrogen Production by Biological Processes: A Survey of Literature. Int. J. Hydrogen Energy 2001, 26, 13–28. [Google Scholar] [CrossRef]
- Yadav, S.; Singh, V.; Mahata, C.; Das, D. Optimization for Simultaneous Enhancement of Biobutanol and Biohydrogen Production. Int. J. Hydrogen Energy 2021, 46, 3726–3741. [Google Scholar] [CrossRef]
- Sekoai, P.T.; Ghimire, A.; Ezeokoli, O.T.; Rao, S.; Ngan, W.Y.; Habimana, O.; Yao, Y.; Yang, P.; Yiu Fung, A.H.; Yoro, K.O.; et al. Valorization of Volatile Fatty Acids from the Dark Fermentation Waste Streams-A Promising Pathway for a Biorefinery Concept. Renew. Sustain. Energy Rev. 2021, 143, 110971. [Google Scholar] [CrossRef]
- Beal, C.M.; Gerber, L.N.; Sills, D.L.; Huntley, M.E.; Machesky, S.C.; Walsh, M.J.; Tester, J.W.; Archibald, I.; Granados, J.; Greene, C.H. Algal Biofuel Production for Fuels and Feed in a 100-Ha Facility: A Comprehensive Techno-Economic Analysis and Life Cycle Assessment. Algal Res. 2015, 10, 266–279. [Google Scholar] [CrossRef] [Green Version]
- Olofsson, M.; Lamela, T.; Nilsson, E.; Bergé, J.P.; del Pino, V.; Uronen, P.; Legrand, C. Seasonal Variation of Lipids and Fatty Acids of the Microalgae Nannochloropsis oculata Grown in Outdoor Large-Scale Photobioreactors. Energies 2012, 5, 1577–1592. [Google Scholar] [CrossRef] [Green Version]
- Chandrasekhar, K.; Raj, T.; Ramanaiah, S.V.; Kumar, G.; Banu, J.R.; Varjani, S.; Sharma, P.; Pandey, A.; Kumar, S.; Kim, S.-H. Algae Biorefinery: A Promising Approach to Promote Microalgae Industry and Waste Utilization. J. Biotechnol. 2022, 345, 1–16. [Google Scholar] [CrossRef]
- Koyande, A.K.; Show, P.-L.; Guo, R.; Tang, B.; Ogino, C.; Chang, J.-S. Bio-Processing of Algal Bio-Refinery: A Review on Current Advances and Future Perspectives. Bioengineered 2019, 10, 574–592. [Google Scholar] [CrossRef] [Green Version]
- Smith, C.; Hill, A.K.; Torrente-Murciano, L. Current and Future Role of Haber-Bosch Ammonia in a Carbon-Free Energy Landscape. Energy Environ. Sci. 2020, 13, 331–344. [Google Scholar] [CrossRef]
- Mogollón, J.M.; Beusen, A.H.W.; van Grinsven, H.J.M.; Westhoek, H.; Bouwman, A.F. Future Agricultural Phosphorus Demand According to the Shared Socioeconomic Pathways. Glob. Environ. Chang. 2018, 50, 149–163. [Google Scholar] [CrossRef]
- Dineshbabu, G.; Goswami, G.; Kumar, R.; Sinha, A.; Das, D. Microalgae–Nutritious, Sustainable Aqua- and Animal Feed Source. J. Funct. Foods 2019, 62, 103545. [Google Scholar] [CrossRef]
- Sayedin, F.; Kermanshahi-pour, A.; He, Q.S.; Tibbetts, S.M.; Lalonde, C.G.E.; Brar, S.K. Microalgae Cultivation in Thin Stillage Anaerobic Digestate for Nutrient Recovery and Bioproduct Production. Algal Res. 2020, 47, 101867. [Google Scholar] [CrossRef]
- Wainaina, S.; Awasthi, M.K.; Sarsaiya, S.; Chen, H.; Singh, E.; Kumar, A.; Ravindran, B.; Awasthi, S.K.; Liu, T.; Duan, Y.; et al. Resource Recovery and Circular Economy from Organic Solid Waste Using Aerobic and Anaerobic Digestion Technologies. Bioresour. Technol. 2020, 301, 122778. [Google Scholar] [CrossRef]
- Norsker, N.H.; Barbosa, M.J.; Vermuë, M.H.; Wijffels, R.H. Microalgal Production—A Close Look at the Economics. Biotechnol. Adv. 2011, 29, 24–27. [Google Scholar] [CrossRef]
- Vázquez-Romero, B.; Perales, J.A.; Pereira, H.; Barbosa, M.; Ruiz, J. Techno-Economic Assessment of Microalgae Production, Harvesting and Drying for Food, Feed, Cosmetics, and Agriculture. Sci. Total Environ. 2022, 837, 155742. [Google Scholar] [CrossRef]
- Schipper, K.; Al-Jabri, H.M.S.J.; Wijffels, R.H.; Barbosa, M.J. Techno-Economics of Algae Production in the Arabian Peninsula. Bioresour. Technol. 2021, 331, 125043. [Google Scholar] [CrossRef]
- Branco-Vieira, M.; Mata, T.M.; Martins, A.A.; Freitas, M.A.V.; Caetano, N.S. Economic Analysis of Microalgae Biodiesel Production in a Small-Scale Facility. Energy Rep. 2020, 6, 325–332. [Google Scholar] [CrossRef]
- Colusse, G.A.; Mendes, C.R.B.; Duarte, M.E.R.; de Carvalho, J.C.; Noseda, M.D. Effects of Different Culture Media on Physiological Features and Laboratory Scale Production Cost of Dunaliella salina. Biotechnol. Rep. 2020, 27, e00508. [Google Scholar] [CrossRef] [PubMed]



| Strain | Protein (%) | EAAs (%) | References |
|---|---|---|---|
| Nannochloropsis salina | 40 | 48.14 | [37] |
| Navicula incerta | 50.38 | 63.5 | [38] |
| Phaeodactylum tricornutum | 28.6 | 57.7 | [39] |
| Isochrysis galbana | 36.4 | 48.7 | [39] |
| Phaeodactylum tricornutum | 70 | N.A. | [40] |
| Tetraselmis sp. | 27.86 | 36.86 | [41] |
| Nannochloropsis granulata | 34 | 17.58 | [42] |
| Pavlova sp. | 66 | 21.25 | [43] |
| Marine Algae Strain | Cultivation Condition | Lipid (% wt./wt.) | Essential Fatty Acids | References | |
|---|---|---|---|---|---|
| EPA (%) | DHA (%) | ||||
| Nannochloropsis salina | Autotrophic | 35 | 28 | N.A | [55] |
| Pavlova lutheri | Autotrophic | 34–36 | 12.10 | 5.69 | [56] |
| Prorocentrum triestinum | Autotrophic | 3.69 | 3.66 | 20.06 | [57] |
| Isochrysis aff. galbana | Autotrophic | 51 | 0.57% | 15.2 | [44] |
| Schizochytrium sp. | Heterotrophic | 17.83 | <1 | 58.25 | [58] |
| Schizochytrium sp. | Heterotrophic | 50.35 | N.A. | 48.95 | [59] |
| Nannochloropsis oceanica | Autotrophic | 35.3 | 28.9 | - | [60] |
| Pavlova sp. | Autotrophic | 16–17 | 26.6 | 8.2 | [43] |
| Algal Strain | Pigments Content | Benefits | References |
|---|---|---|---|
| Tetraselmis suecica Chlorella salina | Lutein, β-carotene | Antioxidant, prevent eye diseases and cancer, skin conditioning | [10,72] |
| Dunaliella salina | β-carotene | Antioxidant, UV protection | [73,74] |
| Navicula incerta | Carotenes | Antioxidant | [74,75] |
| Tetraselmis sp. Picochlorum maculatum | Astaxanthin | Treatment of inflammation, improve blood flow and red blood cells | [76,77] |
| Rhodomonas salina Porphyridium purpureum | Phycoerythrin | Immunodiagnostic, tumor treatment, antioxidant, food colorant | [78,79,80] |
| Spirulina platensis, Phormidium sp. | Phycocyanin | Anti-inflammatory, antioxidant, natural dye, antidiabetic | [81,82] |
| Odontella aurita | Fucoxanthin | Antioxidant, anti-inflammatory, treating chronic diseases | [83] |
| Strain | Type of Polysaccharide | Concentration (mg L−1) | Monomers | References |
|---|---|---|---|---|
| Cylindrotheca closterium | sPS | 3.23–6.10 | Glucose, xylose | [110] |
| Isochrysis galbana | Sulphated EPS | 54.9 * | Glucose, galactose, rhamnose | [109] |
| Porphyridium cruentum | Transparent EPS | - | - | [111] |
| Arthrospira platensis | Calcium spirulan PS | - | Rhamnose, xylose, ribose, fructose | [112] |
| Heterosigma akashiwo | Sulphated EPS | Rhamnose, Galactose, fructose | [107] |
| Microalgae | Lipid Content (%) | Extraction Method | Transesterification Process | Biodiesel Yield (%) | Reference | ||
|---|---|---|---|---|---|---|---|
| °C | min | Catalyst | |||||
| Tetraselmis suecica | 23 | Chloroform-methanol (2:1) | 80 | 20 | H2SO4 | 78 | [149] |
| Nannochloropsis salina | 32.1 | Modified Bligh and Dyer | 40–45 | 180 | NaOH | 60.26 | [150] |
| Nitzchia punctata | 16 | Modified Folch | 40 | 2880 | Lipase | 87.2 | [151] |
| Dunaliella tertiolecta | 69.6 | Bligh and Dyer | 80 | 300 | NaOH | - | [152] |
| Phaeodactylum tricornutum | 36 | Methanol-Hexane (2:3) | N/A | N/A | N/A | 7-11 | [40] |
| Microalgae Strain | Carbohydrate Content (%) | Carbohydrate Extraction | Fermentation Process | Reference | ||
|---|---|---|---|---|---|---|
| Process | Yield (%) | Organism Used | Glucose to Bioethanol Conversion (g/g glucose) | |||
| Tetraselmis seucica | 27 | NaOH, 120 °C | N/A | S. cerevisiae | 0.073 | [157] |
| Synechococcus sp. | 60 | Lysozyme hydrolysis | 80 | S. cerevisiae | 0.37 | [158] |
| Chlorella vulgaris | N/A | H2SO4, 120 °C | 22 | E. coli SJL2526 | 0.4 | [159] |
| Chlorococcum infusionum | 43.8 | NaOH, 120 °C | 79.9 | S. cerevisiae | 0.26 | [160] |
| Dunaliella tertiolecta | 37.8 | Lipidextraction, Chemo-enzymatic | 81.7 | S. cerevisiae | 0.44 | [161] |
| Microalgae | Feedstock Composition | Pretreatment of Biomass | Methane Yield (L CH4/g VS) | Reference | ||
|---|---|---|---|---|---|---|
| Carbohydrate | Protein | Lipid | ||||
| Isochrysis galbana | 6.5 | 15.3 | 22.8 | Acid hydrolysis | 0.016 | [171] |
| Nannochloropsis salina | 11.5 | 17.2 | 37.2 | No treatment | 0.56 | [165] |
| Phaeodactylum tricornutum | 19 | 26.5 | 7.2 | No treatment | 0.34 | [165] |
| Nanofrustulum sp. | 9.0 | 12.5 | 13 | No treatment | 0.51 | [165] |
| Tetraselmis sp. | NA | 11.3 | NA | Supercritical fluid | 0.24 | [172] |
| Microalgae | Feedstock Composition (%) | HTL Condition | Biocrude Yield (%) | Reference | |||||
|---|---|---|---|---|---|---|---|---|---|
| Protein | Lipid | Carbohydrate | Temp (° C) | Time (min) | Solid Content (%) | Catalyst | |||
| Tetraselmis sp. | 37.5 | 14.0 | 18.5 | 350 | 10 | 20 | N/A | 45.4 | [182] |
| Nannochloropsis oculata. | 57.0 | 32.0 | 8.0 | 350 | 60 | 10 | N/A | 38 | [183] |
| Picochlorum sp. | 31.0 | 26.0 | 24.0 | 325 | 30 | 15 | N/A | 39.6 | [174] |
| Dunaliella salina | N/A | N/A | N/A | 300–450 | 60 | 10 | Ni/REHY | 72 | [184] |
| Nannochloropsis salina | 60.0 | 6.0 | 19.0 | 310 | 120 | 25 | N/A | 46 | [185] |
| Pavlova sp. | 46.9 | 13.9 | 28.0 | 350 | >60 | 14 | Na2CO3 | 47.1 | [186] |
| Microalgae | Harvesting Technique | Salinity (%NaCl) | Biomass Density (kg/m3) | Volume Used (L) | Energy Requirement (kWh/kg) | Reference |
|---|---|---|---|---|---|---|
| Picochlorum sp. | Centrifugation | 4.0 | 0.58 | 2500 | 2.49 | [174] |
| Tetraselmis sp. | Crossflow filtration | 4.64 | 0.69 | 200 | 4.65 | [178] |
| Phaeodactylum tricornutum | Electrocoagulation | 3.0 | 0.3–0.6 | 1 | 1.08 | [220] |
| Tetraselmis sp. | Coagulation-flocculation | 4.64 | 0.69 | 50 | 0.49 | [178] |
| Nannochloropsis oceanica | Pulse electrolysis | N.A. | 1.0 | 0.4 | 1.8 | [227] |
| Product | Microalgae Strain | Mode of Cultivation | Cultivation Area (ha) or Volume (m3) * | Cost (USD Kg−1 dry wt) | Reference |
|---|---|---|---|---|---|
| Biomass | Nannochloropsis oceanica | Open pond | 1 | 56.31 | [244] |
| Biomass | - | Open raceway pond | 10 | 3.06–3.70 | [245] |
| Biomass | - | Tubular photobioreactor | 10 | 4.5–5.2 | [245] |
| Biomass | Phaeodactylum tricornutum | Bubble column photobioreactor | 80,000 * | 2.12 | [246] |
| Biodiesel | Phaeodactylum tricornutum | Bubble column photobioreactor | 80,000 * | 0.35 ** | [246] |
| Biomass | Dunaliella salina | Indoor photobioreactor | 10 * | 4.64–301.61 | [247] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahata, C.; Das, P.; Khan, S.; Thaher, M.I.A.; Abdul Quadir, M.; Annamalai, S.N.; Al Jabri, H. The Potential of Marine Microalgae for the Production of Food, Feed, and Fuel (3F). Fermentation 2022, 8, 316. https://doi.org/10.3390/fermentation8070316
Mahata C, Das P, Khan S, Thaher MIA, Abdul Quadir M, Annamalai SN, Al Jabri H. The Potential of Marine Microalgae for the Production of Food, Feed, and Fuel (3F). Fermentation. 2022; 8(7):316. https://doi.org/10.3390/fermentation8070316
Chicago/Turabian StyleMahata, Chandan, Probir Das, Shoyeb Khan, Mahmoud I. A. Thaher, Mohammed Abdul Quadir, Senthil Nagappan Annamalai, and Hareb Al Jabri. 2022. "The Potential of Marine Microalgae for the Production of Food, Feed, and Fuel (3F)" Fermentation 8, no. 7: 316. https://doi.org/10.3390/fermentation8070316
APA StyleMahata, C., Das, P., Khan, S., Thaher, M. I. A., Abdul Quadir, M., Annamalai, S. N., & Al Jabri, H. (2022). The Potential of Marine Microalgae for the Production of Food, Feed, and Fuel (3F). Fermentation, 8(7), 316. https://doi.org/10.3390/fermentation8070316